Nursery Cultivation Strategies for a Widespread Mangrove (Kandelia obovata Sheue & al.): Evaluating the Influence of Salinity, Growth Media, and Genealogy
Abstract
1. Introduction
2. Materials and Methods
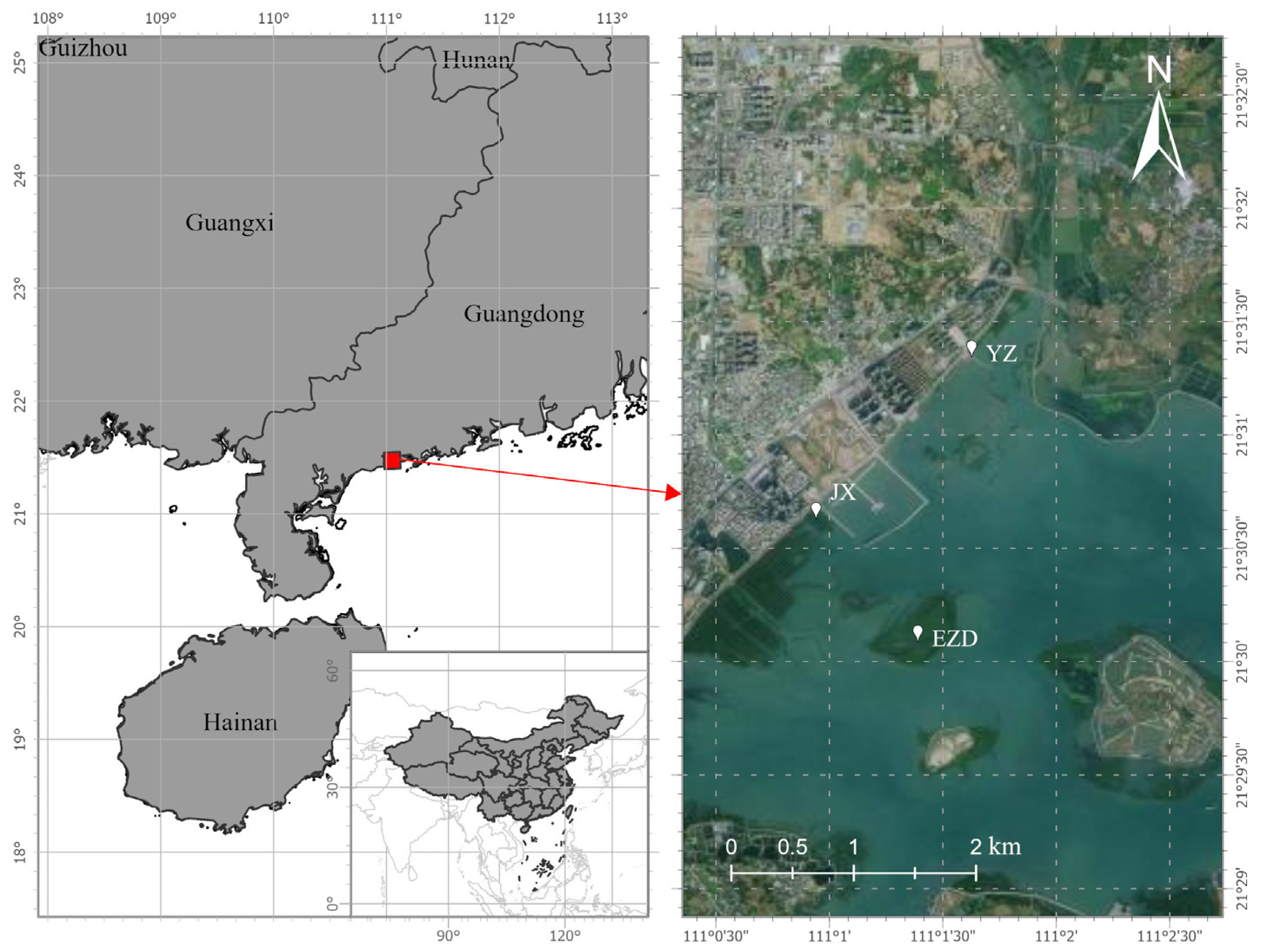
2.1. Experimental Site and Environment
2.2. Plant Material
2.3. Experimental Design and Treatments
2.4. Measurements
2.4.1. Properties of the Media
2.4.2. Seedling Height, Diameter and Mortality Rate
2.4.3. Biomass
2.4.4. Data Processing and Analysis
3. Results
3.1. Changes in Seedling Mortality Rate
3.2. Seedling Height and Diameter
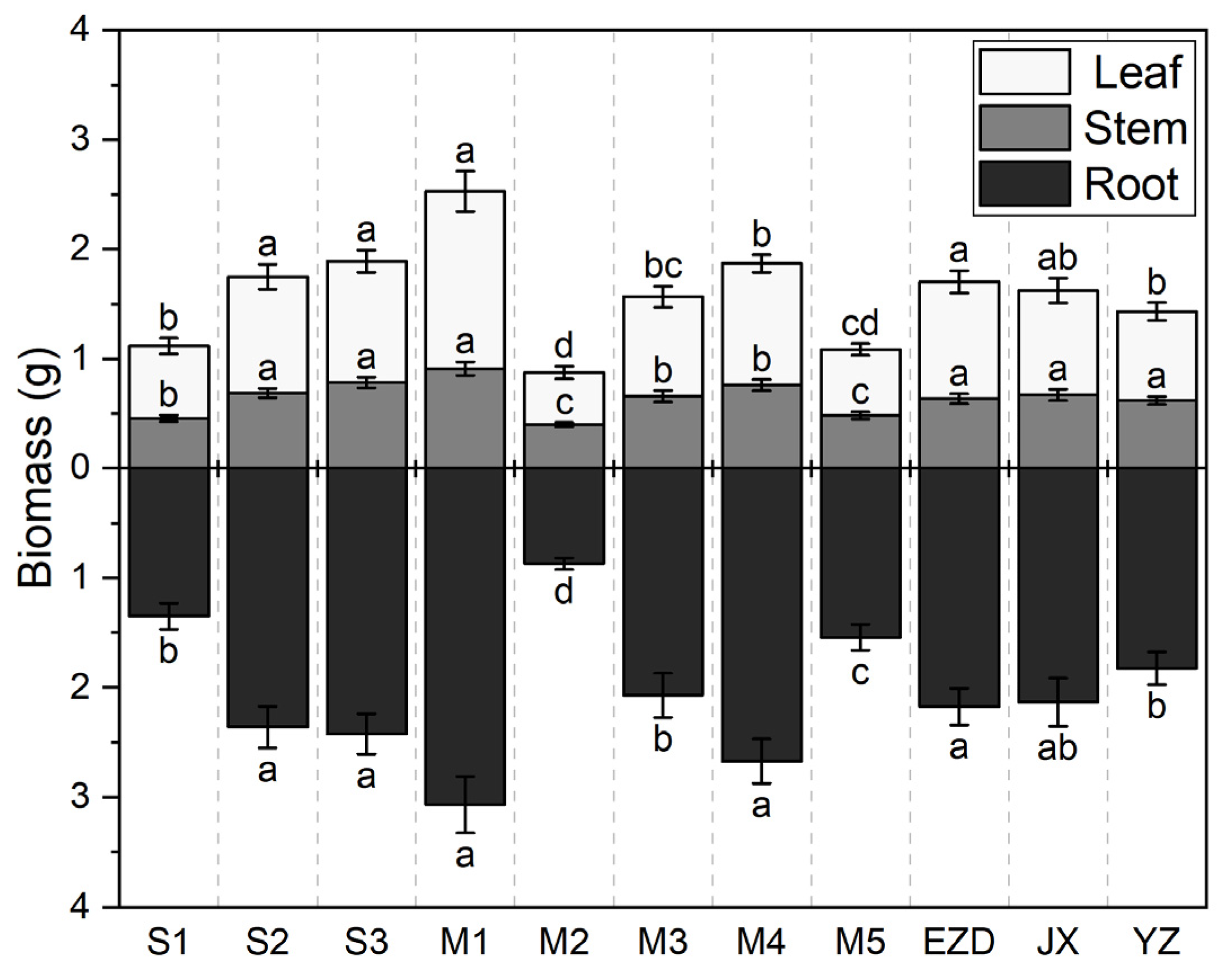
3.3. Biomass Accumulation
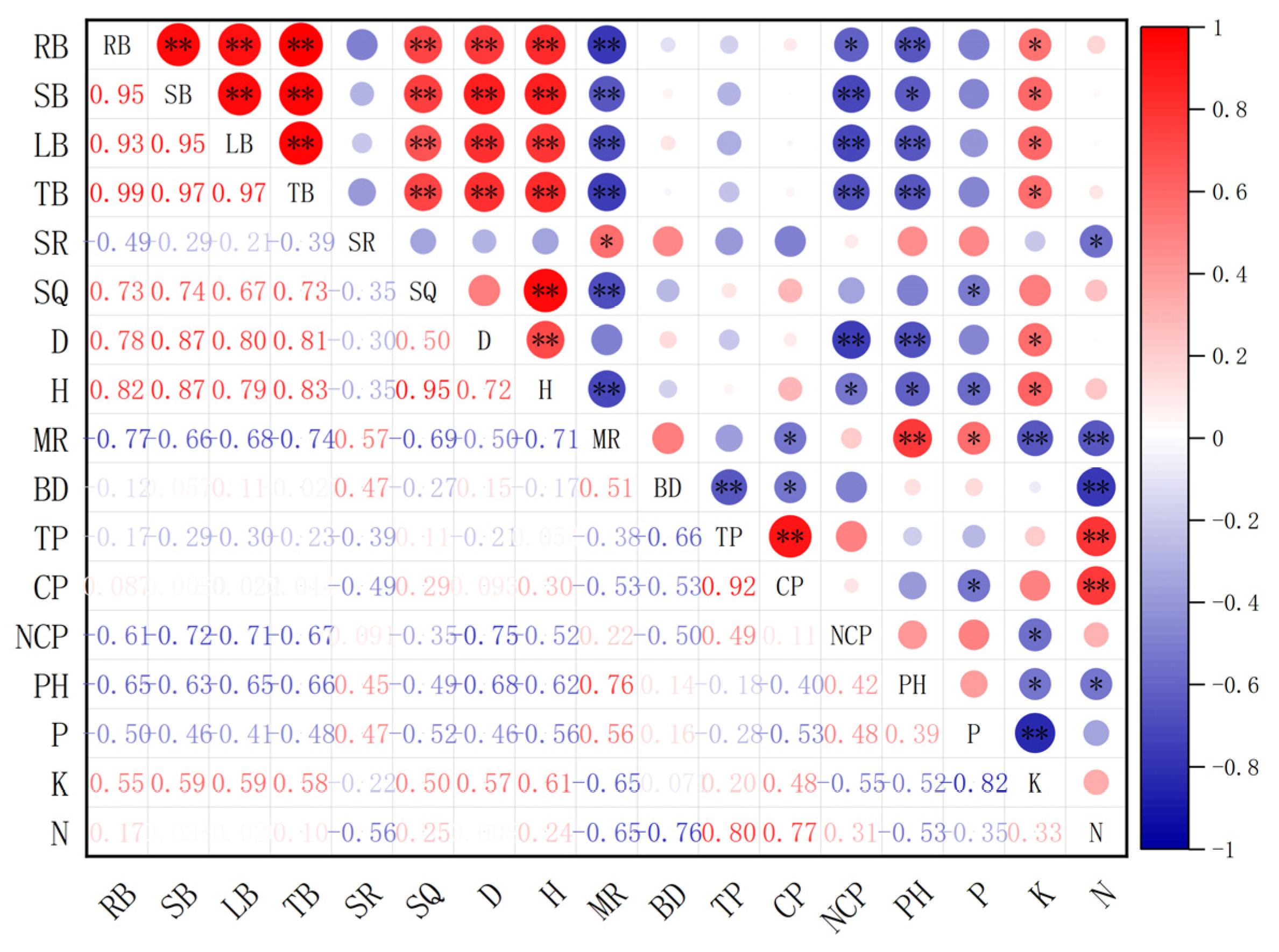
3.4. Difference of the Media Properties and Correlation Analysis
3.5. Evaluate the Influence of Growth Media, Salinity, Genealogy, and Their Combination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Zhang, X.X.; Lin, P.Z.; Chen, X.P. Coastal Protection by Planted Mangrove Forest during Typhoon Mangkhut. J. Mar. Sci. Eng. 2022, 10, 1288. [Google Scholar] [CrossRef]
- Lin, C.Y.; Fu, C.Y.; Liu, Y.; Zhang, M.Q.; Liu, Y.; Wu, W.Y.; Wang, L.X.; Lin, X.H.; Fu, X.M. Assessing the changes of the monetary value of mangrove ecosystem services in China and its application. Front. Environ. Sci. 2022, 10, 1018801. [Google Scholar] [CrossRef]
- Brander, L.M.; Wagtendonk, A.J.; Hussain, S.S.; McVittie, A.; Verburg, P.H.; de Groot, R.S.; van der Ploeg, S. Ecosystem service values for mangroves in Southeast Asia: A meta-analysis and value transfer application. Ecosyst. Serv. 2012, 1, 62–69. [Google Scholar] [CrossRef]
- Fan, H.Q.; Wang, W.Q. Some Thematic Issues for Mangrove Conservation in China. J. Xiamen Univ. Nat. Sci. 2017, 56, 323–330. [Google Scholar]
- Wong, W.Y.; Al-Ani, A.K.I.; Hasikin, K.; Khairuddin, A.S.M.; Razak, S.A.; Hizaddin, H.F.; Mokhtar, M.I.; Azizan, M.M. Water, Soil and Air Pollutants’ Interaction on Mangrove Ecosystem and Corresponding Artificial Intelligence Techniques Used in Decision Support Systems—A Review. IEEE Access 2021, 9, 105532–105563. [Google Scholar] [CrossRef]
- Malik, A.; Fensholt, R.; Mertz, O. Mangrove exploitation effects on biodiversity and ecosystem services. Biodivers. Conserv. 2015, 24, 3543–3557. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Hu, H.Y.; Chen, S.Y.; Wang, W.Q.; Dong, K.Z.; Lin, G.H. Current status of mangrove germplasm resources and key techniques for mangrove seedling propagation in China. Chin. J. Appl. Ecol. 2012, 23, 939–946. [Google Scholar]
- Gu, W.C.; Li, W.Y. Analysis and Suggestions of Benefit Sharing Policies Relating to Forest Tree Germplasm Resources in China. World For. Res. 2007, 20, 66–69. [Google Scholar]
- Kordrostami, M.; Rahimi, M. Molecular markers in plants: Concepts and applications. Genet. Third Millenn. 2015, 13, 4024–4031. [Google Scholar]
- Rossetto, M.; Rymer, P.D. Applications of Molecular Markers in Plant Conservation. In Molecular Markers in Plants; John Wiley & Sons, Ltd: New York, NY, USA, 2012; pp. 81–98. [Google Scholar]
- Budiadi, B.; Widiyatno, W.; Nurjanto, H.H.; Hasani, H.; Jihad, A.N. Seedling Growth and Quality of Avicennia marina (Forssk.) Vierh. under Growth Media Composition and Controlled Salinity in an Ex Situ Nursery. Forests 2022, 13, 684. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Deng, R.; Chen, Q.; Wang, J.; Lu, X. Geographic variations of hypocotyl and seedling growth traits for Kandelia obovata with different provenances. Chin. J. Ecol. 2020, 39, 1769–1777. [Google Scholar]
- Hong, Z.; Liu, X.; Zhang, N.; Yang, Z.; Cui, Z.; Xu, D. Effects of different mediums and container sizes on the growth performance of Dalbergia odorifera at seedling and early afforestation stages. J. Cent. South Univ. For. Technol. 2022, 42, 22–29, 38. [Google Scholar] [CrossRef]
- Li, X.J.; Long, Z.W.; She, Z.L.; Shi, F.H.; Shen, Y.B. Effects of Substrate Composition and Container Size on Growth of Container Seedlings of Tilia miquelianal. J. Northeast. For. Univ. 2023, 51, 46–52. [Google Scholar]
- Liu, B.E.; Liao, B.W.; Han, J. Effect of Different Substrates on Seedlings Growth and Physiological and Biochemical Indices of P. pinnata and H. littoralis. J. Anhui Agric. Sci. 2011, 39, 3449–3453. [Google Scholar] [CrossRef]
- Liu, Z.X.; Li, R.S.; Zou, W.T.; Qiu, Z.F.; Yu, N.; Yang, J.C. Transplantation Effects of Different Nursery Substrates on the Growth of Tissue Culture Seedlings of Mytilaria laosensis. Chin. J. Trop. Crops 2020, 41, 655–660. [Google Scholar] [CrossRef]
- Hanagata, N.; Takemura, T.; Karube, I.; Dubinsky, Z. Salt water relationships in mangroves. Isr. J. Plant Sci. 1999, 47, 63–76. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt tolerance mechanisms in mangroves: A review. Trees 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Reef, R.; Lovelock, C.E. Regulation of water balance in mangroves. Ann. Bot. 2015, 115, 385–395. [Google Scholar] [CrossRef]
- Hayes, M.A.; Jesse, A.; Welti, N.; Tabet, B.; Lockington, D.; Lovelock, C.E. Groundwater enhances above-ground growth in mangroves. J. Ecol. 2019, 107, 1120–1128. [Google Scholar] [CrossRef]
- Santini, N.S.; Reef, R.; Lockington, D.A.; Lovelock, C.E. The use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiologia 2015, 745, 59–68. [Google Scholar] [CrossRef]
- Mitsch, W.; Gosselink, J. Wetlands, 3rd ed.; John Wiley & Sons Inc: New York, NY, USA, 2000; pp. 335–373. [Google Scholar]
- Saenger, P. Mangrove Ecology, Silviculture and Conservation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 11–18. [Google Scholar]
- Suárez, N.; Medina, E. Influence of salinity on Na+ and K+ accumulation, and gas exchange in Avicennia germinans. Photosynthetica 2006, 44, 268–274. [Google Scholar] [CrossRef]
- Ball, M.C. Interactive effects of salinity and irradiance on growth: Implications for mangrove forest structure along salinity gradients. Trees 2002, 16, 126–139. [Google Scholar] [CrossRef]
- Xin, C. Mangroves Distribution Pattern along the River and the Adapted Mechanism to Salt Water and Fresh Water Rotation. Master’s Thesis, Xiamen University, Xiamen, China, 2008. [Google Scholar]
- Werner, A.; Stelzer, R. Physiological responses of the mangrove Rhizophora mangle grown in the absence and presence of NaCl. Plant Cell Environ. 1990, 13, 243–255. [Google Scholar] [CrossRef]
- Wu, W.Z.; Zhao, Z.X.; Yang, S.; Liang, L.C.; Chen, Q.X.; Lu, X.; Liu, X.; Zhang, X.W. The mangrove forest distribution and analysis of afforestation effect in Zhejiang Province. J. Trop. Oceanogr. 2022, 41, 67–74. [Google Scholar]
- Zhao, Y.Z.; Zhong, Y.F.; Ye, C.T.; Liang, P.P.; Pan, X.B.; Zhang, Y.Y.; Zhang, Y.H.; Shen, Y.J. Multi-omics analyses on Kandelia obovata reveal its response to transplanting and genetic differentiation among populations. BMC Plant Biol. 2021, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gu, X.; Chen, X.; Guo, X.; Cai, L.; Lin, L.; Chen, W.; Dong, Y.; Feng, H.; Cai, H.; et al. Coastal wetland restoration based on mangrove Kandelia obovata substitution controlling Spartina alterniflora invasion: A case study of Aojiang Estuary in Zhejiang Province. Chin. J. Ecol. 2020, 39, 1761–1768. [Google Scholar] [CrossRef]
- Liao, B. The Adaptability of Seedlings of Three Mangrove Species to Tide-flooding and Water Salinity. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2010. [Google Scholar]
- Fang, F.; Huiying, G.; Li, Z.; Zhang, X. Physiological Adaptation of Six Mangrove Seedlings to Different Salinity. Bull. Bot. Res. 2023, 43, 881–889. [Google Scholar] [CrossRef]
- Tang, M. A Study on Adaptability of Mangrove Species to Water Salinity. Master’s Thesis, Northeast Normal University, Changchun, China, 2014. [Google Scholar]
- He, Q.F.; Peng, Y.H.; Liu, X.; Jiang, Y.; Lu, G.D. Experiment on hypocotyle seedling growth of Kandelia candel in controlled habitats. China For. Sci. Technol. 2014, 28, 108–110. [Google Scholar]
- Chou, J.B.; Chen, S.B.; Huang, L.; Zheng, C.F.; Chi, W.; Xie, Q.L.; Li, S.L.; Zeng, G.Q. Effects of Different Soils on Hypocotyles Growth of Kandelia candel (L.) Druce. J. Anhui Agric. Sci. 2010, 38, 17514–17517. [Google Scholar]
- Zhang, X.; Wee, K.S.A.; Kajita, T.; Cao, K. Effects of provenance on leaf structure and function of two mangrove species: The genetic adaptation to temperature. Chin. J. Plant Ecol. 2021, 45, 1241–1250. [Google Scholar] [CrossRef]
- Kodikara, K.A.S.; Jayatissa, L.P.; Huxham, M.; Dahdouh-Guebas, F.; Koedam, N. The effects of salinity on growth and survival of mangrove seedlings changes with age. Acta Bot. Bras. 2018, 32, 37–46. [Google Scholar] [CrossRef]
- Li, M.; Guan, W.; Jiang, Z.; Liao, B.; Chen, Y. Dynamics of Plant Community Structures and Soil Properties in Artificial Mangrove Area of Nansha Wetlands in Guangzhou. Wetl. Sci. 2023, 21, 716–722. [Google Scholar] [CrossRef]
- Hutahaean, E.E.; Kusmana, C.; Dewi, H.R. Studi Kemampuan Tumbuh Anakan Mangrove Jenis Rhizophora mucronata, Bruguiera gimnorrhiza, dan Avicennia marina Pada Berbagai Tingkat Salinitas. J. Manaj. Hutan Trop. 1999, 5, 77–85. [Google Scholar]
- Clough, B.F. Growth and Salt Balance of the Mangroves Avicennia marina (Forsk.) Vierh. And Rhizophora stylosa Griff. In Relation to Salinity. Funct. Plant Biol. 1984, 11, 419–430. [Google Scholar] [CrossRef]
- Chang, Y.S.; Peng, L.; Tsuneo, N. Influence of Soil Particle on Salt Effect of Kandelia candel Seedling. J. Nat. Sci. Xiamen 2002, 41, 559–564. [Google Scholar] [CrossRef]
- Lv, X.; Li, D.; Yang, X.; Zhang, M.; Deng, Q. Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species. Forests 2019, 10, 250. [Google Scholar] [CrossRef]
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Zhou, X.H.; Li, Y.Q.; Xiao, Z.Y.; Sun, J.J. Influences of Growth Substrate Ratio, Container Size and SRF on Cunninghamia lanceolata (Lamb.) Hook Container Seedling. Acta Agric. Univ. Jiangxiensis 2017, 39, 72–81. [Google Scholar]
- Ye, Y.; Tan, F.Y.; Lu, C.Y. Effects of soil texture and light on growth and physiology parameters in Kandelia candel. Chin. J. Plant Ecol. 2001, 25, 42–49. [Google Scholar]
- Liu, Q.C.; Ling, W.K.; Xiang, Z.Q.; Hua, L.Q. Test on The Physical and Chemical Characteristics of Several Organic Materials And Compare to The Traditional Peat Moss Cultural Media. North. Hortic. 2007, 7, 37–39. [Google Scholar]
- Auni, A.H.; Bachtiar, B.; Paembonan, S.A.; Larekeng, S.H. Growth analysis of mangrove (Rhizophora apiculata bl) propagule toward differences in types of water and planting media at Makassar mangrove center. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 12137. [Google Scholar] [CrossRef]
- Deng, R. Studies on Physiological Response of Kandelia obovata for Natural and Artificial Cooling. Master’s Thesis, Zhejiang Agriculture & Forest University, Hangzhou, China, 2018. [Google Scholar]
- Ouahzizi, B.; Elbouny, H.; Sellam, K.; Alem, C.; Bakali, A.H. Effects of temperature, provenance, drought stress and salinity on seed germination response and early seedling stage of Thymus atlanticus (Ball) Roussine. J. Appl. Res. Med. Aroma 2023, 34, 100482. [Google Scholar] [CrossRef]
- Qi, W.W.; Ma, H.Y.; Li, S.Y.; Wu, H.T.; Zhao, D.D. Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances. Biology 2023, 12, 1343. [Google Scholar] [CrossRef]
- Saenger, P.; West, P.W. Phenotypic variation of the mangrove species Avicennia marina (Forssk.) Vierh. from seven provenances around Australia. Aquat. Bot. 2018, 149, 28–32. [Google Scholar] [CrossRef]


| M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|
| BD/g·cm−3 | 1.46 ± 0.01 ab | 1.49 ± 0.02 a | 1.50 ± 0.03 a | 1.28 ± 0.05 bc | 1.25 ± 0.06 c |
| TP/% | 43.40 ± 0.45 b | 42.32 ± 0.85 b | 44.07 ± 1.28 b | 45.50 ± 0.87 b | 53.06 ± 0.69 a |
| CP/% | 37.93 ± 0.12 b | 33.43 ± 1.14 b | 37.22 ± 1.31 b | 37.00 ± 0.77 b | 43.52 ± 1.04 a |
| NCP/% | 5.48 ± 0.38 c | 8.88 ± 0.32 a | 6.85 ± 0.16 bc | 8.50 ± 0.66 ab | 9.53 ± 0.41 a |
| PH | 6.72 ± 0.05 c | 8.97 ± 0.39 a | 8.51 ± 0.21 ab | 7.83 ± 0.2 bc | 7.67 ± 0.24 bc |
| P/g·kg−1 | 0.24 ± 0.03 bc | 0.52 ± 0.04 a | 0.18 ± 0.01 c | 0.33 ± 0.03 b | 0.26 ± 0.02 bc |
| K/g·kg−1 | 6.44 ± 0.03 a | 3.68 ± 0.69 b | 5.65 ± 0.45 ab | 5.05 ± 0.37 ab | 5.64 ± 0.37 ab |
| N/g·kg−1 | 0.47 ± 0.03 bc | 0.38 ± 0.01 c | 0.42 ± 0.01 bc | 0.51 ± 0.01 ab | 0.60 ± 0.02 a |
| Source | F Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Height | Diameter | |||||||
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| Salinity (S) | 6.119 ** | 15.657 ** | 15.724 ** | 10.863 ** | 39.433 ** | 31.214 ** | 20.735 ** | 12.347 ** |
| Growth media (M) | 42.357 ** | 39.142 ** | 26.678 ** | 17.055 ** | 54.099 ** | 35.813 ** | 24.792 ** | 15.700 ** |
| Genealogy (G) | 15.552 ** | 2.420 | 0.985 | 0.256 | 21.285 ** | 7.057 ** | 2.602 | 1.415 |
| S × M | 5.754 ** | 5.006 ** | 5.937 ** | 5.197 ** | 2.907 ** | 1.354 | 1.742 | 2.112 * |
| S × G | 1.879 | 0.404 | 0.416 | 0.276 | 3.968 ** | 3.948 ** | 5.191 ** | 3.388 * |
| M × G | 1.578 | 3.990 ** | 3.081 ** | 2.650 ** | 2.274 * | 3.282 ** | 2.040 * | 1.530 |
| S × M × G | 1.497 | 1.86 * | 1.909 * | 1.852 * | 1.838 * | 1.066 | 0.784 | 0.660 |
| Source | F Value | |||
|---|---|---|---|---|
| Root | Stem | Leaf | Total | |
| Salinity (S) | 38.544 ** | 38.604 ** | 13.288 ** | 40.357 ** |
| Growth media (M) | 48.929 ** | 34.996 ** | 27.183 ** | 54.754 ** |
| Genealogy (G) | 3.871 * | 0.943 | 3.621 * | 4.267 * |
| S × M | 5.152 ** | 4.167 ** | 4.404 ** | 6.262 ** |
| M × G | 3.266 ** | 1.479 | 1.419 | 2.802 ** |
| S × G | 2.297 | 0.969 | 0.836 | 1.895 |
| S × M × G | 1.379 | 0.888 | 1.259 | 1.087 |
| Treatment | MI | DQI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity | Media | EZD | JX | YZ | Average | Rank | EZD | JX | YZ | Average | Rank |
| S1 | M1 | 0.548 | 0.390 | 0.330 | 0.423 | 9 | 1.364 | 0.700 | 0.632 | 0.899 | 9 |
| M2 | 0.125 | 0.115 | 0.174 | 0.138 | 15 | 0.480 | 0.279 | 0.382 | 0.380 | 15 | |
| M3 | 0.280 | 0.219 | 0.097 | 0.198 | 13 | 0.966 | 0.524 | 0.393 | 0.628 | 13 | |
| M4 | 0.572 | 0.423 | 0.515 | 0.504 | 6 | 1.159 | 0.971 | 1.282 | 1.137 | 6 | |
| M5 | 0.221 | 0.227 | 0.206 | 0.218 | 12 | 0.583 | 0.580 | 1.123 | 0.762 | 11 | |
| S2 | M1 | 0.923 | 0.878 | 0.784 | 0.862 | 1 | 1.862 | 2.123 | 1.694 | 1.893 | 1 |
| M2 | 0.179 | 0.074 | 0.197 | 0.150 | 14 | 0.424 | 0.687 | 0.602 | 0.571 | 14 | |
| M3 | 0.560 | 0.390 | 0.465 | 0.471 | 7 | 1.211 | 1.161 | 0.936 | 1.103 | 7 | |
| M4 | 0.484 | 0.664 | 0.483 | 0.544 | 5 | 1.097 | 1.716 | 1.212 | 1.342 | 5 | |
| M5 | 0.427 | 0.489 | 0.436 | 0.451 | 8 | 1.362 | 0.691 | 0.798 | 0.950 | 8 | |
| S3 | M1 | 0.769 | 0.932 | 0.636 | 0.779 | 2 | 1.607 | 1.931 | 1.391 | 1.643 | 2 |
| M2 | 0.252 | 0.240 | 0.180 | 0.224 | 11 | 0.603 | 1.051 | 0.604 | 0.753 | 12 | |
| M3 | 0.690 | 0.547 | 0.484 | 0.574 | 4 | 1.577 | 1.308 | 1.160 | 1.348 | 4 | |
| M4 | 0.471 | 0.773 | 0.528 | 0.591 | 3 | 1.282 | 1.719 | 1.131 | 1.377 | 3 | |
| M5 | 0.378 | 0.428 | 0.432 | 0.413 | 10 | 0.762 | 0.667 | 0.901 | 0.777 | 10 | |
| Average | 0.459 | 0.453 | 0.396 | 1.089 | 1.074 | 0.950 | |||||
| Rank | 1 | 2 | 3 | 1 | 2 | 3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yang, J.; Qin, J.; Li, J.; Liu, X.; Wei, P. Nursery Cultivation Strategies for a Widespread Mangrove (Kandelia obovata Sheue & al.): Evaluating the Influence of Salinity, Growth Media, and Genealogy. Forests 2024, 15, 574. https://doi.org/10.3390/f15040574
Zhou J, Yang J, Qin J, Li J, Liu X, Wei P. Nursery Cultivation Strategies for a Widespread Mangrove (Kandelia obovata Sheue & al.): Evaluating the Influence of Salinity, Growth Media, and Genealogy. Forests. 2024; 15(4):574. https://doi.org/10.3390/f15040574
Chicago/Turabian StyleZhou, Jinghang, Jingjun Yang, Jie Qin, Jinhua Li, Xiu Liu, and Penglian Wei. 2024. "Nursery Cultivation Strategies for a Widespread Mangrove (Kandelia obovata Sheue & al.): Evaluating the Influence of Salinity, Growth Media, and Genealogy" Forests 15, no. 4: 574. https://doi.org/10.3390/f15040574
APA StyleZhou, J., Yang, J., Qin, J., Li, J., Liu, X., & Wei, P. (2024). Nursery Cultivation Strategies for a Widespread Mangrove (Kandelia obovata Sheue & al.): Evaluating the Influence of Salinity, Growth Media, and Genealogy. Forests, 15(4), 574. https://doi.org/10.3390/f15040574






