Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Land Uses
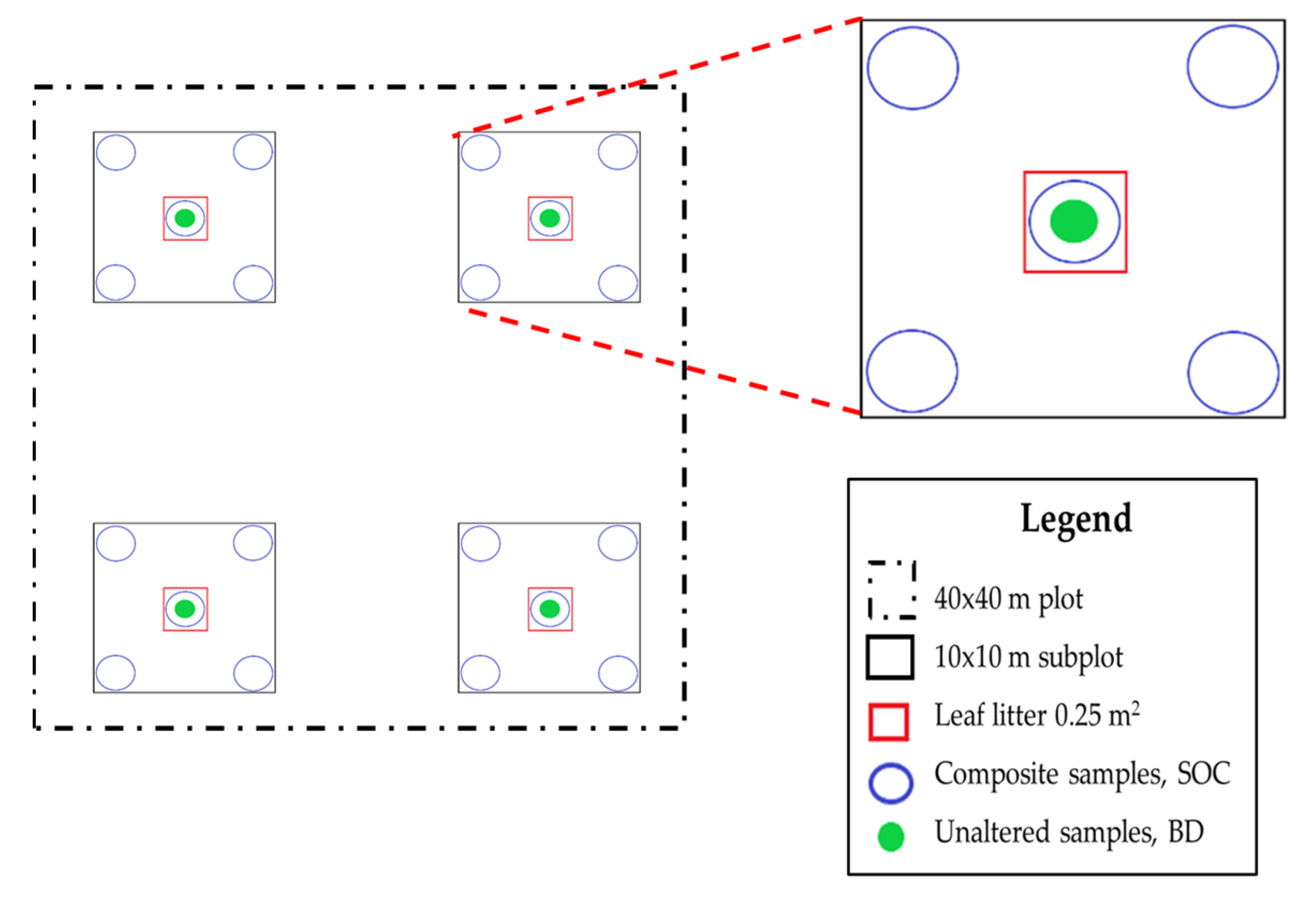
2.2. Field Sampling
2.3. Floristic Composition
2.4. Estimation of Carbon Sequestration
2.5. Data Analysis
3. Results
3.1. Floristic Composition
3.2. Importance Value Index (IVI)
3.3. Diversity Index
3.4. Biomass Importance Value (BIV)
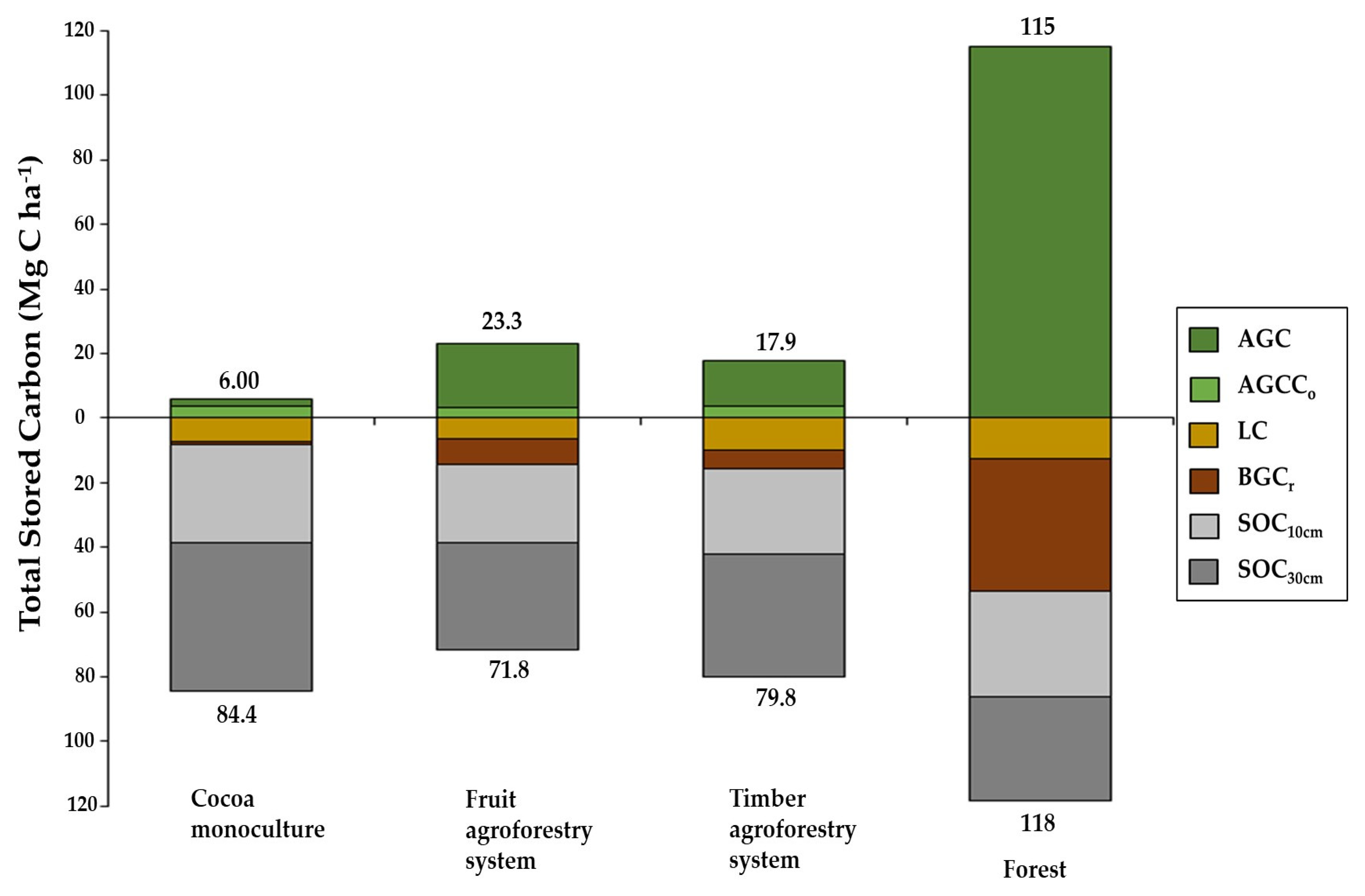
3.5. Total Stored Carbon
4. Discussion
4.1. Floristic Composition
4.2. Stored Biomass Carbon
4.3. Carbon Stored in the Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lombo, D.F.; Burbano, E.; Arias, J.A.; Rivera, M. Carbon Storage in Tree Biomass Dispersed in Pastures in the Arid Caribbean Region of Colombia. For. Syst. 2023, 32, e002. [Google Scholar] [CrossRef]
- Pocomucha, V.S.; Alegre, J.; Abregú, L. Análisis Socioeconómico y Carbono Almacenado En Sistemas Agroforestales de Cacao (Theobroma Cacao L.) En Huánuco. Ecol. Appl. 2016, 15, 107–114. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Salas-Ruiz, A.; Changoluisa, D.; Bravo-Medina, C. Towards Sustainable Urban Planning for Puyo (Ecuador): Amazon Forest Landscape as Potential Green Infrastructure. Sustainability 2020, 12, 4768. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Chakraborty, S.; Nair, V.D. Changes in Soil Carbon Stocks across the Forest-Agroforest-Agriculture/Pasture Continuum in Various Agroecological Regions: A Meta-Analysis. Agric. Ecosyst. Environ. 2018, 266, 55–67. [Google Scholar] [CrossRef]
- Del Cisne Jiménez-Torres, A. La Diversidad Mejora El Almacenamiento de Carbono En Los Bosques Tropicales. Recimundo 2021, 5, 316–323. [Google Scholar] [CrossRef]
- Andrade, H.J.; Segura, M.A.; Suárez, J.C. Growth and Carbon Sequestration in Biomass of Cordia Alliodora in Andean Agroforestry Systems with Coffee. Agrofor. Syst. 2023, 97, 1435–1446. [Google Scholar] [CrossRef]
- Jurado Riascos, M.A.; Ordóñez Jurado, H.R.; Lagos Burbano, T.C. Carbon Storage Evaluation in Coffee Production Systems (Coffea Arabica L.), Consacá, Nariño, Colombia. Rev. Luna Azul 2020, 51, 166–181. [Google Scholar] [CrossRef]
- Luis Enrique Arteaga, N.; Jairo Efrén Burbano, N. Efectos Del Cambio Climático: Una Mirada al Campo. Rev. Cienc. Agric. 2018, 35, 79–91. [Google Scholar] [CrossRef]
- Avellán Rivera, A.R.; Barreto Dolin, E.; Peralta Tercero, E.D.J. Carbono En Biomasa Aérea, Sistema Agroforestal de Theobroma Cacao L. Laboratorio Natural, Los Laureles 2018. Rev. Univ. Del Caribe 2020, 24, 98–106. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- Rojas-Vargas, E.P.; Silva-Agudelo, E.D.; Guillén-Motta, A.Y.; Motta-Delgado, P.A.; Herrera-Valencia, W. Carbono Almacenado En Estrato Arbóreo de Sistemas Ganaderos y Naturales Del Municipio de Albania, Caquetá, Colombia. Cienc. Agric. 2019, 16, 35–46. [Google Scholar] [CrossRef]
- García-Quintana, Y.; Arteaga-Crespo, Y.; Torres-Navarrete, B.; Bravo-Medina, C.; Robles-Morillo, M. Aerial Biomass of Botanical Families in Piedmont Evergreen Forest Subject to Intervention Levels. Colomb. For. 2021, 24, 45–59. [Google Scholar] [CrossRef]
- Jones, I.L.; DeWalt, S.J.; Lopez, O.R.; Bunnefeld, L.; Pattison, Z.; Dent, D.H. Above- and Belowground Carbon Stocks Are Decoupled in Secondary Tropical Forests and Are Positively Related to Forest Age and Soil Nutrients Respectively. Sci. Total Environ. 2019, 697, 133987. [Google Scholar] [CrossRef] [PubMed]
- García-Cox, W.; López-Tobar, R.; Herrera-Feijoo, R.J.; Tapia, A.; Heredia-R, M.; Toulkeridis, T.; Torres, B. Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador. Forests 2023, 14, 1406. [Google Scholar] [CrossRef]
- Jadán, O.; Quizhpe, W.; Edwin, P.; González, M.; Ponce, E.; Aguirre, Z.; Peña, D. Richness Floristic and Stored Carbon on Three Altitudinal Floors of Amazon Forests Zamora Chinchipe, Ecuador. Bosques Latid. Cero 2017, 7, 56–71. [Google Scholar]
- Vicuña-Miñano, E.; Baker, T.R.; Banda, R.K.; Honorio, E.; Monteagudo, A.; Phillips, O.L.; Del Castillo, D.; Farfan, W.; Flores, G.; Huaman, D.; et al. El Sumidero de Carbono En Los Bosques Primarios Amazónicos Es Una Oportunidad Para Lograr La Sostenibilidad de Su Conservación. Folia Amaz. 2019, 27, 101–109. [Google Scholar] [CrossRef]
- Solis, R.; Vallejos-Torres, G.; Arévalo, L.; Marín-Díaz, J.; Ñique-Alvarez, M.; Engedal, T.; Bruun, T.B. Carbon Stocks and the Use of Shade Trees in Different Coffee Growing Systems in the Peruvian Amazon. J. Agric. Sci. 2020, 158, 450–460. [Google Scholar] [CrossRef]
- Ballesteros-Possú, W.; Valencia, J.C.; Navia-Estrada, J.F. Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia. Sustainability 2022, 14, 9447. [Google Scholar] [CrossRef]
- Casanova-Lugo, F.; Petit-Aldana, J.; Solorio-Sánchez, J. Los Sistemas Agroforestales Como Alternativa a La Captura de Carbono En El Trópico Mexicano. Rev. Chapingo Ser. Cienc. For. Ambiente 2011, XVII, 133–143. [Google Scholar] [CrossRef]
- Hernández Nuñez, H.E.; Andrade, H.J.; Suárez Salazar, J.C.; Sánchez, A.J.R.; Gutiérrez, S.D.R.; Gutiérrez García, G.A.; Trujillo, E.; Casanoves, F. Almacenamiento de Carbono En Sistemas Agroforestales En Llanos Orientales de Colombia. Rev. Biol. Trop. 2021, 69, 352–368. [Google Scholar] [CrossRef]
- Alcívar Torres, A.; García Vásquez, G.; Cadena Piedrahita, D.; Sánchez Vásquez, V. Evaluación y Planificación de Sistemas Agroforestales Sustentables de Cacao (Theobroma Cacao L.) y Bambú (Guadua angustifolia K.), Montalvo, Ecuador. Rev. Cienc. Investig. 2019, 4, 10–21. [Google Scholar] [CrossRef]
- Schneidewind, U.; Niether, W.; Armengot, L.; Schneider, M.; Sauer, D.; Heitkamp, F.; Gerold, G. Carbon Stocks, Litterfall and Pruning Residues in Monoculture and Agroforestry Cacao Production Systems. Exp. Agric. 2019, 55, 452–470. [Google Scholar] [CrossRef]
- Rodríguez, L.; Suárez, J.C.; Rodriguez, W.; Artunduaga, K.J.; Lavelle, P. Agroforestry Systems Impact Soil Macroaggregation and Enhance Carbon Storage in Colombian Deforested Amazonia. Geoderma 2021, 384, 114810. [Google Scholar] [CrossRef]
- Jadán, O.; Torres, B.; Günter, S. Influencia Del Uso de La Tierra Sobre Almacenamiento de Carbono En Sistemas Productivos y Bosque Primario En Napo, Reserva de Biosfera Sumaco, Ecuador. Cienc. Tecnol. 2012, 1, 173–186. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Cerri, C.E.P.; Tormena, C.A.; Cerri, C.C. A Soil Management Assessment Framework (SMAF) Evaluation of Brazilian Sugarcane Expansion on Soil Quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226. [Google Scholar] [CrossRef]
- Martínez, H.E.; Fuentes, E.J.P.; Acevedo, H.E. Soil Organic Carbon and Soil Properties. Rev. Cienc. Suelo Nutr. Veg. 2008, 8, 68–96. [Google Scholar]
- Naoki, K.; Gómez, M.I.; Schneider, M. Selection of Different Cacao (Theobroma cacao, Malvaceae) Production Systems by Birds in Alto Beni, Bolivia-a Cafeteria Experiment in the Field. Ecol. Boliv. 2017, 52, 100–115. [Google Scholar]
- Hairiah, K.; van Noordwijk, M.; Sari, R.R.; Saputra, D.D.; Widianto; Suprayogo, D.; Kurniawan, S.; Prayogo, C.; Gusli, S. Soil Carbon Stocks in Indonesian (Agro) Forest Transitions: Compaction Conceals Lower Carbon Concentrations in Standard Accounting. Agric. Ecosyst. Environ. 2020, 294, 106879. [Google Scholar] [CrossRef]
- Marques-Monroe, P.H.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Laís-Carvalho, V. Carbon and Nitrogen Occluded in Soil Aggregates under Cacao-Based Agroforestry Systems in Southern Bahia, Brazil. J. Soil. Sci. Plant Nutr. 2022, 22, 1326–1339. [Google Scholar] [CrossRef]
- Aryal, D.R.; Gómez-González, R.R.; Hernández-Nuriasmú, R.; Morales-Ruiz, D.E. Carbon Stocks and Tree Diversity in Scattered Tree Silvopastoral Systems in Chiapas, Mexico. Agrofor. Syst. 2019, 93, 213–227. [Google Scholar] [CrossRef]
- Jose, S.; Bardhan, S. Agroforestry for Biomass Production and Carbon Sequestration: An Overview. Agrofor. Syst. 2012, 86, 105–111. [Google Scholar] [CrossRef]
- Arango, P.C.Z. Composition and Structure of Shade Canopy in Coffee Agroforestry Systems of Three Municipalities of Cundinamarca, Colombia. Cienc. Florest. 2019, 29, 685–697. [Google Scholar] [CrossRef]
- Cárdenas, A.; Moliner, A.; Hontoria, C.; Ibrahim, M. Ecological Structure and Carbon Storage in Traditional Silvopastoral Systems in Nicaragua. Agrofor. Syst. 2019, 93, 229–239. [Google Scholar] [CrossRef]
- Torres, B.; Bravo, C.; Torres, A.; Tipán-Torres, C.; Vargas, J.C.; Herrera-Feijoo, R.J.; Heredia-R, M.; Barba, C.; García, A. Carbon Stock Assessment in Silvopastoral Systems along an Elevational Gradient: A Study from Cattle Producers in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2023, 15, 449. [Google Scholar] [CrossRef]
- Caguana-Muyolema, J.A.; Román-Cáceres, D.A.; Cevallos-Rodríguez, J.P.; Roman-Robalino, D.A. Estudio Florístico En El Ecosistema Páramo de La Quebrada Galgalán, comunidad de Atillo. Polo Del Conoc. 2020, 5, 1020–1042. [Google Scholar]
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; García, A. Structure and above Ground Biomass along an Elevation Small-Scale Gradient: Case Study in an Evergreen Andean Amazon Forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- González-Molina, P. Ecología e Interpretación Del Paisaje. UF0733; Tutot Formación; La Rioja: Logroño, Spain, 2018. [Google Scholar]
- Alberto Mora Donjuán, C.; Alanís Rodríguez, E.; Jiménez Pérez, J.; Aurelio González Tagle, M.; Israel Yerena Yamallel, J.; Cuellar, C. Estructura, Composición Florística y Diversidad Del Matorral Espinoso Tamaulipeco, México. Ecol. Apl. 2013, 12, 29–34. [Google Scholar] [CrossRef][Green Version]
- López-Santiago, J.G.; Casanova-Lugo, F.; Villanueva-López, G.; Díaz-Echeverría, V.F.; Solorio-Sánchez, F.J.; Martínez-Zurimendi, P.; Aryal, D.R.; Chay-Canul, A.J. Carbon Storage in a Silvopastoral System Compared to That in a Deciduous Dry Forest in Michoacán, Mexico. Agrofor. Syst. 2019, 93, 199–211. [Google Scholar] [CrossRef]
- Pradhan, B.M.; Awasthi, K.D.; Bajracharya, R.M. Soil Organic Carbon Stocks under Different Forest Types in Pokhare Khola Sub-Watershed: A Case Study from Dhading District of Nepal. WIT Trans. Ecol. Environ. 2012, 157, 535–546. [Google Scholar] [CrossRef]
- Dantas, D.; de Castro Nunes Santos Terra, M.; Pinto, L.O.R.; Calegario, N.; Maciel, S.M. Above and Belowground Carbon Stock in a Tropical Forest in Brazil. Acta Sci. Agron. 2020, 43, e48276. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, A.C.; Brown, A.S.; Cairns, A.M.A.; Chambers, J.Q.; Eamus, A.D.; Foïster, A.H.; Fromard, A.F.; Higuchi, N.; Kira, A.T.; et al. Ecosystem Ecology Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecología 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. Secuestro de Carbono En Sistemas Agroforestales de Cacao y Café Ubicados En La Reserva de Biosfera Sumaco; Hess, B., Fedlmeier, C., Moreno, A., Eds.; GESORED; Tena: Quito, Ecuador, 2011. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root Biomass Allocation in the World’s Upland Forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties-Agronomy Monograph No. 9; Segoe Rd.: Madison, WI, USA; West Lafayette, IN, USA, 1982; pp. 539–579. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; SSSA Book Series; Klute, A., Ed.; ASA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar] [CrossRef]
- Pla, I. Medición y Evaluación de Propiedades Fisicas de Los Suelos: Dificultades y Errores Más Frecuentes. I-Propiedades Mecánicas. Suelos Ecuat. 2011, 40, 75–93. [Google Scholar]
- Hernández-Hernández, R.M.; Ramírez, E.; Castro, I.; Cano, S. Cambios En Indicadores de Calidad de Suelos de Ladera Reforestados Con Pinos. Agrociencia 2008, 42, 253–266. [Google Scholar]
- Moreno, C.E. Métodos Para Medir La Biodiversidad; M&T–Manuales y Tesis SEA: Zaragoza, Spain, 2001; Volume 1, pp. 1–88. [Google Scholar]
- Rojas-Molina, J.; Ramos-Calderon, P.F.; Castro-Zabala, M.A.; Pesca-Moreno, A.; Vargas-Valenzuela, Y.; Escobar-Pachajoa, L. Structure and Floristic Composition of Forests Associated to Theobroma Species in the Colombian Amazon. Rev. Mex. Cienc. For. 2021, 12, 128–150. [Google Scholar] [CrossRef]
- Carmo Lima, R.; Marques da Silva Silva, B.; Doff Sotta, E.; Couteron, P.; da Silva Aparício, P.; Ferreira dos Santos, V.; Lima Bueno, R.; Klaus Santos dos Santos, Y.; Bruno Brito Ramos, M. Análise Fitossociológica de Um Trecho de Floresta Ombrófila Densa Na Amazônia Oriental. Rev. Arq. Cient. IMMES Macapa AP 2019, 2, 89–100. [Google Scholar]
- Tian, D.; Xiang, Y.; Seabloom, E.; Wang, J.; Jia, X.; Li, T.; Li, Z.; Yang, J.; Guo, H.; Niu, S. Soil Carbon Sequestration Benefits of Active versus Natural Restoration Vary with Initial Carbon Content and Soil Layer. Commun. Earth Environ. 2023, 4, 83. [Google Scholar] [CrossRef]
- Sierra, C.A.; Del Valle, J.I.; Restrepo, H.I. Total Carbon Accumulation in a Tropical Forest Landscape. Carbon Balance Manag. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Agus, C.; Putra, P.B.; Faridah, E.; Wulandari, D.; Napitupulu, R.R.P. Organic Carbon Stock and Their Dynamics in Rehabilitation Ecosystem Areas of Post Open Coal Mining at Tropical Region. Procedia Eng. 2016, 159, 329–337. [Google Scholar] [CrossRef][Green Version]
- Calderón-Balcázar, A.; Cárdenas, C.D.; Díaz-Vasco, O.; Fandiño, E.; Márquez, T.; Pizano, C. Biomass and Carbon Stocks of Four Vegetation Types in the Llanos Orientales of Colombia (Mapiripán, Meta). Trees For. People 2023, 12, 100380. [Google Scholar] [CrossRef]
- Aynekulu, E.; Suber, M.; Noordwijk, M.; Arango, J.; Roshetko, J.M.; Rosenstock, T.S. Carbon Storage Potential of Silvopastoral Systems of Colombia. Land 2020, 9, 309. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]


| Types of Land Uses | Description |
|---|---|
| Chakra-type agroforestry system (AFS) | These arise as an alternative for management and production that is friendly to the ecosystem, since they resemble the succession of a natural forest [27,28,29], and generally adaptation in the tropics is very high [19]. In the Amazon of Ecuador, this type of management has been traditionally and culturally practiced directly and indirectly, with the implementation of the so-called Amazon chakras, part of the cultural identity of the populations that inhabit the Amazon, and little by little it has been established as a diversified farming model in association with cocoa, coffee, and timber species, among others, and may vary according to the purpose, type of soil, and management practices in relation to geographical location [4,28], since it provides food, medicinal, construction, habitat, and nutrient cycling resources and contributes to carbon storage [27,30,31]. Therefore, it captures atmospheric CO2 and promotes the conservation of biodiversity [22,32]. |
| Chakra | As traditional production systems similar to agrarian management of the forest on a family scale and an alternative to industrial and intensive crops, these systems or polycultures called chakra arise, which have been traditionally developed by Amazonian populations for the purposes of family subsistence. The chakras or diversified production systems are associated with different kind of crops, like: yucca, banana, naranjilla, coffee, cocoa, etc., as well as fruit trees (e.g., Inga edulis, Citrus sinensis, Terminalia oblonga, Citrus aurantiifolia, Bactris gasipaes, etc.), timber trees (e.g., Cordia alliodora, Piptocoma discolor, Schefflera morototoni, Persea americana, etc.), and fauna species in a natural state, without neglecting the variety of medicinal plants. The ideology of these communities is to maintain a balance between chakra and nature, without altering the forest, the life that inhabits that space, or the soil, the main support of life. |
| Cocoa monoculture (CMC) | Considered as a production system devoid of tree species, dependent on the use of agrochemicals, fertilizers, and amendments. From an economic point of view, it can be considered a very efficient production system, but in the long term, it can become a threat to the remaining natural resource that still remains. Faced with this scenario, and the interest in maintaining tropical forests and conserving their biodiversity, alternatives should be chosen that involve economic, social, cultural, and ecological interests, promoting the conversion and implementation of nature-friendly management systems such as forestry, agroforestry, or silvopastoral [2]. |
| Forest | Land covered with exuberant natural vegetation, home to a great biodiversity of tree plant species (e.g., Otoba glycycarpa, Inga sp., Cecropia sciadophylla, Apeiba membranacea, Mabea standleyi, Protium sagotianum, Iriartea deltoidea, Chimarrhis glabriflora, Sterculia colombiana, Virola flexuosa, Annona papilionella, etc.), with a high carbon storage potential in plant biomass that contributes to a notable reduction of greenhouse gases [5]. In addition to being recognized for maintaining a balance between all elements, it is highly efficient and at the same time has the capacity to withstand changes [2,5]. |
| Parameter | Equation | References | |
|---|---|---|---|
| Basal area (Ba) | [14,34] | (1) | |
| Relative density (RD) | [14] | (2) | |
| Relative dominance (RDom) | [14] | (3) | |
| Importance value index (IVI) | [14,35] | (4) | |
| Biomass importance value (BIV) | [36] | (5) | |
| Simpson index (S) | [30,35] | (6) | |
| Margalef index (D) | [14,33,37,38] | (7) | |
| Shannon–Wiener index (H’) | [38] | (8) |
| Family | Species | N° ind. | RD % | RDom % | IVI % |
|---|---|---|---|---|---|
| Cocoa monoculture (CMC) | |||||
| Boraginaceae | Cordia alliodora | 4 | 30.8 | 34.2 | 32.5 |
| Arecaceae | Bactris gasipaes | 3 | 23.1 | 17.0 | 20.0 |
| Lauraceae | Persea americana | 2 | 15.4 | 14.1 | 14.7 |
| Urticaceae | Pourouma cecropiifolia | 1 | 7.69 | 12.5 | 10.1 |
| Malvaceae | Ceiba samauma | 1 | 7.69 | 11.2 | 9.46 |
| Subtotal | 11 | 84.6 | 89.0 | 86.8 | |
| Fruit agroforestry system (FAFS) | |||||
| Fabaceae | Inga edulis | 40 | 46.0 | 35.8 | 40.9 |
| Boraginaceae | Cordia alliodora | 13 | 14.9 | 27.8 | 21.3 |
| Rutaceae | Citrus sinensis | 13 | 14.9 | 8.03 | 11.5 |
| Rutaceae | Citrus aurantiifolia | 9 | 10.3 | 4.86 | 7.60 |
| Meliaceae | Cedrela odorata | 3 | 3.45 | 5.62 | 4.54 |
| Subtotal | 78 | 89.7 | 82.0 | 85.9 | |
| Timber agroforestry system (TAFS) | |||||
| Boraginaceae | Cordia alliodora | 21 | 25.3 | 30.2 | 27.8 |
| Asteraceae | Piptocoma discolor | 26 | 31.3 | 22.0 | 26.7 |
| Fabaceae | Inga edulis | 6 | 7.23 | 7.85 | 7.54 |
| Araliaceae | Schefflera morototoni | 3 | 3.61 | 9.00 | 6.31 |
| Arecaceae | Bactris gasipaes | 6 | 7.23 | 4.39 | 5.81 |
| Subtotal | 62 | 74.7 | 73.5 | 74.1 | |
| Forest | |||||
| Myristicaceae | Otoba glycycarpa | 24 | 8.30 | 9.11 | 8.71 |
| Fabaceae | Inga sp. | 9 | 3.11 | 5.91 | 4.51 |
| Urticaceae | Cecropia sciadophylla | 8 | 2.77 | 6.20 | 4.48 |
| Malvaceae | Apeiba membranacea | 8 | 2.77 | 6.18 | 4.47 |
| Euphorbiaceae | Mabea standleyi | 9 | 3.11 | 5.41 | 4.26 |
| Burseraceae | Protium sagotianum | 10 | 3.46 | 3.31 | 3.38 |
| Arecaceae | Iriartea deltoidea | 11 | 3.81 | 1.98 | 2.89 |
| Rubiaceae | Chimarrhis glabriflora | 8 | 2.77 | 2.76 | 2.76 |
| Malvaceae | Sterculia colombiana | 6 | 2.08 | 2.31 | 2.19 |
| Myristicaceae | Virola flexuosa | 7 | 2.42 | 1.95 | 2.18 |
| Subtotal | 100 | 34.6 | 45.1 | 39.9 | |
| Rest of species (71) | 189 | 65.4 | 54.9 | 60.1 | |
| Index | CMC | FAFS | TAFS | Forest | Significance |
|---|---|---|---|---|---|
| Shannon H’ | 0.977 b (±0.389) | 0.873 b (±0.424) | 1.39 b (±0.482) | 3.49 a (±0.122) | *** |
| Simpson S | 0.577 ab (±0.176) | 0.423 b (±0.230) | 0.655 ab (±0.141) | 0.960 a (±0.000) | ** |
| Margalef D | 1.34 b (±0.577) | 1.24 b (±0.397) | 1.79 b (±0.804) | 9.34 a (±1.16) | *** |
| Ba ha−1 | 0.793 c (±0.177) | 5.18 b (±0.613) | 4.06 bc (±1.39) | 27.1 a (±2.47) | *** |
| AGB ha−1 | 5.34 c (±1.29) | 42.9 b (±4.25) | 30.7 bc (±10.5) | 245 a (±22.1) | *** |
| Family | Species | RD % | Ba % | AGB % | BIV % |
|---|---|---|---|---|---|
| Cocoa monoculture (CMC) | |||||
| Boraginaceae | Cordia alliodora | 30.8 | 34.2 | 38.1 | 34.4 |
| Arecaceae | Bactris gasipaes | 23.1 | 17.0 | 12.8 | 17.6 |
| Lauraceae | Persea americana | 15.4 | 14.1 | 16.7 | 15.4 |
| Malvaceae | Ceiba samauma | 7.69 | 11.3 | 14.4 | 11.1 |
| Urticaceae | Pourouma cecropiifolia | 7.69 | 12.5 | 10.1 | 10.1 |
| Subtotal | 84.6 | 89.0 | 92.2 | 88.6 | |
| Fruit agroforestry system (FAFS) | |||||
| Fabaceae | Inga edulis | 46.0 | 35.8 | 30.9 | 37.5 |
| Boraginaceae | Cordia alliodora | 14.9 | 27.8 | 27.6 | 23.4 |
| Rutaceae | Citrus sinensis | 14.9 | 8.03 | 7.56 | 10.2 |
| Combretaceae | Terminalia oblonga | 1.15 | 6.87 | 12.5 | 6.85 |
| Rutaceae | Citrus aurantiifolia | 10.3 | 4.86 | 4.28 | 6.49 |
| Subtotal | 87.4 | 83.3 | 82.9 | 84.5 | |
| Timber agroforestry system (TAFS) | |||||
| Boraginaceae | Cordia alliodora | 25.3 | 30.2 | 30.4 | 28.6 |
| Asteraceae | Piptocoma discolor | 31.3 | 22.0 | 17.0 | 23.5 |
| Fabaceae | Inga edulis | 7.23 | 7.85 | 8.34 | 7.80 |
| Araliaceae | Schefflera morototoni | 3.61 | 9.00 | 9.66 | 7.42 |
| Lauraceae | Persea americana | 4.82 | 4.69 | 5.27 | 4.93 |
| Subtotal | 72.3 | 73.8 | 70.7 | 72.3 | |
| Forest | |||||
| Myristicaceae | Otoba glycycarpa | 8.30 | 9.11 | 7.46 | 8.29 |
| Urticaceae | Cecropia sciadophylla | 2.77 | 6.20 | 8.55 | 5.84 |
| Fabaceae | Inga sp. | 3.11 | 5.91 | 6.41 | 5.15 |
| Euphorbiaceae | Mabea standleyi | 3.11 | 5.41 | 6.90 | 5.14 |
| Malvaceae | Apeiba membranácea | 2.77 | 6.18 | 4.73 | 4.56 |
| Burseraceae | Protium sagotianum | 3.46 | 3.31 | 3.51 | 3.43 |
| Rubiaceae | Chimarrhis glabriflora | 2.77 | 2.76 | 4.02 | 3.18 |
| Annonaceae | Annona papilionella | 1.38 | 2.65 | 2.88 | 2.30 |
| Arecaceae | Iriartea deltoidea | 3.81 | 1.98 | 0.90 | 2.23 |
| Malvaceae | Sterculia colombiana | 2.08 | 2.31 | 1.99 | 2.13 |
| Subtotal | 33.6 | 45.8 | 47.4 | 42.2 | |
| Units | Components | Types of Land Uses | Significance | |||
|---|---|---|---|---|---|---|
| CMC | FAFS | TAFS | Forest | |||
| Mg C ha−1 | AGC | 2.51 c (±0.61) | 20.2 b (±2.00) | 14.4 bc (±4.93) | 115 a (±10.4) | *** |
| AGCCo | 3.49 a (±1.30) | 3.10 a (±1.14) | 3.49 a (±0.62) | --- | n/s | |
| TAGC | 6.00 c (±1.83) | 23.3 b (±3.09) | 17.9 bc (±5.16) | 115 a (±10.4) | *** | |
| BGCr | 1.02 c (±0.22) | 7.79 b (±0.65) | 5.65 bc (±1.92) | 40.7 a (±3.15) | *** | |
| LC | 7.33 a (±3.15) | 6.37 a (±2.30) | 10.1 a (±3.95) | 12.8 a (±2.37) | n/s | |
| SOC10cm | 30.0 a (±5.61) | 24.6 a (±7.10) | 26.3 a (±8.72) | 32.6 a (±1.89) | n/s | |
| SOC30cm | 46.0 a (±1.90) | 33.1 a (±5.64) | 37.8 a (±14.2) | 32.3 a (±5.32) | n/s | |
| TSC | 90.4 b (±4.23) | 95.1 b (±15.2) | 97.8 b (±16.2) | 233 a (±7.68) | *** | |
| Mg CO2 ha−1 | AGC | 9.20 c (±2.23) | 73.9 b (±7.32) | 53.0 bc (±18.1) | 421 a (±38.1) | |
| AGCCo | 12.8 a (±4.78) | 11.4 a (±4.17) | 12.8 a (±2.28) | --- | ||
| TAGC | 22.0 c (±6.73) | 85.3 b (±11.3) | 65.8 bc (±18.9) | 421 a (±38.1) | ||
| BGCr | 3.75 c (±0.82) | 28.6 b (±2.39) | 20.7 bc (±7.05) | 149 a (±11.5) | ||
| LC | 26.9 a (±11.6) | 23.4 a (±8.44) | 37.0 a (±14.5) | 46.9 a (±8.69) | ||
| SOC10cm | 110 a (±20.6) | 90.1 a (±26.0) | 96.3 a (±32.0) | 119 a (±6.92) | ||
| SOC30cm | 169 a (±6.98) | 121 a (±20.7) | 139 a (±52.2) | 119 a (±19.5) | ||
| CO2eq total | 331 b (±15.5) | 349 b (±55.9) | 358 b (±59.5) | 856 a (±28.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huera-Lucero, T.; Lopez-Piñeiro, A.; Torres, B.; Bravo-Medina, C. Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon. Forests 2024, 15, 557. https://doi.org/10.3390/f15030557
Huera-Lucero T, Lopez-Piñeiro A, Torres B, Bravo-Medina C. Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon. Forests. 2024; 15(3):557. https://doi.org/10.3390/f15030557
Chicago/Turabian StyleHuera-Lucero, Thony, Antonio Lopez-Piñeiro, Bolier Torres, and Carlos Bravo-Medina. 2024. "Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon" Forests 15, no. 3: 557. https://doi.org/10.3390/f15030557
APA StyleHuera-Lucero, T., Lopez-Piñeiro, A., Torres, B., & Bravo-Medina, C. (2024). Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon. Forests, 15(3), 557. https://doi.org/10.3390/f15030557









