Abstract
Soil nutrient transformation and the microbial metabolism are primarily regulated by soil microorganisms, including fungi and bacteria, which exhibit distinct growth patterns, energy substrate utilization, and survival strategies. Despite their significance, our understanding of the key microorganisms governing the soil microbial metabolism and multifunctionality in subtropical woodlands remains limited. To address this knowledge gap, we conducted a large-scale investigation and assessment of the soil microbial metabolic limitation and soil multifunctionality in Camellia oleifera Abel and Pinus massoniana Lamb. woodlands in subtropical China. Our results reveal that the microbial phosphorus limitation was more severe in C. oleifera compared to P. massoniana woodlands. Nonetheless, the pattern of carbon metabolic limitation for microbes and soil multifunctionality was similar in both types of woodland. Specifically, the microbial carbon limitation was positively associated with both bacterial and fungal richness, while the microbial phosphorus limitation was significantly correlated with fungi including the richness and community structure in the P. massoniana woodland. By contrast, we did not observe significant correlations between microbial metabolic limitation indices and microbial parameters in C. oleifera woodlands. Regarding soil multifunctionality, the results reveal a strong positive correlation between the soil multifunctionality and fungal community in both P. massoniana and C. oleifera woodlands. Furthermore, our structural equation modeling revealed that the soil fungal community, rather than the bacterial community, had a significant effect on the microbial metabolic limitation and soil multifunctionality. Overall, our study provides profound insights into the relative importance of bacterial and fungal communities in shaping the soil microbial metabolic limitation and soil multifunctionality in subtropical woodlands. The findings of our study have important implications for the management and conservation of subtropical woodlands.
1. Introduction
Microorganisms play a pivotal role in driving the Earth’s biogeochemical cycles and maintaining soil ecosystem functions. The metabolic parameters of microbial communities can serve as critical indicators of ecosystem processes and functioning [1]. Extracellular enzymes produced by soil microbes are essential agents of biological metabolism, breaking down complex organic matter, depolymerizing macromolecules, and facilitating microbial nutrient acquisition [2,3]. Ecoenzymatic stoichiometry provides a theoretical framework for predicting soil microbial processes and nutrient limitations based on the activities of extracellular enzymes. For example, the ratio of β-1,4-glucosidase (BG) to β-1,4-N-acetylglucosaminidase (NAG) (BG/NAG) is a widely used indicator of the microbial carbon (C) versus nitrogen (N) limitation, with higher ratios indicating a greater C limitation [4,5]. Furthermore, the enzyme vector model was proposed to reveal the relative C vs. nutrient limitation using the vector length and reveal the relative phosphorus (P) vs. N limitation by using the vector angle, reflecting the relative demands of the microbial community in a manner independent of total enzyme activity [6,7].
Previous studies have demonstrated that the microbial resource limitation is primarily governed by the C, N, and P substrates [8]. Our recent study conducted in a subtropical plantation ecosystem has highlighted the co-limiting role of both C and P in regulating the soil microbial metabolism [9]. Traditionally, it has been believed that soil parameters such as temperature, pH, and moisture are the primary regulators of microbial resource limitation patterns. A case study conducted along an elevation gradient found that the mean annual temperature explained a substantial proportion of the variations in the microbial N limitation, suggesting that temperature plays a key role in shaping microbial nutrient acquisition and utilization strategies [10]. It should be noted that the soil microbial metabolism can be influenced by the microbial community composition, as different microbial communities are involved in diverse metabolic processes and exhibit varying levels of synthetic ecoenzyme activity [11]. For instance, soil bacteria grow faster and have higher turnover rates than fungi, leading to higher levels of N, P, and organic compounds in bacteria. This can cause changes in the microbial nutrient demand and the activities of extracellular enzymes when the microbial community composition changes [12,13]. Nevertheless, the question of whether bacterial or fungal communities dominate the soil microbial metabolic limitation and microbial processes in terrestrial ecosystems remains a fundamental and unresolved issue, with important implications for ecosystem functioning and global biogeochemical cycles.
Soil multifunctionality refers to the ecosystem services provided by soil via multiple physical, chemical, and biological processes [14,15]. Previous studies have focused on the contribution of soil chemical and physical parameters to soil multifunctionality due to rapid changes in soil biological parameters, such as microbial biomass, soil moisture, and pH [16,17,18]. However, recent studies have shown that the soil biodiversity and microbial community composition are crucial determinants of soil ecosystem multifunctionality, including carbon sequestration, greenhouse gas emission, and nutrient leaching [19,20]. Notably, soil fungal diversity, but not bacterial diversity, has been positively correlated with soil multifunctionality in boreal forest ecosystems [21], while the opposite was found in subtropical forest ecosystems [22]. Interestingly, in a degraded alpine meadow, bacterial diversity was negatively correlated with potential soil multifunctionality [23]. These findings suggest that the relationship between microbial diversity and ecosystem multifunctionality is likely to be ecosystem-dependent, potentially due to differential metabolite production and a division of labor among bacterial and fungal groups during soil processes [24]. Taking the soil carbon process as an example, fungi primarily break down complex organic matter, such as particulate terrigenous carbon, while bacteria mainly recycle simple organic compounds. Furthermore, changes in both bacterial and fungal community structures can cause changes in the soil function under different environmental conditions [25]. Therefore, it is vital to quantify the relative importance of bacterial and fungal communities for soil multifunctionality, as this can provide appropriate management practices to improve the soil function in a given ecosystem.
Camellia oleifera Abel and Pinus massoniana Lamb. are widely cultivated tree species in the subtropical region of southern China. The former is one of the major woody oil plants globally, while the latter is a crucial timber species. These two tree species exhibit distinct physiological traits. Specifically, C. oleifera is a broad-leaved evergreen woody shrub or a small tree that grows slowly, usually not exceeding 3 m in height, whereas P. massoniana is a coniferous evergreen tree that grows rapidly, with high productivity [26,27]. The marked difference in plant growth rates between the two species can have a profound impact on soil nutrient cycling and the microbial community. Furthermore, P. massoniana leaves have a higher lignin concentration and lignin/N ratio than C. oleifera leaves, which leads to slower litter decomposition, resulting in lower-quantity and -quality carbon resources for the soil microbial community [28]. Consequently, changes in the microbial community, which are influenced by the tree species, can affect the microbial resource limitation and soil multifunctionality. To investigate the link between the microbial community and soil metabolic limitation and multifunctionality in a particular ecosystem, we examined soil enzyme activities as well as bacterial and fungal communities in both C. oleifera and P. massoniana woodlands across the subtropical region of southern China. Specifically, our study aims to (i) quantify the patterns of the soil microbial metabolic limitation and multifunctionality in the two woodlands and (ii) explore whether there is a similar link between bacterial and fungal communities and the microbial metabolic limitation and soil multifunctionality in the aforementioned woodlands.
2. Materials and Methods
2.1. Site Description and Soil Sampling
Thirty-two sites that have both C. oleifera and P. massoniana woodlands were randomly selected across the subtropical region in Southern China, which spans from 25.36° N to 29.70° N and 110.47° E to 115.28° E (Figure S1). The climate in this region is classified as a subtropical monsoon climate, with the average mean annual temperature and mean annual precipitation across the study sites being 16 °C and 1375 mm, respectively [29]. In each study site, the distance between C. oleifera and P. massoniana woodlands did not exceed 1 km to ensure consistent microclimate and topography conditions. Before collecting soil samples, we selected typical C. oleifera and P. massoniana woodlands in each study site, namely, C. oleifera and P. massoniana were the dominant species within these two woodlands, respectively. In the selected C. oleifera woodlands, simple artificial management practices such as branch thinning have been implemented, while the P. massoniana woodlands represent a naturally growing forest. The tree density in the C. oleifera woodlands typically ranged from 750 to 1500 plants per hectare, while the density for the P. massoniana woodlands was approximately between 2000 and 5000 plants per hectare. Within each 10 × 10 m quadrat, 12 plots were used to collect the soil and subsequently blended into a single composite soil sample. The size of the woodland of both C. oleifera and P. massoniana at each study site was varied. To ensure representative sampling, the woodland size was required to be at least 0.7 hectares, with the majority exceeding 2 hectares. In each woodland for one study site, three 10 × 10 m quadrats were designated to collect the soil samples. This means that each study site had three soil samples from the C. oleifera woodland and three soil samples from the P. massoniana woodland. All soil samples were transported to the laboratory using boxes with ice bags. The soil samples were sieved (<2 mm) to remove plant litter and gravel in the laboratory, and then divided into three subsamples. One subsample was stored at –80 °C for microbial DNA analysis, another was stored at 4 °C for soil microbial biomass and enzyme activity determination, and the last one was air-dried for physicochemical parameter analysis.
2.2. Soil Physicochemical and Biological Parameters Analysis
Soil pH was measured in a deionized water suspension with a water-to-soil mass ratio of 1:2.5 using a digital pH meter (Mettler-Toledo 320, Shanghai, China). Soil organic carbon (SOC) was determined using the Walkley–Black method with potassium dichromate [30]. Soil total nitrogen (TN) was measured using the Kjeldahl digestion method with flow injection analysis [31]. Soil Olsen-P content was extracted using 0.5 M NaHCO3 and determined using the ammonium molybdate method on a UV spectrophotometer (Shimadzu UV-2550, Shimadzu, Kyoto, Japan) at 882 nm [32].
Soil microbial biomass C, N, and P contents (MBC, MBN, and MBP, respectively) were measured using the chloroform-fumigation method. Briefly, trichloromethane-fumigated and non-fumigated soil samples were incubated at 25 °C for 24 h. After fumigant removal, the samples were extracted with 0.5 M K2SO4 for the MBC and MBN content and 0.5 M NaHCO3 for the MBP content. Finally, MBC, MBN, and MBP were calculated as the difference between the fumigated and non-fumigated C, N, and P contents. The derived conversion factors were 0.45, 0.54, and 0.40 for MBC, MBN, and MBP, respectively [33].
The activities of five soil nutrient-acquisition enzymes, including two C-acquisition enzymes (β-1,4-glucosidase [BG] and β-D-cellobiosidase [CBH]), two N-acquisition enzymes (L-leucine aminopeptidase [LAP] and β-N-acetylglucosaminidase [NAG]) and a P-acquisition enzyme (acid phosphatase [AP]), were determined using standard fluorometric techniques [34] (Table S1). Soil enzyme activities were normalized as nanomoles of substrate released per hour per gram of SOC (nmol g SOC−1 h−1).
2.3. Assessment of Soil Microbial Metabolic Limitation and Soil Multifunctionality
The pattern of the soil microbial metabolic limitation in the woodlands was evaluated using two independent methods. First, a scatter plot of soil enzymatic activities based on stoichiometric and metabolic theories, represented by the N/P versus C/N enzymatic activity ratios, was exhibited to reflect the four limitation statuses, including P limitation, N limitation, C&P limitation, and N&P limitation [4]. The second method was the vector model represented by the vector length (L) and angle (A°) to describe the soil microbial nutrient limitation. An increasing vector length indicated the high relative C limitation of microbes, whereas microbial P and N limitations were suggested by a vector angle of >45° and <45°, respectively [6]. The vector length and angle (°) were calculated using the following formulas:
X = (CBH + BG)/(CBH + BG + AP)
y = (CBH + BG)/(CBH + BG + NAG + LAP)
Vector Length = SQRT (x2 + y2)
Vector angle (°) = DEGREES (ATAN2 (x,y))
Soil multifunctionality was assessed using both the averaging and multiple-threshold approaches [35]. The averaging approach aimed to combine a collection of soil functions related to C, N, and P cycling, including soil extracellular enzyme activities, microbial biomass, SOC, TN, and Olsen-P, which were Z score-transformed and then averaged into a single index to obtain a quantitative soil multifunctionality index. The multiple-threshold approach was also applied for calculating the soil multifunctionality (Figures S2 and S3) [36]. Considering that averaging multifunctionality was strongly correlated with the maximum observed function number at the 25%, 50%, and 75% thresholds (r = 0.87, p < 0.001; r = 0.90, p < 0.001; r = 0.79, p < 0.001, respectively, Table S2), we used the value of the averaging approach to represent the soil multifunctionality in the main analyses.
2.4. Soil DNA Extraction, High-Throughput Sequencing, and Data Analysis
Soil DNA was extracted using the PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA), following the manufacturer’s manual. The quality and quantity of the DNA extracts were evaluated using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Omaha, NE, USA). The bacterial 16S rRNA gene V3-V4 region was amplified using the primer set 343F (5′-TACGGRAGGCAGCAG-3′)/798R (5′-AGGGTATCTAATCCT-3′). The fungal ITS1 region was amplified using the common primer set ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′)/ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′). The primers for each sample amplification contained a unique 8-mer tag barcode that allowed the distinguishing of samples in the sequencing pools. PCR reactions were performed in 25 μL reaction mixtures, consisting of 12.5 μL of 2 × KAPA HiFi HotStart ReadyMix, 5 μL of each primer (1 μM), and 2.5 μL of diluted DNA (5 ng/μL). The PCR cycling program was as follows: 95 °C for 3 min, 30 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. The amplicon was purified using AMPure XP Beads (Beckman Coulter, Oxon, UK). Purified PCR amplicons were pooled in equimolar amounts and then paired-end sequenced (2 × 300) on an Illumina MiSeq platform at Shanghai Hanyu Biotech (Shanghai, China), following standard protocols.
The pair-end raw reads were assembled, screened, and trimmed using the mothur software (v.1.32.1). Quality-based trimming was first used to minimize sequencing error effects. Sequences were removed based on the following criteria using the “screen.seqs” command: average quality score of 50 bp windows < 25, homopolymers of more than eight bases, primer sequence, ambiguous base call, and read length < 200 bp. The unique sequence set was classified into operational taxonomic units (OTUs) with a threshold of 97% identity using the “cluster” command. Representative sequences of OTUs of 16S and ITS were analyzed using the “classify.seqs” command against the Greengenes Database (release 13.5v) and the UNITE Database for taxonomic annotation with a confidence threshold of 90% [37]. Each soil bacterial 16S sequence was rarified to the same sequencing depth of 6996 sequences per sample for community analysis, while soil fungal ITS sequences were rarified to the depth of 3373 sequences. The raw reads of bacteria and fungi were jointly deposited in the NCBI Sequence Read Archive (SRA) under accession number PRJNA903957.
2.5. Statistical Analysis
The data were primarily analyzed using R software version 4.0.5. The alpha diversity of the soil bacterial and fungal communities was indicated by the richness and Shannon index, which were calculated using the “vegan” package. Principal coordinates analysis (PCoA) based on Bray–Curtis distance, conducted using the “vegan” package, was used to reveal the overall structural variety of the soil bacterial and fungal communities. The effect of the woodland type on the soil microbial community structure was examined using permutational multivariate analysis of variance (PERMANOVA) with the “adonis” function in the “vegan” package. Redundancy analysis (RDA), also using the “vegan” package, was used to evaluate the relationships between the soil edaphic factors and microbial groups. A heatmap illustrating the Pearson correlation between environmental and microbial factors with vector indices and soil multifunctionality was visualized using the “pheatmap” package. Random forest analysis was performed using the “randomForest” and “rfPermute” packages to identify the main predictors of vector indices and soil multifunctionality.
Structural equation modeling (SEM) was conducted to test the direct and indirect effects of the soil pH, soil nutrients (SOC, TN, and Olsen-P), microbial biomass, bacterial community, and fungal community on the vector indices and soil multifunctionality. To reduce the complexity of the SEM, the parameters including SOC, TN, and Olsen-P were integrated as a soil nutrient index, which was calculated based on PCoA analysis using the “vegan” package. The first axis of the soil nutrient characteristic was picked for the SEM. The first axis of the PCoA for bacterial and fungal community composition was used for demonstrating the bacterial and fungal community, separately. The overall fit model of the SEM was evaluated using the maximum-likelihood estimation method. The model fitness was checked using chi-square (χ2), p value (>0.05), and the root mean square error of approximation (0 ≤ RMSEA ≤ 0.05). All the SEM analyses were performed using AMOS 28.0 (AMOS IBM, New York, NY, USA).
For the variables of the edaphic parameters, extracellular enzyme activities, microbial biomass, vector indices, soil multifunctionality, and microbial alpha diversity, the paired t-test was employed to evaluate the significant difference between the two subtropical woodlands. The normality of data and homoscedasticity of variances were assessed using Shapiro–Wilk and Levene’s tests, respectively. As some variables did not meet the assumptions, non-parametric Wilcox tests were employed for analysis of variances. Linear correlation analysis was applied to assess the relationships among the edaphic parameters, vector indices, soil multifunctionality, and microbial diversity. The geographic information of the study region and sampling sites was visualized using ArcMap 10.5 software.
3. Results
3.1. Soil Physicochemical Properties, Extracellular Enzyme Activities, and Microbial Biomass
The soils from C. oleifera displayed a higher pH and lower SOC content than those in the P. massoniana woodland. TN and Olsen-P concentrations showed no significant difference between the two subtropical woodlands (p > 0.05) (Table S3). Higher N-acquiring enzyme activities (NAG + LAP) were observed in P. massoniana than in C. oleifera woodland soils, whereas the C-acquiring enzymes (BG + CBH), P-acquiring enzyme (AP) activities, and all microbial biomass parameters, including MBC, MBN, and MNP, showed no significant difference between the two woodland soils according to the paired t-test (Figure 1).

Figure 1.
Soil enzymatic activities and microbial biomass between Camellia oleifera Abel and Pinus massoniana Lamb woodlands. (a) C acquisition enzymes (CBH + BG); (b) N acquisition enzymes (LAP + NAG); (c) P acquisition enzymes (AP); (d) Microbial biomass carbon; (e) Microbial biomass nitrogen, (f) Microbial biomass phosphorus. ns: non-significant difference; * p < 0.05.
3.2. Soil Microbial Metabolic Limitation and Soil Multifunctionality
The vector length, which indicates microbial C limitation, was consistent between the two subtropical woodlands (Figure 2a). The vector angle, representing the microbial N/P limitation, consistently exceeded 45°, indicating a strong soil microbial P limitation in both subtropical woodlands. The vector angle value for soil microbes from C. oleifera was higher than that of the P. massoniana woodlands (Figure 2b). The scatter plot of ecoenzymatic stoichiometry showed that the soil microbes in both subtropical woodlands were limited by P or by both C and P (Figure 2c). Soil multifunctionality remained unchanged in both subtropical woodlands (Figure 2d).
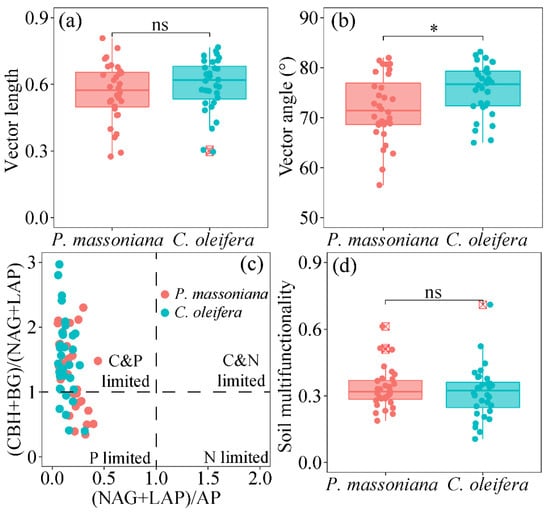
Figure 2.
Microbial metabolic limitation and soil multifunctionality between between Camellia oleifera Abel and Pinus massoniana Lamb woodlands. (a) Vector length; (b) vector angle; (c) scatter plots of soil ecoenzymatic stoichiometry; (d) soil multifunctionality across two subtropical woodlands. (CBH + BG)/(NAG + LAP): The ratio of C-acquisition enzyme activities and N-acquisition enzyme activities. (NAG + LAP)/AP: The ratio of N-acquisition enzyme activities and P-acquisition enzyme activities. ns: non-significant difference; * p < 0.05.
Pearson’s analysis indicated that the soil multifunctionality in the P. massoniana woodlands showed a significant positive correlation with SOC, Olsen-P, MBC, and MBN (p < 0.05) (Figure S4). Similarly, the soil multifunctionality in the C. oleifera woodlands was significantly and positively related to SOC, TN, Olsen-P, MBC, MBN, and MBP (p < 0.05) (Figure S4). Notably, the vector length in the P. massoniana woodlands was positively related to soil pH, while the vector angle in the C. oleifera woodlands was negatively correlated with soil pH (p < 0.05). No significant correlation was observed between the vector indices and other environmental variables (apart from soil pH) in the subtropical woodlands (p > 0.05).
3.3. Bacterial and Fungal Community Diversity and Composition
Although there was an increasing trend in the bacterial richness in C. oleifera compared to P. massoniana, only the Shannon index was significant. There was no significant difference in the alpha diversity of fungal communities between the two subtropical woodlands (Figure S5). Across the tested soils, the bacterial phylum was dominated by Proteobacteria (38.99%), Acidobacteria (30.77%), and Actinobacteria (23.11%) (Figure 3c). Within the Proteobacteria phylum, the class Alphaproteobacteria was the most abundant (25.02%) (Figure S6). Members of Ascomycota (35.03%), Basidiomycota (30.42%), and Zygomycota (1.15%) were prevalent fungal groups in both subtropical woodlands’ soils (Figure 3f). The fungal communities showed a significant difference between the two subtropical woodlands (PERMANOVA: p = 0.024), while the soil bacterial communities did not show a significant difference (PERMANOVA: p = 0.105) (Figure 3a,d).
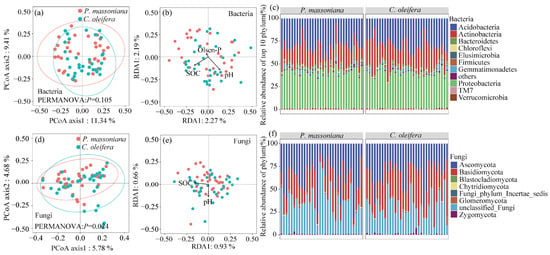
Figure 3.
Principal coordinate analysis (PCoA) based on the Bray–Curtis distances showing the effects of the woodland types on (a) bacterial and (d) fungal communities. Redundancy analysis (RDA) of the relationships between the (b) bacterial and (e) fungal communities and selected environmental variables for the two subtropical woodlands. Relative abundances of bacterial (c) and fungal (f) phyla in the two subtropical woodlands.
The effects of environmental factors on the microbial communities were evaluated using RDA analysis based on the Bray–Curtis dissimilarity of OTUs. The forward selection identified pH (p = 0.001), SOC (p = 0.001) and Olsen-P (p = 0.016) as significant explanatory variables for bacterial communities and pH (p = 0.016) and SOC (p = 0.008) for fungal communities (Figure 3b,e). Pearson’s correlation analysis revealed that the bacterial richness was significantly and positively correlated with soil pH (p < 0.05) and negatively correlated with MBC (p < 0.05) in the P. massoniana woodlands, while both bacterial and fungal richness was only negatively correlated with soil pH (p < 0.05) in the C. oleifera woodlands (Figure S4).
3.4. Linkage between Microbial Communities with Soil Metabolic Limitation and Soil Multifunctionality
In the P. massoniana woodlands, the vector length was significantly correlated with the richness of both soil bacteria and fungi, while the vector angle showed a linearly positive correlation with the fungal community (r = 0.37, p = 0.039) and a negative correlation with the fungal richness (Figure 4). By contrast, both the vector length and vector angle had no significant correlation with any microbial parameters in the C. oleifera woodlands. Similarly, no significant relationship was observed between the vector indices and any microbial parameters in all the tested data collected from the subtropical woodlands (Figure S7). Linear correlation analysis showed that the soil multifunctionality was positively related to the fungal community in both the C. oleifera and P. massoniana woodlands (Figure 5). In contrast to the bacterial community, the soil fungal community in all the tested data showed a strong positive correlation with the potential soil multifunctionality (Figure S8).
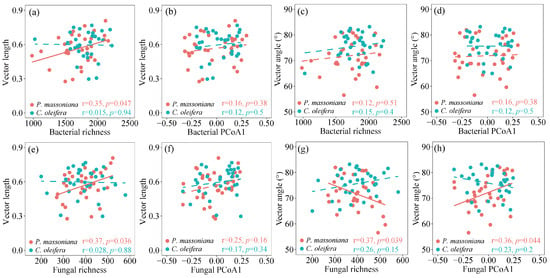
Figure 4.
Liner correlation between vector length and soil microbial parameters in two subtropical woodlands: (a) bacterial richness; (b) bacterial PCoA1; (e) fungal richness; (f) fungal PCoA1. Liner correlation between vector angle and soil microbial parameters in two subtropical woodlands: (c) bacterial richness; (d) bacterial PCoA1; (g) fungal richness; (h) fungal PCoA1. Solid and dashed lines denote statistically significant (two-sided p ≤ 0.05) and nonsignificant (two-sided p > 0.05) relationships, respectively.
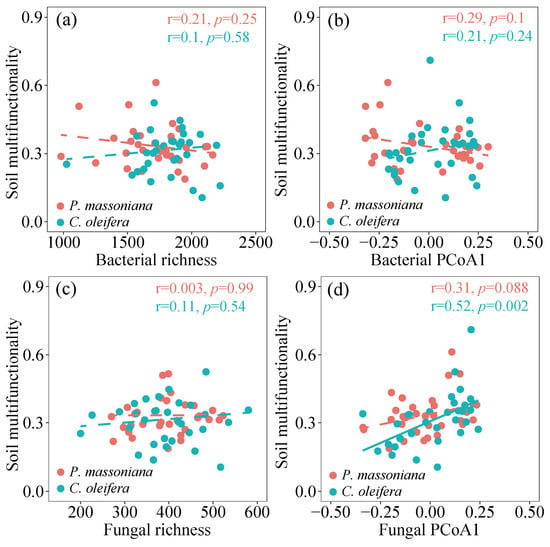
Figure 5.
Liner correlation between soil multifunctionality and microbial parameters in two subtropical woodlands: (a) soil bacterial richness; (b) soil bacterial PCoA1; (c) soil fungal richness; (d) soil fungal PCoA1. Solid and dashed lines denote statistically significant (two-sided p ≤ 0.05) and nonsignificant (two-sided p > 0.05) relationships, respectively.
3.5. Environmental Predictors for Soil Metabolic Limitation and Soil Multifunctionality
Random forest modeling was used to identify key environmental variables for predicting the soil metabolic limitation and soil multifunctionality in two woodlands. In the P. massoniana woodlands, the SOC and bacterial richness were selected to explain the vector length, while the soil fungal community and richness were identified as predictors for the vector angle (Figure 6). However, none of the environmental factors were considered as significant predictors for the vector length, and only the SOC was found to predict the vector angle in the C. oleifera woodlands. Regarding soil multifunctionality, the most important factors influencing soil multifunctionality contents were TN, SOC, Olsen-P, and MBN in the P. massoniana woodlands. In addition to the soil nutrient parameters, which included TN, SOC, Olsen-P, MBN, and MBC as significant predictors, the structure of the soil fungal community also had a significant impact on the soil multifunctionality in the C. oleifera woodlands (Figure 6).
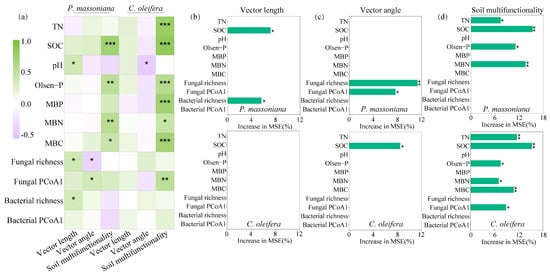
Figure 6.
(a) Pearson correlations between soil environmental and microbial factors with vector indices and soil multifunctionality in the two subtropical woodlands. The relative importance (% of increase of the mean square error, MSE) of the environmental and microbial factors on the (b) vector length, (c) vector angle, and (d) soil multifunctionality based on random forest methods in two subtropical woodlands. TN: total nitrogen; SOC: soil organic carbon; Olsen-P: Olsen phosphorus; MBP: microbial biomass phosphorus; MBN: microbial biomass nitrogen; MBC: microbial biomass carbon; Fungal PCoA1: the first axis of PCoA for fungal community composition; Bacterial PCoA1: the first axis of PCoA for bacterial composition. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.6. Direct and Indirect Impact of Environmental Variables on the Soil Microbial Metabolic Limitation and Soil Multifunctionality
The direct and indirect effects of the five main soil parameters, including pH, microbial biomass, soil nutrients, and bacterial and fungal communities, on the microbial metabolic limitation and soil multifunctionality were inferred using the SEM. In the P. massoniana woodlands, the tested factors explained 36.2% and 50.6% of the variance in the vector length and the vector angle, respectively. The bacterial richness had a strong positive direct effect on the vector length, while both fungal richness and communities contributed to the variations in the vector angle. Notably, the soil nutrients were the most critical factor for the soil multifunctionality. However, limited direct effects were observed for both the bacterial and fungal richness and communities on the soil multifunctionality. Correspondingly, in the C. oleifera woodlands, only the fungal richness had a positive effect on the vector angle, while no soil environmental factors or microbial parameters were found to explain the vector length. On the whole, the fungal community had a stronger positive impact on the soil multifunctionality than the bacterial community (Figure 7).
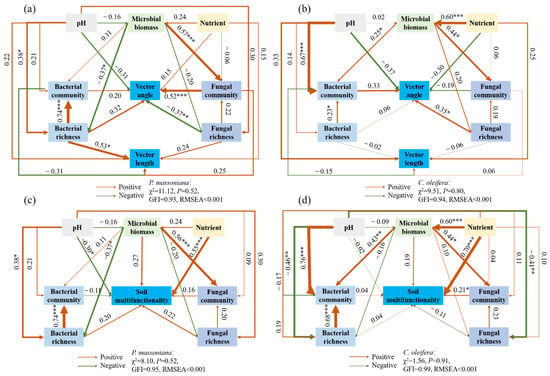
Figure 7.
Structural equation models for the effects of environmental and microbial variables on the vector indices in the (a) P. massoniana and (b) C. oleifera woodlands and the soil multifunctionality in the (c) P. massoniana and (d) C. oleifera woodlands. Red and green arrows indicate positive and negative relationships, respectively. The thickness of the arrow is proportional to the magnitude of the standardized path coefficients and indicative of the strength of the relationship. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
4.1. The Pattern of Soil Microbial Limitation in the Two Woodlands
The main pattern of soil microbial P limitation was observed in our tested subtropical woodlands, which is consistent with the study of soil microbial metabolic limitations across Chinese forests [38]. Most subtropical forests and woodlands are considered to be P-limited since the available P is strongly adsorbed by aluminum and iron oxides, resulting in it not being easy to be acquired by both plants and microbes [39]. In this study, we emphasized the varied impact of tree species, extensively cultivated in Southern China, on the metabolic limitation of soil microorganisms. Interestingly, the microbial P limitation in soils of C. oleifera was significantly more severe than that in P. massoniana woodlands (Figure 2). Two possible mechanisms may be responsible for this notable variation in this microbial P limitation pattern between the two woodlands. First, P. massoniana, as a typical pioneer tree, can reduce soil pH through the secretion of high levels of organic acids, activating insoluble aluminum-bound phosphate or iron phosphate, resulting the alleviation of the microbial P limitation [40]. Indeed, we observed that the soil pH was lower in the P. massoniana woodlands compared to the C. oleifera woodlands (Table S3). Second, needle-leaved trees, including P. massoniana, have a higher C-P ratio compared to broad-leaved trees, suggesting that P. massoniana requires less P for its growth than C. oleifera. Moreover, the annual harvest of oil-tea fruit in C. oleifera woodlands leads to the removal of substantial amounts of P from the soil. As a result, there is increased competition for phosphorus between trees and soil microorganisms, leading to a greater phosphorus limitation for the microorganisms in the C. oleifera than in the P. massoniana woodlands.
4.2. Soil Multifunctionality Remains Consistent between the Two Woodlands
A large number of studies found that different plant species have varying capacities to affect soil organic carbon accumulation, soil nutrient availability, and the composition of soil microbial communities [41,42,43], suggesting a varied impact of plant species on the potential soil multifunctionality. However, our results reveal no significant difference in the soil multifunctionality between P. massoniana and C. oleifera woodlands (Figure 2). One possible reason is that under similar climate and soil conditions, the soil multifunctionality of the P. massoniana and C. oleifera woodlands is similar. Additionally, the two mentioned plant species had limited effects on the soil physicochemical properties, enzyme activities, and microbial communities. For example, among the soil biological parameters tested, including the enzymes involved in C, N, and P acquisition, MBC, MBN, and MBP, as well as bacterial and fungal communities, only N-acquisition enzymes and the fungal community showed a significant difference between the woodland types mentioned above. It is worth mentioning that while the plant species had a significant impact on the soil SOC content, the mean difference between the two woodlands was less than 1 mg kg−1. This suggests that the small variation in SOC between the two woodlands is unlikely to result in changes in the potential multifunctionality of the soil. Moreover, the soil pH values of both woodlands were relatively low, which therefore makes it not likely to significantly impact the soil potential multifunctionality via affecting the bacterial community.
Plant community characteristics, such as the plant species, diversity, and community structure, have shown varied effects on soil multifunctionality [44,45]. The positive correlation between plant diversity and soil multifunctionality is usually attributed to the increase in the heterogeneity of the carbon litter, leading to a higher quality and quantity of the SOC pool and also a more stable microbial structure of the soil, thereby supporting a higher soil function [46,47]. In our investigated woodlands, the simple structure of vegetation in both woodlands resulted in a low plant diversity, suggesting that the effect of the diverse carbon input pathway on the soil multifunction was limited.
4.3. Relationships between Soil microbial Metabolic Limitation and Microbial Community Depend on the Woodland Type
The relationships between the microbial community and microbial metabolic limitation were different in the C. oleifera and P. massoniana woodlands. Significant and positive linkages between both the bacterial and fungal richness and microbial C limitation were demonstrated in the P. massoniana woodlands (Figure 4), indicating that the microbial C limitation was collectively regulated by bacterial and fungal species. Fungi and bacteria are thought to be the main decomposers of organic carbon substrates, and they exhibit distinct substrate-degradation strategies. For example, most of the bacteria have an appetite for the labile carbon substrate, and fungal species have the ability to degrade refractory carbon [48]. It should be noted that bacterial diversity, rather than fungal diversity, was selected to predict the soil microbial C limitation in the P. massoniana woodlands based on the random forest analysis and the SEM model (Figure 6 and Figure 7), suggesting that more bacterial species are associated with a higher carbon substrate demand because of the bacterial species being specialists for certain substrates. Alternatively, the microbial carbon limitation was insensitive to the fungal diversity due to the reason that the fungi, which are characterized by their diverse carbon mineralization pathways, might not be critically dependent on the secretion of β-D-cellobiosidase and β-1,4-glucosidase under a C-limitation situation. In the C. oleifera woodlands, our study did not identify any factors that could predict a microbial carbon limitation. The reason could potentially be attributed to the influence of certain unidentified microbial parameters on the microbial carbon limitation, such as the growth rates of bacteria and fungi, which have been shown to be associated with the microbial carbon limitation [49].
What attracted our interest was that the soil fungal richness was negatively related to the microbial P limitation in the P. massoniana woodlands (Figure 4 and Figure 7). This result indicates that fungi could be a dominant contributor to the mitigation of microbial P deficiency. Previous studies demonstrated that compared to bacteria, phosphorus-solubilizing fungi demonstrate a remarkable tenfold increase in their capacity to secrete organic acids [50], suggesting that the process of P solubilization by the fungi significantly increases the P availability for microbial utilization. Additionally, P. massoniana exhibits ectomycorrhizal colonization [51] and demonstrates an increased mycelial extension and density in response to low phosphorus conditions, facilitating enhanced co-absorption of phosphorus from the soil [52]. On the other hand, ectomycorrhiza can expresses a high-affinity phosphorus transporter that promotes efficient phosphorus uptake under low phosphorus stress [53]. This enhances the mobilization of bound P, making it more available as labile P [54,55]. It should be noted that fungal richness had an opposite effect on the microbial P limitation in the C. oleifera woodlands (Figure 7), suggesting that the process of P mobilization associated with the species of phosphate-solubilizing fungi in the C. oleifera woodlands could be distinctive. The microbial P limitation increased with fungal richness, suggesting that the species of fungi in the C. oleifera woodlands have weaker P-mobilization abilities compared to those in the P. massoniana woodlands. Another possibility was that the taxonomic groups of phosphate-solubilizing fungi within the overall fungal population in the C. oleifera woodlands are relatively limited, and that increasing the number of fungal species could lead to a higher P demand. Indeed, the significant difference observed in the fungal communities between the C. oleifera and P. massoniana woodlands supports this viewpoint. It should be noted that the relationship between the soil microbial metabolic limitation and the microbial community could also be affect by the seasons due to the seasonal dynamics of microbes. A study with a temporal dimension may offer a more robust and compelling argument [56].
4.4. Fungal Community Regulate Soil Multifunctionality, Rather Than Bacterial Community
The soil fungal community, rather than the bacterial community, showed a significant and positive correlation with potential soil multifunctionality (Figure S8), suggesting that fungal communities might have the potential to regulate changes in soil multiple functions in subtropical woodlands. This discovery is consistent with a prior study conducted in a boreal forest, which similarly emphasized the substantial influence of fungal diversity, rather than bacterial diversity, on soil multifunctionality [21]. However, it is worth noting that in the context of grassland ecosystems, bacterial diversity is typically recognized as a key determinant affecting soil multifunctionality [23,57]. Usually, the high proportion of labile compounds in the grass litter has been shown to facilitate the bacterial decomposers, due to their efficient capacity of fast carbon cycling in soil [58,59]. The heightened importance of fungi over bacteria in forest soils, potentially due to the presence of abundant recalcitrant substrates like lignin polymers, which are associated with the strong degradative capacity of fungi, may provide an explanation for this disparity. Interestingly, a prior study discovered that in agroecosystems, soil multifunctionality was jointly regulated by both bacterial and fungal communities [60]. This observation underscores the significance of adequate organic material inputs, which encompass a diverse array of both labile and recalcitrant carbon substrates, highlighting the interconnected role of both bacterial and fungal communities in shaping soil multifunctionality. Therefore, the relationship between soil multifunctionality and microbial groups may be related to the characteristics of the carbon substrates of microbial groups.
In the present study, surprisingly, we found different correlations between the fungal community and soil multifunctionality when the C. oleifera and P. massoniana woodlands were separately analyzed. In the P. massoniana woodlands, the fungal community exhibited a minimal influence on the soil multifunctionality. Conversely, within the C. oleifera woodlands, the fungal community exerted pronounced effects on the soil multifunctionality (Figure 7). One plausible explanation for this phenomenon is that the soil ecosystem functionality might be linked to specific fungal species through niche complementarity and/or fungal interactions. Consequently, variations in the fungal community composition may give rise to disparities in the relationship between the fungi and soil multifunctionality.
5. Conclusions
In this study, we found that the soil microbial communities in both C. oleifera and P. massoniana woodlands were limited by C and P. Notably, microbial P limitation was more pronounced in C. oleifera soils compared to P. massoniana soils. Similarity patterns in microbial C limitation and soil multifunctionality were identified between P. massoniana and C. oleifera woodlands. Furthermore, bacterial richness was considered as a key indicator for predicting the microbial carbon limitation in P. massoniana woodland soil, while no factors had an effect on the microbial carbon limitation in C. oleifera woodland soil. The microbial phosphorus limitation in P. massoniana woodland soil was significantly influenced by both the composition and richness of the fungal community, whereas only the fungal richness positively contributed to the microbial P limitation in C. oleifera woodland soil. With regard to soil multifunctionality, a robust correlation was observed between the fungal community and the soil multifunctionality in the subtropical woodlands. These findings can provide a scientific basis for the effective management and maintenance of subtropical woodlands, ensuring their long-term sustainability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15030527/s1. Table S1: A description of C, N and P acquisition extracellular enzymes in this study; Table S2: Pearson correlations between averaging multifunctionality index (used in this study) and the number of functions at or above a threshold (25, 50 and 75%) of the maximum observed function (n = 64); Table S3: Soil physicochemical properties in two subtropical woodlands. p-values are estimated using paired-samples t test; Figure S1: Geographic location of the study areas and sampling sites across two subtropical woodlands in southern China; Figure S2: Relationships between microbial community parameter and multiple-threshold soil multifunctionality above a series of sequential thresholds (from 1 to 99% at 1% intervals) of the maximum observed soil function; Figure S3: The slopes of the relationships between microbial community parameter and the number of functions at or above a threshold of some proportion of the maximum observed function, at different threshold values (x-axis); Figure S4: Pearson correlations between soil environmental factors with vector indices, soil multifunctionality and microbial richness in two subtropical woodlands; Figure S5: Richness and Shannon diversity for (a) bacterial and (b) fungal communities in two subtropical woodlands; Figure S6: Relative abundance of soil (a) bacterial and (b) fungal classes in two subtropical woodlands; Figure S7: Liner correlation between vector length and soil microbial parameter in two subtropical woodlands; Figure S8: Liner correlation between soil multifunctionality and microbial parameter in two subtropical woodlands.
Author Contributions
Conceptualization, H.Q., Y.S. and Y.H.; Methodology, H.Q., C.L., Q.S., S.D., X.D., L.C., X.C., Y.S. and Y.H.; Software, H.Q., Q.S., S.D., X.D. and L.C.; Formal analysis, H.Q., C.L., X.C. and Y.H.; Investigation, C.D., L.C. and Y.H.; Writing—original draft, H.Q. and C.L.; Writing—review & editing, H.Q., C.L. and Y.H.; Visualization, H.Q.; Supervision, Y.H.; Project administration, X.C., Y.S. and Y.H.; Funding acquisition, X.C., Y.S. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key Research Program (2017YFC0505503) and the National Natural Science Foundation of China (42177295, 42277309).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, P.L.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Peng, X.Q.; Wang, W. Stoichometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil. Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Mori, T.; Rosinger, C.; Margenot, A.J. Enzymatic C: N: P stoichiometry: Questionable assumptions and inconsistencies to infer soil microbial nutrient limitation. Geoderma 2023, 429, 116242. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil. Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric models of microbial metabolic limitation in soil systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Mao, Q.; Xiao, K.; Wang, K. Resource limitation of soil microbes in karst ecosystems. Sci. Total Environ. 2019, 650, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, L.; Hu, Y.; Deng, C.; Sun, Q.; Deng, S.; Chen, X.; Mei, L.; Wu, J.; Su, Y. Soil Microbial Resource Limitations and Community Assembly along a Camellia oleifera Plantation Chronosequence. Front. Microbiol. 2021, 12, 736165. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Zhou, Z.; Deng, J.; Zhao, F.; Guo, Y.; Han, X.; Yang, G.; Feng, Y.; Ren, G.; et al. Resource limitation and modeled microbial metabolism along an elevation gradient. Catena 2022, 209, 105807. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- McGuire, K.L.; Bent, E.; Borneman, J.; Majumder, A.; Allison, S.D.; Treseder, K.K. Functional diversity in resource use by fungi. Ecology 2010, 91, 2324–2332. [Google Scholar] [CrossRef]
- Elser, J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Vogel, H.J.; Bartke, S.; Daedlow, K.; Helming, K.; Kögel-Knabner, I.; Lang, B.; Rabot, E.; Russell, D.; Stößel, B.; Weller, U.; et al. A systemic approach for modeling soil functions. Soil 2018, 4, 83–92. [Google Scholar] [CrossRef]
- Creamer, R.E.; Barel, J.M.; Bongiorno, G.; Zwetsloot, M.J. The life of soils: Integrating the who and how of multifunctionality. Soil. Biol. Biochem. 2022, 166, 108561. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Zhu, M.; Zhu, C.; Feng, W.; Duan, G.; Cernava, T.; Jin, D. Di-n-butyl phthalate stress hampers compost multifunctionality by reducing microbial biomass, diversity and network complexity. Bioresour. Technol. 2023, 376, 128889. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, S.; Gao, X.; Yang, M.; Li, S.; Shen, H.; Xiao, J.; Han, Y.; Zhang, J.; Li, Y.; et al. Aboveground community composition and soil moisture play determining roles in restoring ecosystem multifunctionality of alpine steppe on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2021, 305, 107163. [Google Scholar] [CrossRef]
- Dong, Z.; Li, H.; Xiao, J.; Sun, J.; Liu, R.; Zhang, A. Soil multifunctionality of paddy field is explained by soil pH rather than microbial diversity after 8−years of repeated applications of biochar and nitrogen fertilizer. Sci. Total Environ. 2022, 853, 158620. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Trivedi, P.; He, J.Z.; Wang, J.T.; Singh, B.K. Identity of biocrust species and microbial communities drive the response of soil multifunctionality to simulated global change. Soil. Biol. Biochem. 2017, 107, 208–217. [Google Scholar] [CrossRef]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Zhu, Y.N.; Singh, B.K. Fungal richness contributes to multifunctionality in boreal forest soil. Soil. Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Han, S.; Tan, S.; Chen, W.; Huang, Q. Bacterial rather than fungal diversity and community assembly drive soil multifunctionality in a subtropical forest ecosystem. Environ. Microbiol. Rep. 2022, 14, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Liu, G.; Zhang, C.; Wang, G. Bacterial richness is negatively related to potential soil multifunctionality in a degraded alpine meadow. Ecol. Indic. 2021, 121, 106996. [Google Scholar] [CrossRef]
- Fabian, J.; Zlatanovic, S.; Mutz, M.; Premke, K. Fungal–bacterial dynamics and their contribution to terrigenous carbon turnover in relation to organic matter quality. ISME J. 2017, 11, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rai, A.; Banyal, R.; Chauhan, P.S.; Singh, N. Plant community regulates soil multifunctionality in a tropical dry forest. Ecol. Indic. 2018, 95, 953–963. [Google Scholar] [CrossRef]
- Zheng, H.; Ouyang, Z.; Xu, W.; Wang, X.; Miao, H.; Li, X.; Tian, Y. Variation of carbon storage by different reforestation types in the hilly red soil region of southern China. For. Ecol. Manag. 2008, 255, 1113–1121. [Google Scholar] [CrossRef]
- Dong, H.; Ge, J.; Sun, K.; Wang, B.; Xue, J.; Wakelin, S.A.; Wu, J.; Sheng, W.; Liang, C.; Xu, Q.; et al. Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. For. Ecol. Manag. 2021, 482, 118817. [Google Scholar] [CrossRef]
- Tu, C.; Lu, Q.; Zhang, Y.; Tian, J.; Gao, Y.; Liu, Y.; Yang, H.; Chen, L.; Zhang, J.; Wang, J.; et al. The soil nematode community indicates the soil ecological restoration of the Pinus massoniana plantation gap replanted with Cinnamomum longipaniculatum. Ecol. Indic. 2022, 136, 108678. [Google Scholar] [CrossRef]
- Bai, Y.S.; Liu, M.X.; Yi, J.; Zhang, H.L. Temporal stability analysis of soil moisture along a coniferous forest hillslope with subtropical monsoon climate in southwest China. J. Mt. Sci. 2021, 18, 2900–2914. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture Circular 939; US Department of Agriculture: Washington, DC, USA, 1954.
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil. Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil. Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.E.; Gamfeldt, L.; Isbell, F.; Lefcheck, J.S.; Griffin, J.N.; Hector, A.; Cardinale, B.J.; Hooper, D.U.; Dee, L.E.; Duffy, J.E.; et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 2014, 5, 111–124. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, P.; Xu, T.; Zhou, Y.; Chen, R.; Zhu, H.; Zhou, K. Development of a one-step multiplex PCR assay for differential detection of four species (Enterobacter cloacae, Enterobacter hormaechei, Enterobacter roggenkampii, and Enterobacter kobei) belonging to Enterobacter cloacae complex with clinical significance. Front. Cell. Infect. Microbiol. 2021, 11, 677089. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bing, H.; Moorhead, D.L.; Delgado-Baquerizo, M.; Ye, L.; Yu, J.; Zhang, S.; Wang, X.; Peng, S.; Guo, X.; et al. Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun. Earth Environ. 2022, 3, 184. [Google Scholar] [CrossRef]
- Haynes, R.J. Effects of liming on phosphate availability in acid soils: A critical review. Plant Soil. 1982, 68, 289–308. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Shan, Q.; Fang, L.; Jiang, D. Organic acid exudation from the roots of Cunninghamia lanceolata and Pinus massoniana seedlings under low phosphorus stress. Front. For. 2008, 3, 117–120. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Lawson, C.S.; Hedlund, K.; Edwards, A.R.; Brook, A.J.; Igual, J.M.; Mortimer, S.R.; Van der Putten, W.H. Plant species and functional group effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J. Ecol. 2006, 94, 893–904. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Yu, M.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Glob. Ecol. Conserv. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Fry, E.L.; Savage, J.; Hall, A.L.; Oakley, S.; Pritchard, W.J.; Ostle, N.J.; Pywell, R.F.; Bullock, J.M.; Bardgett, R.D. Soil multifunctionality and drought resistance are determined by plant structural traits in restoring grassland. Ecology 2018, 99, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.; Gross, N.; Quero, J.L.; Carmona, C.P.; Ochoa, V.; Gozalo, B.; Delgado-Baquerizo, M.; Dumack, K.; Hamonts, K.; Singh, B.K.; et al. Cascading effects from plants to soil microorganisms explain how plant species richness and simulated climate change affect soil multifunctionality. Glob. Chang. Biol. 2018, 24, 5642–5654. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Borja, M.E.; Delgado-Baquerizo, M. Plant diversity and soil stoichiometry regulates the changes in multifunctionality during pine temperate forest secondary succession. Sci. Total Environ. 2019, 697, 134204. [Google Scholar] [CrossRef]
- Wen, Z.; Zheng, H.; Zhao, H.; Xie, S.; Liu, L.; Ouyang, Z. Land-use intensity indirectly affects soil multifunctionality via a cascade effect of plant diversity on soil bacterial diversity. Glob. Ecol. Conserv. 2020, 23, e01061. [Google Scholar] [CrossRef]
- Pastorelli, R.; Costagli, V.; Forte, C.; Viti, C.; Rompato, B.; Nannini, G.; Certini, G. Litter decomposition: Little evidence of the “home-field advantage” in a mountain forest in Italy. Soil. Biol. Biochem. 2021, 159, 108300. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Chang. Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Zúñiga-Silgado, D.; Rivera-Leyva, J.C.; Coleman, J.J.; Sánchez-Reyez, A.; Valencia-Díaz, S.; Serrano, M.; de-Bashan, L.E.; Folch-Mallol, J.L. Soil type affects organic acid production and phosphorus solubilization efficiency mediated by several native fungal strains from Mexico. Microorganisms 2020, 8, 1337. [Google Scholar] [CrossRef]
- Huang, J.; Nara, K.; Zong, K.; Wang, J.; Xue, S.; Peng, K.; Shen, Z.; Lian, C. Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province, China. Fungal Ecol. 2014, 22, 589–602. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Ectomycorrhizal fungi: The symbiotic route to the root for phosphorus in forest soils. Plant Soil 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, X.P.; Ding, G.J. Ectomycorrhizal symbiosis enhances tolerance to low phosphorous through expression of phosphate transporter genes in masson pine (Pinus massoniana). Acta Physiol. Plant 2017, 39, 101. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Luo, X.; Hou, E.; Zheng, M.; Zhang, L.; He, X.; Shen, W.; Wen, D. Mycorrhizal fungi and phosphatase involvement in rhizosphere phosphorus transformations improves plant nutrition during subtropical forest succession. Soil Biol. Biochem. 2021, 153, 108099. [Google Scholar] [CrossRef]
- Keyes, S.; Van Veelen, A.; McKay Fletcher, D.; Scotson, C.; Koebernick, N.; Petroselli, C.; Williams, K.; Ruiz, S.; Cooper, L.; Mayon, R.; et al. Multimodal correlative imaging and modelling of phosphorus uptake from soil by hyphae of mycorrhizal fungi. New Phytol. 2022, 234, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.P.; Firestone, M.K. Seasonal dynamics of microbial community composition and function in oak canopy and open grassland soils. Microb. Ecol. 2006, 52, 470–479. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Cheng, J.; Wei, G.; Lin, Y. Above–and belowground biodiversity drives soil multifunctionality along a long–term grassland restoration chronosequence. Sci. Total Environ. 2021, 772, 145010. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Wang, Y.; Boydston, A.; Guo, D.; Li, X.; Wen, H.; Qin, Y.; Fu, H. Litter decomposition in fenced and grazed grasslands: A test of the home-field advantage hypothesis. Geoderma 2019, 354, 113876. [Google Scholar] [CrossRef]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Jia, J.; Zhang, J.; Zhang, F. Bacterial taxa and fungal diversity are the key factors determining soil multifunctionality in different cropping systems. Land Degrad. Dev. 2021, 32, 5012–5022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).