Abstract
Assessments of synergies and trade-offs between climate change mitigation and forest biodiversity conservation have focused on set-aside areas. We evaluated a more comprehensive portfolio of silvicultural management adaptations to climate change and conservation measures exemplary for managed European beech forests. Based on the available literature, we assessed a range of common silvicultural management and conservation measures for their effects on carbon sequestration in forest and wood products and for substituting more carbon-intensive products. We complemented this review with carbon sequestration simulations for a typical mountainous beech forest region in Austria. We propose three priority actions to enhance the synergies between climate change mitigation and biodiversity. First, actively increase the proportion of European beech in secondary Norway spruce forests, even though beech will not be unaffected by expected water supply limitations. Secondly, optimize the benefits of shelterwood systems and promote uneven-aged forestry, and thirdly, enhance mixed tree species. Targeted conservation measures (deadwood, habitat trees, and old forest patches) increase the total C storage but decrease the annual C sequestration in forests, particularly in wood products. The establishment of a beech wood market with an extended product portfolio to reduce the use of fuelwood is essential for sustainable climate change mitigation. Since there are limitations in the production of saw timber quality beech wood on low fertility sites, C accumulation, and biodiversity can be emphasized in these areas.
1. Introduction
With its EU Green Deal [1], the European Commission provided a strategy to face two of the most critical societal challenges of our time: The climate crisis and the biodiversity crisis. European forests are considered part of the solution to both, first by sequestering a significant share of greenhouse gas (GHG) emissions from fossil fuel burning [2] and, second, by preserving and restoring diverse forest habitats for the multitude of species living there [3]. Past and current management strategies seem not to meet these challenges adequately. Although not fully attributable to forest management, the GHG sink strength by forests decreased during the last decade in many European countries [4]. In addition, biodiversity is still on the decline, not only in highly intensified agricultural areas but also in forests [3,5,6,7]. Also, conservation targets set by the European Commission for forests through the Habitats Directive have not been achieved [8].
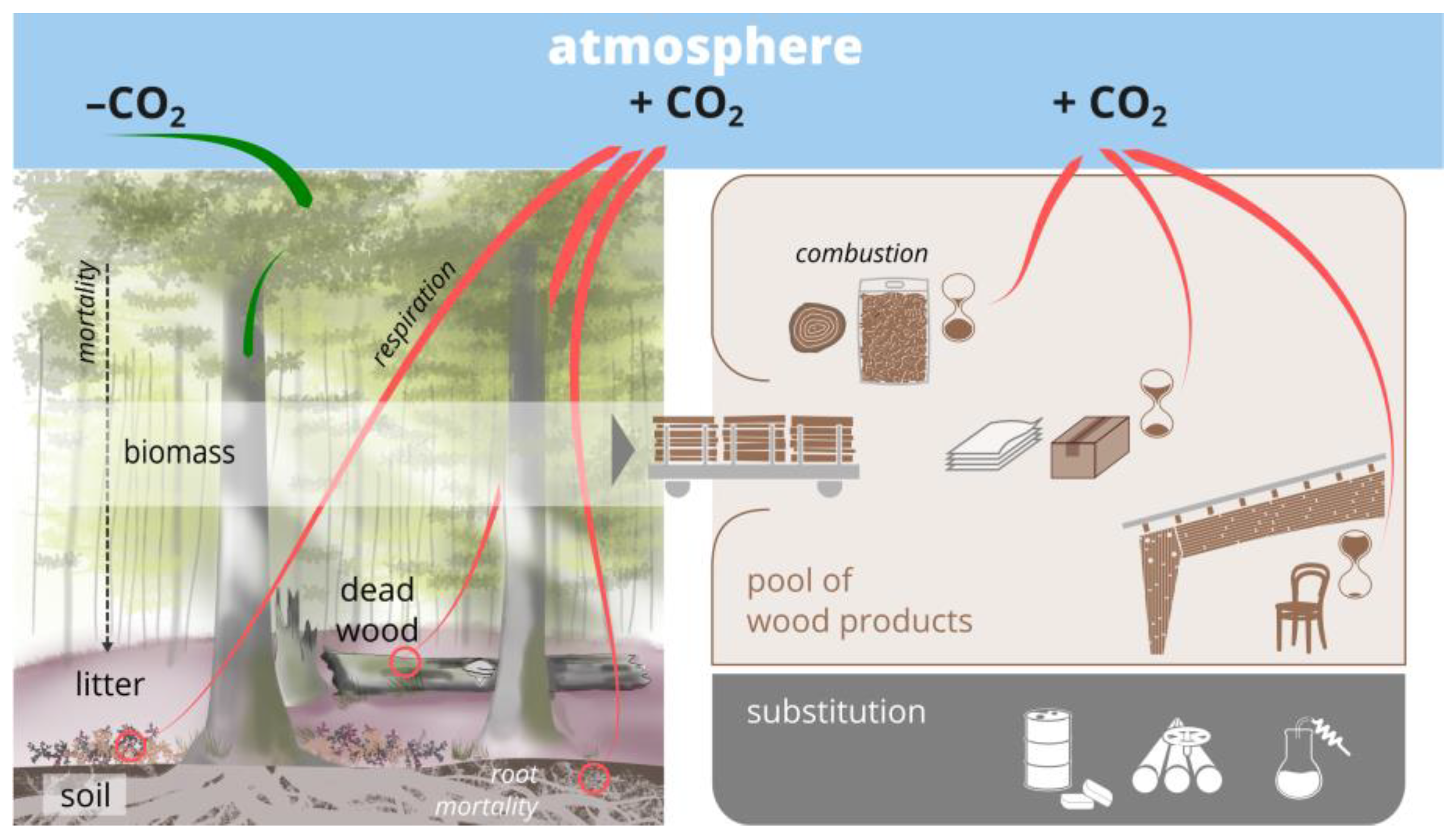
In addition to separating forests with high conservation value (e.g., primary forests) from managed forests by strict protection, biodiversity conservation can also be integrated into managed forests. Indeed, this is needed due to the sheer smallness of the remnants of primary forests [9], the long history of intensive human use, and the tight interplay of management and natural processes, which has affected forest biodiversity over time [10]. In addition, and notwithstanding the importance of protected forests for GHG sequestration [11], forest-based climate mitigation to a significant extent includes potential carbon sinks and substitution effects leveraged through long-lived wood products and bioenergy [12,13] (see, e.g., for Austria [14], Switzerland [15], and Germany [16]) (Figure 1). Hence, from a climate mitigation perspective, wood production on extensive forest land is indispensable to optimize the forest carbon sink, so that also from this perspective, biodiversity conservation has to be implemented in managed forests.
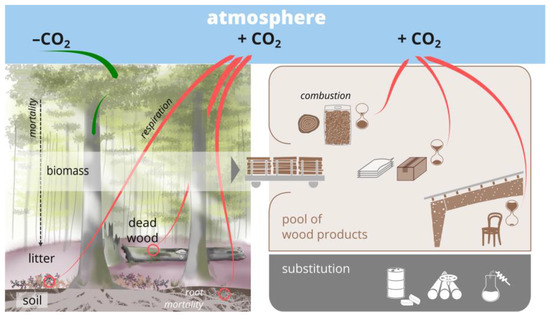
Figure 1.
The assessment of climate change mitigation through silvicultural management and conservation measures involves the carbon fluxes (tree growth, mortality, harvest, and respiration) and pools (tree biomass, soil, deadwood) in a forest, in wood products (storage and combustion after the end of the life cycle), and via the substitution of carbon-intensive products by timber.
In order to achieve climate and biodiversity targets, we have to upscale integrated measures, considering the local and regional characteristics of forests, their management, the legal and policy framework, adaptation to climate change, social practices, and the wood market [17,18]. An integrated approach may possibly ease conflicts between timber production and non-timber services, such as GHG sequestration, which are generally severe at the stand-level [19,20] but may be solved across the entire landscape [21,22]. In any case, a more systematic and systemic approach is needed, as it has recently been framed in “climate-smart forestry” [23].
Comprehensive knowledge of the integration of biodiversity conservation in managed forests exists [24,25]. The portfolio of measures includes retention of old-growth habitat structures and provision of connectivity, increase in tree species diversity, enhancement of deadwood amount and quality, and habitat trees while also allowing for natural disturbance processes. Currently, attempts to assess synergies and tradeoffs between forest-based climate mitigation and conservation have focused on optimizing set-aside forests for biodiversity, which simultaneously have a high potential carbon density [21,22,26]. However, to our knowledge, an assessment of the synergies and tradeoffs of a more comprehensive portfolio of conservation measures is not yet available.
Integrating the climate mitigation and biodiversity functions of forests can hardly be pursued without considering adaptation to climate change [23,27]. In our work, we focused on the conversion of Norway spruce to beech forests in Austria. Climate change and the historical legacy of monotonous Norway spruce forests that were planted on sites of native beech and other, mostly deciduous forests have already caused considerable forest dieback in many areas in Europe in recent years [28,29,30]. European beech is less sensible to water limitation than Norway spruce [31]; hence, it is recommended as a “climate fit” stand-forming alternative to spruce, even though at the dry end of the beech distribution, considerable range will likely be lost in the future [32,33]. Apart from shifting from highly productive to the least vulnerable tree species, continuous-cover forestry and the creation of mixed forest stands are among the most important climate adaptation measures in European forests [34,35]. These adaptations are important aspects within the portfolios of the integrated forest management [24,25]; hence, they hold substantial biodiversity co-benefits.
Integration of silvicultural management measures for optimizing forest-based carbon sinks and biodiversity has to be context-specific, taking into account the forest habitat structure and the related species pool [20]. Here, we take concrete biodiversity conservation measures applicable in central European beech forests and assess their potential and tradeoffs to sequester carbon in the forest stands and their wood products or substitute carbon emissions from non-wood products (Figure 1). Our aim is to provide a portfolio of integrated silvicultural management measures as decision support and recommendations for forest and conservation policymakers as well as forest managers.
2. Materials and Methods
2.1. Silvicultural Management Measures
In order to render practicability in integrating biodiversity conservation into sustainable forest management of European beech forests, we choose three silvicultural management measures (SMMs) currently applied to adapt to climatic changes and three frequently applied integrated biodiversity conservation measures (BCMs) in managed forests (Table 1).

Table 1.
Silvicultural management measures (SMMs) and biodiversity conservation measures (BCMs) and their relation to biodiversity.
Silvicultural management schema varies a lot among countries and forest owners. Therefore, we defined typical management practices in Austria in order to keep our assessment comparable.
2.1.1. Change from Spruce to Beech (SMM-1)
Exchanging Norway spruce with more climate change-resistant tree species is the most prominent adaptation measure in Austrian forests [29,36]. European beech is among the stand-forming trees used as climate-fit alternatives, where possible [32,33]. This change renders positive biodiversity effects on sites where these species would naturally occur. Though important for adaptation on beech forest sites to future climate change, we do not address sessile and common oak in this paper.
2.1.2. Tree Species Diversity (SMM-2)
Tree species diversification has been acknowledged to increase the growth and stability of forests [37]. With regard to climate adaptation, it aims to distribute climate risks to a higher number of species, hence increasing resilience. Tree species diversity by itself is increasing biodiversity but has positive indirect effects through increasing structural diversity [24,25]. We aim to extract the tree-mixing effects of typical mixtures with beech. For reasons of comparability, we assume a shelterwood-cut system, as defined below (Section 2.1.3), in a single-tree mixture (and not a group mixture).
2.1.3. Shelterwood and Continuous Cover Forestry (SMM-3)
Keeping a continuous forest cover instead of a rotation clear-cutting system is generally thought to improve forest resilience and biodiversity [38]. Note that effects on biodiversity vary by the concrete application of the different continuous cover forestry systems across a landscape [39]. We differentiated two management practices and compared them with a clear-cut system: (SMM-3a) even-aged shelterwood system and (SMM-3b) uneven-aged continuous cover forestry.
SMM-3a: Typically, an even-aged stand is repeatedly thinned to stimulate the growth of the remaining cohort and natural regeneration. Indeed, age classes are evenly distributed across larger areas of a forest enterprise. Note that shelterwood systems and irregular shelterwood systems represent the typical beech forest management in Austria.
SMM-3b: Uneven-aged trees (at least three age classes) with various sizes are distributed randomly in a forest stand. Typically, single trees and groups of trees are harvested to open the canopy and stimulate regeneration.
2.1.4. Old Forests and Forest Patches (BCM-1)
Protecting old-growth forests from management [40] and maintaining patches of old forests of various sizes in otherwise managed forest land [25,41] is a classical measure to protect forest biodiversity. We assume no management in the patches and ages significant beyond the commonly applied rotation periods. Natural beech forests are characterized by rather small-scale gap dynamics (gaps < 200 m2 are dominating [42,43,44]). Therefore, the recommended minimum areas for the protection of natural beech forests are also small, amounting to 20–50 ha [45,46]. A typical network of old forest in a managed forest landscape would consist of one-hectare patches [47] and a 1–2 km distance between these patches [48]. Other than deadwood (BCM-2) and habitat trees (BCM-3), BCM-1 is a measure primarily focused on the landscape and not on the stand scale.
2.1.5. Deadwood (BCM-2)
Keeping a certain amount and quality of deadwood beyond the thresholds for the survival of deadwood-dependent (saproxylic) organism groups is an important goal in the integrated forest management [49]. We assume 30–50 m3 of standing and lying deadwood, a threshold used for deciduous forests [50], for beech forests. It has to be noted that stem diameter > 20 cm is disproportionately useful for these organism groups.
2.1.6. Habitat Trees (BCM-3)
Large, old trees, dead or living, provide microhabitats for a number of forest species [51,52,53] and are therefore recommended as conservation measures in central European forests [25]. We assume 5 to 10 trees per hectare, with a diameter at breast height (DBH) > 60 cm.
2.2. Assessment of Carbon Sink Strength
Based on the available literature, we assessed each SSM regarding its effect size on carbon sequestration in the forest, in wood products, and for the substitution of more carbon-intensive products (Figure 1). In addition, we exemplified the effects of SSMs and BCMs for a typical mountainous beech forest region in Austria (File S1).
We applied three newly established yield tables together with allometric biomass functions, turnover of leaves, (fine and coarse) roots, and branches to calculate net primary production (NPP). We then estimated heterotrophic respiration (Rh) using a number of empirical relationships [54]. Finally, we derived the net ecosystem production (NEP) as the difference between NPP and Rh [55]. Using this approach, which is described in detail in File S1, NEP could be estimated for each site and age class available in the yield tables. The yield tables used here mirror typically managed pure spruce and beech forests in a mountain region in Austria: Fichte Tirol (later called Spruce Sawn Wood) [56], Buche Tirol (later called Beech Sawn Wood) [57], and Buche Nordalpe (later called Beech Fuel Wood) on calcareous bedrock [58]. Beech Sawn Wood is targeted toward trees with larger stem diameters (DBH) (most likely sawn wood) on sites with intermediate and high-yield classes. Beech Fuel Wood is characterized by lower stem diameters than Beech Sawn Wood (Table S1 in File S1). The latter reflects a typical management towards fuel wood. Spruce Sawn Wood is a traditional management for sawn spruce wood (Table S1 in File S1).
Since spruce shows higher wood volume productivity (diameter > 7 cm) than beech at the same sites [59], we exemplarily also compared the NPP, Rh, and NEP of defined forest site types growing on Kalklehm–Rendzina (an intermediary between Rendzic Leptosol and Chromic Cambisol) and Kalkbraunlehm (Chromic Cambisol) between 800 and 1600 m a.s.l. in the Northern Limestone Alps of Tyrol. At the Kalklehm–Rendzina sites, beech and spruce showed maximum tree heights at year 100 of 11 and 14 m (interquartile range: 8–17 m) and 20 and 26 (interquartile range: 17–31 m. At the Kalkbraunlehm sites, beech and spruce showed maximum tree heights at year 100 of 16 and 19 m (interquartile range: 13–24 m) and 29 and 29 m (interquartile range: 23–35 m). Therefore, we filtered the maximum tree height at year 100 (m) of the yield tables Beech Sawn Wood and Beech Fuel Wood for the interquartile ranges 8–17 m (Kalklehm–Rendzina) and 13–24 m (Kalkbraunlehm) and of the yield table Spruce Sawn Wood for 17–31 m (Kalklehm–Rendzina) and 23–35 m (Kalkbraunlehm), and calculated the mean values of NPP, Rh and NEP.
We estimated the total stored and annual sequestration of C in the stem and branches of a single habitat tree with a stem diameter of 70 cm and stands of yield table Beech Fuel Wood using the approach described above and detailed in File S1.
We estimated CO2 respiration from the beech stem deadwood based on published total decay rates, integrating snag decay, snag fall rates, and log decay [60]. These values are robust since they were modeled based on four deadwood inventories of the Swiss National Forest Inventory covering a large number of trees (n = 1016) and large climatic gradients (mean annual temperature: −2 °C to 12 °C, mean annual precipitation sums: 540 to 2300 mm). We used the first ten years of the decay process to derive the rates for the decadal periods in the yield tables. Mean annual deadwood respiration and its variation were calculated by converting the defined deadwood volumes to biomass by the abovementioned shrinking and wood density ratios for beech and applying loss ratios. Variations in decay rates were addressed by using the 15 published decay rates presented in Hararuk et al. [60].
3. Results
3.1. NEP Comparison between Pure Spruce and Beech Forests
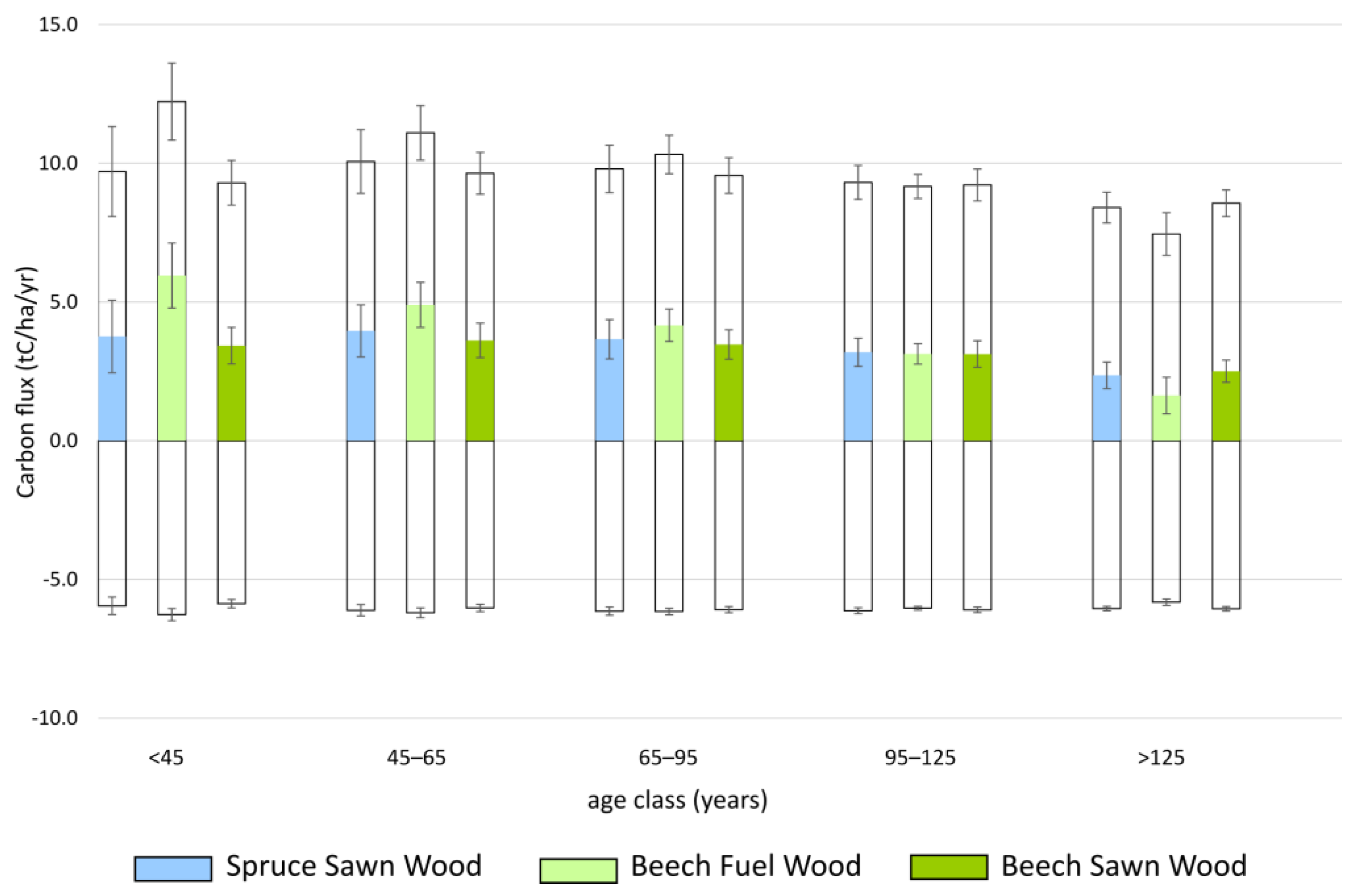
The estimated NPP, Rh, and NEP compare well with published numbers (see File S2). Pure beech stands with high thinning intensity (Beech Sawn Wood) reached the highest mean NPP (10.1 t C ha−1 y−1) and mean NEP (4.0 t C ha−1 y−1) across all yield classes (Figure 2, Table S3 in File S1). Spruce Sawn Wood and Beech Fuel Wood were rather similar: SpruceSawn Wood (NPP = 9.5 t C ha−1 y−1, NEP = 3.4 t C ha−1 y−1); Beech Fuel Wood: (NPP = 9.3 t C ha−1 y−1, NEP = 3.2 t C ha−1 y−1) (Figure 2, Table S3 in File S1). NPP and NEP increased from the intermediate to the high-yield class (Table S3 in File S1).
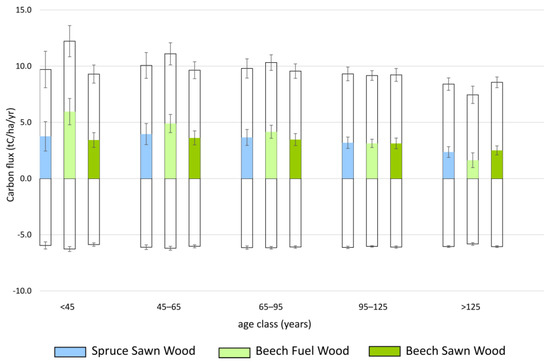
Figure 2.
Net ecosystem production (NEP, color bars) as the difference between net primary production (NPP, unshaded upper bar) and heterotrophic respiration (Rh, unshaded lower bar) by age classes of three typical forest stands on intermediate yield classes in Tyrol, Austria. Error bars represent the standard deviation of the mean of all yield classes within the intermediate group.
At both site types, Spruce Sawn Wood reached higher NPP (Kalklehm–Rendzina: 9.0 t C ha−1 y−1; Kalkbraunlehm: 10.4 t C ha−1 y−1) and NEP (Kalklehm–Rendzina: 3.0 t C ha−1 y−1; Kalkbraunlehm: 4.1 t C ha−1 y−1) than Beech Sawn Wood (NPP: 8.9 C ha−1 y−1, 9.3 C ha−1 y−1; NEP: 3.0 C ha−1 y−1, 3.3 C ha−1 y−1) and Beech Fuel Wood (NPP: 7.7 C ha−1 y−1, 8.6 C ha−1 y−1; NEP: 1.9 C ha−1 y−1, 2.7 C ha−1 y−1) (age classes 45 to 125 years).
3.2. Habitat Trees
Ten beech trees with stem diameters of 70 cm store 23.1 t C in stem and branch wood. The C stored in these trees is equivalent to the mean annual stem and branch NPP of 6.9 stands (ha) (SD = 2.1 stands), respectively, of beech forest of the type of Beech Fuel Wood in high (5.4 stands (ha) SD = 1.1 stands) and intermediate (8.3 stands (ha), SD = 2.0 ha) yield classes. The numbers differ according to age and yield class, where lower fertile sites obviously resulted in higher equivalent areas. Ten habitat trees (60–75 cm diameter) occupy 0.12–0.14 ha per hectare stand area (crown projection). Without the regeneration process in this area, NPP decreases by 0.14–0.23 t C ha−1 (< 2.2%).
3.3. Deadwood
We used the pure beech stand reflected in the yield table Beech Fuel Wood as a reference for NEP. Taking into account various climatic conditions, the average annual respiration flux of the first ten years of the decay process of 30 and 50 m3 per hectare deadwood was 0.19 and 0.32 t C. These are 4.4 to 10.1% of the respective amount of NEP in the high and intermediate yield classes (Table 2). Since deadwood decay will very likely be lower in the low-yield classes and higher in the high-yield classes, the 25 percentiles for the low (4.8 and 8% for 30 and 50 m3 ha−1 deadwood) and the 75 percentiles for the high-yield classes (5.6 and 9.3% for 30 and 50 m3 ha−1 deadwood) may be used as a more robust estimate.

Table 2.
Amount (mean ± SD) of annual C respiration of 30 and 50 m3/hectare deadwood amounts and comparison with total forest stand net ecosystem production (NEP) of the exemplary beech forest Beech Fuel Wood. The magnitude of deadwood respiration is shown as the mean and 25 and 75% percentiles.
4. Discussion
4.1. Co-Benefits of Forest Adaptation for Climate Mitigation
We suggest, from the variety of forest adaptation measures to cope with adverse climate effects [35,61,62,63], three priority actions to increase synergies between climate mitigation and biodiversity in Austrian beech forest. First, actively increasing the proportion of less vulnerable tree species, such as European beech, sessile, and common oak, in artificial Norway spruce, black pine, and Scots pine forests is already widely acknowledged. European beech will continue to play an important role as a stand-forming species in these changes, even though it will not be unaffected by the expected limitations in the water supply [32,64,65,66,67]. Second, optimizing of the proven shelterwood system with natural regeneration and promoting uneven-aged forestry, and third, enhancing tree species mixing.
A change in tree species directly affects C stocks and growth increments [68,69]. The lower volume growth of beech compared to spruce is compensated by its higher wood density (beech: 0.68 t/m3; spruce: 0.43 t/m3) and different carbon allocation characteristics to tree compartments (e.g., branches) when calculating NEP [70,71]. By taking this into account, NEP is much more driven by management strategies and yield class than by tree species. Using typical yield tables characterizing pure spruce and beech stands in Tyrol, Austria; we elaborated upon these differences. Whereas beech and spruce forests with high thinning intensity targeted at sawn-wood resulted in the highest (and very similar) NEP in the intermediate and high-yield classes, beech forest stands aiming at wood volume maximization showed lower NEP (maximal 1 t C ha−1 y−1). In lower-yield classes, where sawn timber is usually not the management target, both beech stands show higher NEP than spruce stands. From a climate mitigation perspective, this might be seen as positive since various management portfolios will result in reasonable NEP. Fuel timber production instead of sawn-wood forestry with beech will very likely pertain in the future due to high management costs on sites with low yields. A good example is the vast mountainous areas of the European Alps, where beech is predicted to increase in the future [72]. Hence, NEP can be optimized efficiently in these areas even when focusing on wood volume maximization. In contrast, medium and higher productive beech sites with intermediate and good yield classes could focus on C sequestration in long-lived wood products while simultaneously keeping high NEP values.
A number of factors can additionally affect NEP when beech is exchanging spruce. The rotation periods of beech forests are currently generally longer than those of secondary pure spruce stands [73,74,75]; hence, in the long term, this would also result in smaller total emissions during stand replacement in the case of clear-cut management. Beech forest soils are less rich in C than spruce forests [76,77,78]. During the transformation of spruce forests to beech forests, elevated heterotrophic C respiration very likely occurs [79] as a result of higher soil organic matter turnover [80,81]. Higher C respiration in beech compared to spruce forests will also result from faster decay rates of beech deadwood [82].
Though not necessarily, a change in tree species from spruce to beech very likely results in a change in stand establishment concepts, management systems, and rotation periods [83,84,85]. Here, we focused on the most important aspects of clear-cut versus shelterwood systems and uneven-aged continuous cover forests. However, it is particularly noted that the shelterwood regeneration method is most frequently applied in Austrian monodominant beech or mixed forests [38]. The essential aspect in shelterwood systems is the gradual removal of mature trees during final harvesting and the simultaneous establishment of a natural regeneration rich in stem numbers and vitality [86,87,88,89,90]. The removal of the entire stand by clearcutting negatively affects carbon levels in the forest floor [91,92,93]. In comparison to clearcutting, continuous C assimilation, litter input and circulation of nutrients, and the avoidance of elevated soil C respiration through canopy cooling effects maintain a positive NEP during thinning and final harvest events, assuming vital tree regeneration [94,95,96]. However, the harvest of high numbers of mature trees (>50%) in a short period can still lead to a net loss of C [97]. Albeit differences between sites and tree mixtures are obvious, a harvest intensity of 50% of the stem volume seems to result in a good regeneration [88], and < 30% minimizes the C loss [98,99,100,101,102]. In addition to these direct thinning and harvest effects, shelterwood systems optimize growth by continuously reducing stand density. The growth of older beech stands respond positively to thinning and hence are effective in storing C across their entire lifespan [103,104,105,106]. This characteristic also results in positive NEP effects and total C storage when extending the rotation period [107]. We conclude that when changing from even-aged spruce to beech forests, NEP is optimized when combined with shelterwood systems, applying reasonable thinning intensity and longer rotation periods.
Uneven-aged continuous forest cover systems with beech vary a lot in their characteristics [108]. In general, a wide range of age classes occur within a stand, keeping a relatively closed canopy even when harvesting mature trees. Hence, the main difference with even-aged rotation forests is their ability to continuously store C at a relatively constant rate [109,110,111]. Transformation of age-class Norway spruce forests into uneven-aged single tree selection forests has the ability to increase the soil C stock [112].
European beech often occurs in monodominant stands, although in both natural and silvicultural systems, while many forms of mixing exist as well [73]. Actually, beech was initially promoted as an admixture to Norway spruce plantations in order to ameliorate soils [72]. Today, the diversification of tree species also aims at distributing climate risks [34,35]. Regarding NEP, the positive effect of tree species diversity on stand densities, productivity, and aboveground biomass is the most important [113,114,115,116]. The effect also holds for the traditional Austrian beech mixtures, such as beech/spruce [117,118], beech/oak [119], beech/pine [120], and spruce/fir/beech [121]. Effects on soil carbon will, in general, also be similar to a change from spruce to beech when admixing beech into previously pure conifer forests [80,122]. The high competitiveness of beech results in the need for more intensive management in order to keep high-value timber from other trees. From a pure NEP perspective, group mixing is less favorable since the positive mixing effect on C assimilation is either weakened or entirely diminished [123].
4.2. Co-Benefits of Forest Adaptation for Biodiversity
Our priority measures relating to adaptation to climate changes render a number of co-benefits for forest biodiversity. For a good reason, ensuring a natural tree species composition, substituting clear-cut with continuous forest cover systems, and enhancing tree diversity are key features in forest conservation portfolios [24,25]. In contrast to European deciduous tree species providing a higher level of biodiversity-enhancing microhabitats than conifer species [51,52], a number of, very likely more influential, indirect effects exist via stand and landscape scale structural diversity.
Structural diversity is one of the key components in a variety of organism groups in forests, such as mosses, lichens, and fungi on deadwood [124], as well as for birds and mammals [125] and insects [126]. Structural diversity on the stand level improves in uneven-aged compared to even-aged forests [127] and when admixing species in monodominant forest stands. Therefore, managed, monodominant, even-aged beech forests may have low structural diversity. High tree species diversity, on the other hand, optimizes the diversity of appropriate habitats for deadwood-dwelling organisms [128].
The distribution of structurally diverse patches (regarding development stages, deadwood, old trees) in the landscape and temporal continuity also plays a decisive role [41]. Conservation plans aim at optimizing structural diversity not only on the stand but also on the landscape scale [25]. However, without dedicated conservation measures, managed forest landscapes can hold a considerable number of species. For example, higher gamma diversity can be reached in even-aged shelterwood beech forest landscapes with different development ages, as opposed to uneven-aged continuous cover forests (“Plenterwald”), as a result of heterogeneous microclimate conditions in the understory at the landscape level [39]. However, such examples should not be generalized since effects on biodiversity depend strongly on the group of organisms considered or even on the specific requirements of a single (keystone) species. While birds benefit from uneven-aged forest structures at the local level [129], typical beetles, fungi, or mosses in beech forests need deadwood structures or old trees, which are often less available in intensively managed forests [130].
4.3. Carbon Sink Effects of Targeted Conservation Measures
Notwithstanding the significance of adaptation measures in forests for biodiversity, the degree of positive effects strongly depends on the management intensity and spatial heterogeneity. Natural forests generally show a particularly high density and diversity of the different forest development phases (“patches”) and a small-scale heterogeneous stand structure [131]. Managed forest landscapes commonly are less structured, have low or no share of terminal and decay phases, and have less deadwood [10,129]. Among the targeted measures recommended to overcome this issue, we reviewed the retention of old trees and forest patches and deadwood accumulation for their effect on C sequestration in central European beech forests and wood products, as well as on the substitution of more C-intensive products other than timber.
Without large-scale disturbance, natural and near-natural forests are very likely C sinks up to an advanced stand age of 200 to 400 years [11,132]. Note that species associated with old-growth beech forests rely on stand ages of 100–170 years in sub-montane and 160–220 years in mixed montane forests [133]. While C accumulates when ceasing tree harvests [105,134,135], NEP decreases in older stands as a result of competition, mortality, and deadwood decay [136]. As recently shown by Pretzsch et al. [123], one-third of the total production in monospecific, up to 150 years stands in Europe, would flow to the debris pool when unmanaged, thus, lowering C sequestration in the aboveground living biomass and thereby the potential allocation of C stored in wood products. The rather small part of the deadwood C not respired is transferred to the soil organic matter. Soils in central European primeval beech forests show larger C pools than managed forests [137].
Outside protected areas, usually quite small forest patches are retained to mimic old-growth structures and hence provide key habitat features for forest biodiversity. Size, extent, and configuration are important considerations for conservation. The smallest area necessary to allow for natural dynamics and all forest development stages of beech forests is thought to range between 20 and 50 ha [45,46]. Since such patch sizes are unrealistic in most managed forest land, much smaller patches are usually retained together with habitat trees in a bigger network, ideally additionally connecting protection forest [10].
A typical network may consist of one-hectare patches [47] with a 1–2 km distance between these patches [48], resulting in approximately 2%–7% relative area. As for abandoned forests, C accumulates in these patches, though with a lower NEP than the managed surroundings.
Since these distances are too far for many forest species and the size too ambitious for most forest owners, identified habitat trees, protected from felling, serve as stepping stones [138]. Old beech trees of reasonable size (diameter > 60–70 cm) are very well suited because they provide a particularly dense and rich variety in microhabitats [51,52,53]. Due to their size, they have high biomass and can account for a significant proportion of total stored carbon in forest stands [134,139,140]. Old trees not only act as C storage but also very actively add to the NEP [141]. In uneven-aged forests, for example, the strongest individual trees together can account for the largest proportion of growth increment despite a smaller proportion of the total volume [111]. In temperate zones of central Europe, dominant beech trees with diameters > 60 cm on vigorous sites can also be expected to have tree ages of at least 100 years [142,143]. Recent studies showed that the highest annual mass increment of dominant trees is beyond the common terminal ages of 100–200 years [144]. Beech trees with a diameter > 80 cm may have larger mean annual ring widths and larger mean increases in basal area than trees with a diameter between 30 and 80 cm [145]. We exemplarily estimated the climate mitigation potential of habitat trees. Accordingly, 5–29 hectares of a typical beech forest in Tyrol, Austria (high to intermediate-yield classes) are necessary to sequester the same amount of C, which is stored in 10 habitat trees with a stem diameter of 70 cm. Apart from its considerable C storage, habitat trees usually remain during stand replacement, thereby buffering the C emissions [146] and limiting the space for young trees with higher annual growth rates. The latter effect is, however, very small.
In addition to the significance of islands of old-growth structures, a lack of deadwood can lead to habitat fragmentation and isolation, especially for species with low dispersal ability and mobility [147]. Accordingly, deadwood connectivity is critical to the long-term survival of many forest species [148]. It has been clearly shown that when deadwood abundance and diversity are high, the diversity and abundance of old-growth and deadwood-associated species generally increase in European forests [50,149]. Accordingly, when deadwood disappears, the diversity of these species also decreases significantly [150,151]. Managed forests generally have lower amounts of deadwood due to the utilization of the wood [152]. Deadwood thresholds in managed forests aim to protect the majority of species [10] and range between 30 and 40 m3 ha−1 in European mixed mountain forests and between 30 and 50 m3 ha−1 in lowland deciduous forests [50]. In addition to deadwood quantity and spatial distribution, deadwood diversity has a positive effect on biodiversity. Strong deadwood, whether lying or standing, is a particularly valuable key structure for many biotic communities and harbors not only several but also many rare and protected species [150,153].
Regarding deadwood, impacts on NEP relate to the transfer of C from living to dead trees and decomposition towards soil organic matter, thereby releasing CO2. Deadwood storage is therefore determined by the rate of tree mortality and decomposition [152,154]. An increase in deadwood quantity leads to an increase in heterotrophic respiration, lowering the NEP [97,123,155]. Taking the NEP estimates from typical beech forests in Austria (yield table Beech Fuel Wood) and the recommended 30–50 m3 ha−1 deadwood amount, mean deadwood respiration remained < 10.1% of the amount of NEP. In intact stands with only a small gap to patch scale dynamics (i.e., no disturbed areas with large amounts of deadwood), the increase in deadwood does not lead to net C emissions [60,156,157,158]. C respiration from deadwood in the amounts given above is just too low. For example, in our estimation, it was an order of magnitude lower than total heterotrophic respiration (Rh). Slow decay rates of deadwood [60,82] result in C accumulation in deadwood once mortality or artificial felling increases and the wood remains in the forest [134,152], adding to total C storage in beech forests [135]. Note that turnover in beech deadwood is lower than the turnover of C pools in most wood products, thus, lowering the total C sequestration [123]. C in soil organic matter benefits from the small portion of deadwood that is not respired [159,160,161]. It is generally thought that high soil C pools in pristine beech forests relate to deadwood decomposition [107,137].
4.4. Climate Mitigation with Beech Wood Products and Substitution Effects
C storage in wood products and the substitution of fossil fuel-based emissions significantly add to NEP-based climate mitigation in European forests [12,13,14,15,16] (Figure 1). Technical wood characteristics of tree species and harvested wood quality assortments, apart from the market, wood imports, etc., steer the potential mitigation effects. With regard to European beech, a number of specific factors have to be considered. First, beech wood is currently much less used in long-lived woods than fuel wood. For example, only 1.3% of the total sawmill Roundwood in Austria was beech in the year 2021 [162]; two-thirds of the harvested beech wood directly goes towards bioenergy use. Second, the majority of the European wood industry is adapted to Norway spruce [29]. Third, high-quality beech Roundwood for long-lived wood products is very difficult to produce under unfavorable site conditions, which are widespread (e.g., shallow limestone soils) [83].
Increasing the share of beech at potentially favorable sites, where Norway spruce is expected to lose ground due to climate change, will help in maintaining the forest C sink in European forests. However, in order to fully exploit these changes for climate mitigation, a transformation of the spruce-dominated wood industry to beech and other deciduous tree species is indispensable. Braun et al. [14] exemplified for Austria that a scenario favoring bioenergy involving increased harvest, thinning, and shorter rotation periods has a lower climate change mitigation efficiency per volume of utilized wood than scenarios assuming increased material use. It is, therefore, unsatisfactory that beech wood only, to a small extent, enters the wood material chain. Furthermore, increased imports of spruce timber from other regions (e.g., northern Europe) would also be undesirable from a climate change perspective and would have a negative impact on the income of Austrian forest owners. Research efforts to improve the suitability of European beech wood for load-bearing applications and engineered wood products have recently increased [162]. Even though for engineered wood, beech offers an even higher mechanical performance compared to spruce, further improvements are necessary to be competitive. Moreover, silvicultural methods to increase stem wood quality potentially broaden the suitability for a higher variability and more durable wood products, storing and substituting more of the emitted C over a longer period [163]. Silvicultural management models are available to improve the Roundwood quality of beech [83,164,165,166]. Nonetheless, since there are limitations regarding site conditions [73,83,85], a focus may be lain on C accumulation and biodiversity rather than fuel wood in the areas with low-quality sites, whereas high-value sites may emphasize sawmill Roundwood production for, e.g., load-bearing applications.
5. Conclusions
European beech will play an important role in adapting spruce forests, which are and will continue to be highly impacted by climatic changes in large areas of Austria and beyond. Other tree species can also help maintain C assimilation in mixed forests, and closed canopy forestry can limit C emissions during stand replacement. These measures support the long-term C sink in forests and simultaneously provide substantial co-benefits for biodiversity conservation. Old forest patches and the protection of larger unmanaged areas, habitat trees, and deadwood are key elements in forest biodiversity conservation and forest health. They may also offer flexibility in reaching short-term climate targets by retaining and storing C in the forest. To support the switch from predominant fuel wood use to long-lived products, the establishment of a large-scale beech wood material chain should be promoted. Instead of advocating for an either–or approach, this time could be used to prepare for both the production of Beech sawn-wood where it is possible and economically viable, as well as the implementation of integrated biodiversity measures in as many forests as possible.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f15020359/s1. Refs [167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185] are cited in the Supplementary Materials.
Author Contributions
Conceptualization and methodology, J.K. and T.D.; writing—review and editing and formal analysis, J.K.; writing—review and editing, G.P.; original draft preparation, T.D.; Review and editing, E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Climate Research Fund (ACRP13—ManageBeech—KR20AC0K17973).
Data Availability Statement
No new data were created in this review article.
Acknowledgments
Ivo Offenthaler designed Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Commission. The European Green Deal: COM(2019) 640 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2019:640:FIN (accessed on 1 October 2023).
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Available online: https://ipbes.net/global-assessment (accessed on 1 October 2023).
- European Environmental Agency. European Union Emission Inventory Report 1990–2020: Under the UNECE Air Convention; EEA Report No 03/2022; European Environmental Agency: Copenhagen, Denmark, 2022. [Google Scholar]
- Staab, M.; Gossner, M.M.; Simons, N.K.; Achury, R.; Ambarlı, D.; Bae, S.; Schall, P.; Weisser, W.W.; Blüthgen, N. Insect decline in forests depends on species’ traits and may be mitigated by management. Commun. Biol. 2023, 6, 338. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Staude, I.R.; Waller, D.M.; Bernhardt-Römermann, M.; Bjorkman, A.D.; Brunet, J.; de Frenne, P.; Hédl, R.; Jandt, U.; Lenoir, J.; Máliš, F.; et al. Replacements of small- by large-ranged species scale up to diversity loss in Europe’s temperate forest biome. Nat. Ecol. Evol. 2020, 4, 802–808. [Google Scholar] [CrossRef]
- European Environment Agency. State of Nature in the EU: Results from Reporting under the Nature Directives 2013–2018; EEA Report; European Environment Agency: Copenhagen, Denmark, 2020. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Kraus, D.; Krumm, F. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Joensuu, Finland, 2013; ISBN 9789525980073. [Google Scholar]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, P.; Cardellini, G.; González-García, S.; Hurmekoski, E.; Sathre, R.; Seppälä, J.; Smyth, C.; Stern, T.; Verkerk, P.J. Substitution Effects of Wood-Based Products in Climate Change Mitigation; EFI: Joensuu, Finland, 2018; ISBN 9789525980691. [Google Scholar]
- Knauf, M.; Köhl, M.; Mues, V.; Olschofsky, K.; Frühwald, A. Modeling the CO2-effects of forest management and wood usage on a regional basis. Carbon Balance Manag. 2015, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Fritz, D.; Weiss, P.; Braschel, N.; Büchsenmeister, R.; Freudenschuß, A.; Gschwantner, T.; Jandl, R.; Ledermann, T.; Neumann, M.; et al. A holistic assessment of greenhouse gas dynamics from forests to the effects of wood products use in Austria. Carbon Manag. 2016, 7, 271–283. [Google Scholar] [CrossRef]
- BAFU. CO2-Effekte der Schweizer Wald- und Holzwirtschaft; Umwelt-Wissen; BAFU: Ittigen, Switzerland, 2007. [Google Scholar]
- Mund, M. Klimaschutzwirkung des Wald- und Holzsektors: Schutz- und Nutzungsszenarien für Drei Modellregionen in Thüringen: Ergebnisse des F+E-Vorhabens “Ökosystemleistungen Naturnaher Wälder in der Wald- und Klimapolitik” (FKZ 3511 84 0200); BfN: Bonn, Germany, 2015; ISBN 978-3-89624-131-3. [Google Scholar]
- Bruna-Garcia, X.; Marey-Perez, M.F. Public participation: A need of forest planning. iForest 2014, 7, 216–226. [Google Scholar] [CrossRef]
- Martins, H.; Borges, J.G. Addressing collaborative planning methods and tools in forest management. For. Ecol. Manag. 2007, 248, 107–118. [Google Scholar] [CrossRef]
- Pohjanmies, T.; Triviño, M.; Le Tortorec, E.; Salminen, H.; Mönkkönen, M. Conflicting objectives in production forests pose a challenge for forest management. Ecosyst. Serv. 2017, 28, 298–310. [Google Scholar] [CrossRef]
- Sabatini, F.M.; de Andrade, R.B.; Paillet, Y.; Ódor, P.; Bouget, C.; Campagnaro, T.; Gosselin, F.; Janssen, P.; Mattioli, W.; Nascimbene, J.; et al. Trade-offs between carbon stocks and biodiversity in European temperate forests. Glob. Change Biol. 2019, 25, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Buotte, P.C.; Law, B.E.; Ripple, W.J.; Berner, L.T. Carbon sequestration and biodiversity co-benefits of preserving forests in the western United States. Ecol. Appl. 2020, 30, e02039. [Google Scholar] [CrossRef] [PubMed]
- Reside, A.E.; VanDerWal, J.; Moran, C. Trade-offs in carbon storage and biodiversity conservation under climate change reveal risk to endemic species. Biol. Conserv. 2017, 207, 9–16. [Google Scholar] [CrossRef]
- Kauppi, P.; Hanewinkel, M.; Lundmark, T.; Nabuurs, G.J.; Peltola, H.; Trasobares, A.; Hetemäki, L.; European Forest Institute. Climate Smart Forestry in Europe. Available online: https://www.efi.int/sites/default/files/files/publication-bank/2018/Climate_Smart_Forestry_in_Europe.pdf (accessed on 15 October 2023).
- Oettel, J.; Lapin, K. Linking forest management and biodiversity indicators to strengthen sustainable forest management in Europe. Ecol. Indic. 2021, 122, 107275. [Google Scholar] [CrossRef]
- Krumm, F.; Schuck, A.; Rigling, A. How to Balance Forestry and Biodiversity Conservation—A View across Europe. Available online: https://forbiodiv.wsl.ch/de/the-book.html (accessed on 1 November 2023).
- Gregor, K.; Knoke, T.; Krause, A.; Reyer, C.P.O.; Lindeskog, M.; Papastefanou, P.; Smith, B.; Lansø, A.-S.; Rammig, A. Trade-Offs for Climate-Smart Forestry in Europe Under Uncertain Future Climate. Earth’s Future 2022, 10, e2022EF002796. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Change 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Zhiqiang, Y.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef]
- Jandl, R. Climate-induced challenges of Norway spruce in Northern Austria. Trees For. People 2020, 1, 100008. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Change 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Renneberg, H. Potential risk for European beech (Fagus sylvatica L.) in a changing climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Lexer, M.J.; Hönninger, K.; Scheifinger, H.; Matulla, C.; Groll, N.; Kromp-Kolb, H.; Schadauer, K.; Starlinger, F.; Englisch, M. The sensitivity of Austrian forests to scenarios of climatic change: A large-scale risk assessment based on a modified gap model and forest inventory data. For. Ecol. Manag. 2002, 162, 53–72. [Google Scholar] [CrossRef]
- Kolström, M.; Lindner, M.; Vilén, T.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Kremer, A.; Delzon, S.; Barbati, A.; et al. Reviewing the Science and Implementation of Climate Change Adaptation Measures in European Forestry. Forests 2011, 2, 961–982. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Ammer, C. Converting Norway spruce stands with beech—A review of arguments and techniques. Austrian J. For. Sci. 2008, 125, 3–26. [Google Scholar]
- Cordonnier, T.; Kunstler, G.; Courbaud, B.; Morin, X. Managing tree species diversity and ecosystem functions through coexistence mechanisms. Ann. For. Sci. 2018, 75, 65. [Google Scholar] [CrossRef]
- Mason, W.L.; Diaci, J.; Carvalho, J.; Valkonen, S. Continuous cover forestry in Europe: Usage and the knowledge gaps and challenges to wider adoption. For. Int. J. For. Res. 2022, 95, 1–12. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Barredo, J.; Brailescu, C.; Teller, A.; Sabatini, F.M.; Mauri, A.; Janouskova, K. Mapping and Assessment of Primary and Old-Growth Forests in Europe; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Fahrig, L. Why do several small patches hold more species than few large patches? Glob. Ecol. Biogeogr. 2020, 29, 615–628. [Google Scholar] [CrossRef]
- Rugani, T.; Diaci, J.; Hladnik, D. Gap dynamics and structure of two old-growth beech forest remnants in Slovenia. PLoS ONE 2013, 8, e52641. [Google Scholar] [CrossRef] [PubMed]
- Zeibig, A.; Diaci, J.; Wagner, S. Gap disturbance patterns of a Fagus sylvatica virgin forest remnant in the mountain vegetation belt of Slovenia. For. Snow Landsc. Res. 2005, 79, 69–80. [Google Scholar]
- Hobi, M.L.; Ginzler, C.; Commarmot, B.; Bugmann, H. Gap pattern of the largest primeval beech forest of Europe revealed by remote sensing. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Tichy, K.; Frank, G. Österreichisches Programm Naturwaldreservate; Bundesministerium für Land- und Forstwirtschaft: Vienna, Austria, 1995. [Google Scholar]
- Projektgruppe Naturwaldreservate. Empfehlungen für die Einrichtung und Betreuung von Naturwaldreservaten in Deutschland. Forstarchiv 1993, 64, 122–129. [Google Scholar]
- Müller, M.; Lachat, T.; Bütler, R. Wie gross sollen Altholzinseln sein? Schweiz. Z. Forstwes. 2012, 163, 49–56. [Google Scholar] [CrossRef]
- Brunet, J.; Isacsson, G. Restoration of beech forest for saproxylic beetles—Effects of habitat fragmentation and substrate density on species diversity and distribution. Biodivers. Conserv. 2009, 18, 2387–2404. [Google Scholar] [CrossRef]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of tree characteristics and forest management on tree microhabitats. Biol. Conserv. 2011, 144, 441–450. [Google Scholar] [CrossRef]
- Großmann, J.; Schultze, J.; Bauhus, J.; Pyttel, P. Predictors of Microhabitat Frequency and Diversity in Mixed Mountain Forests in South-Western Germany. Forests 2018, 9, 104. [Google Scholar] [CrossRef]
- Asbeck, T.; Pyttel, P.; Frey, J.; Bauhus, J. Predicting abundance and diversity of tree-related microhabitats in Central European montane forests from common forest attributes. For. Ecol. Manag. 2019, 432, 400–408. [Google Scholar] [CrossRef]
- Chen, S.; Zou, J.; Hu, Z.; Chen, H.; Lu, Y. Global annual soil respiration in relation to climate, soil properties and vegetation characteristics: Summary of available data. Agric. For. Meteorol. 2014, 198–199, 335–346. [Google Scholar] [CrossRef]
- Lovett, G.; Cole, J.; Pace, M. Is Net Ecosystem Production Equal to Ecosystem Carbon Accumulation? Ecosystems 2006, 9, 152–155. [Google Scholar] [CrossRef]
- Eckmüllner, O. Empfohlene Ertragstafeln für Nord- und Osttirol; Amt der Tiroler Landesregierung, Abt. Forstplanung: Innsbruck, Austria, 2004. [Google Scholar]
- Eckmüllner, O. Ertragstafel Buche Tirol; Amt der Tiroler Landesregierung, Abt. Forstplanung: Innscbruck, Austria, 2011. [Google Scholar]
- Eckmüllner, O. Buchenertragstafel Österreich für Nordalpen; Amt der Tiroler Landesregierung, Abt. Forstplanung: Innsbruck, Austria, 2011. [Google Scholar]
- Amt der Tiroler Landesregierung, Abteilung Forstplanung. Waldtypenbechreibung Tirols Teil 3; Amt der Tiroler Landesregierung: Innsbruck, Austria, 2023. [Google Scholar]
- Hararuk, O.; Kurz, W.A.; Didion, M. Dynamics of dead wood decay in Swiss forests. For. Ecosyst. 2020, 7, 36. [Google Scholar] [CrossRef]
- Coșofreț, C.; Bouriaud, L. Which Silvicultural Measures Are Recommended to Adapt Forests to Climate Change? A Literature Review. Bull. Transilv. Univ. Braşov 2020, 12, 13–14. [Google Scholar] [CrossRef]
- Weiss, P.; Braun, M.; Fritz, D.; Gschwantner, T.; Hesser, F.; Jandl, R.; Kindermann, G.; Koller, T.; Ledermann, T.; Ludvig, A.; et al. Klimakrise Managen: Ausblick für Wald und Holznutzung; BFW Praxisinformationen: Vienna, Austria, 2020; Volume 51. [Google Scholar]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Freudenschuss, A.; Gschwantner, T.; Weiss, P. Strategies for Climate-Smart Forest Management in Austria. Forests 2018, 9, 592. [Google Scholar] [CrossRef]
- Pretzsch, H.; Grams, T.; Häberle, K.H.; Pritsch, K.; Bauerle, T.; Rötzer, T. Growth and mortality of Norway spruce and European beech in monospecific and mixed-species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees 2020, 34, 957–970. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Kopp, G.; Ruff, M.; Leuschner, C. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees 2017, 31, 673–686. [Google Scholar] [CrossRef]
- Knutzen, F.; Dulamsuren, C.; Meier, I.C.; Leuschner, C. Recent Climate Warming-Related Growth Decline Impairs European Beech in the Center of Its Distribution Range. Ecosystems 2017, 20, 1494–1511. [Google Scholar] [CrossRef]
- del Castillo, E.M.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; de Dios, V.R.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- De Simon, G.; Alberti, G.; Delle Vedove, G.; Zerbi, G.; Peressotti, A. Carbon stocks and net ecosystem production changes with time in two Italian forest chronosequences. Eur. J. For. Res. 2012, 131, 1297–1311. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Herrmann, V.; Banbury Morgan, R.; Bond-Lamberty, B.; Cook-Patton, S.C.; Ferson, A.E.; Muller-Landau, H.C.; Wang, M.M.H. Carbon cycling in mature and regrowth forests globally. Environ. Res. Lett. 2021, 16, 53009. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Horodecki, P. Differences in biomass production and carbon sequestration between highland and lowland stands of Picea abies (L.) H. Karst. and Fagus sylvatica L. For. Ecol. Manag. 2020, 474, 118329. [Google Scholar] [CrossRef]
- Neumann, M.; Moreno, A.; Mues, V.; Härkönen, S.; Mura, M.; Bouriaud, O.; Lang, M.; Achten, W.M.; Thivolle-Cazat, A.; Bronisz, K.; et al. Comparison of carbon estimation methods for European forests. For. Ecol. Manag. 2016, 361, 397–420. [Google Scholar] [CrossRef]
- Jandl, R.; Foldal, C.B.; Ledermann, T.; Kindermann, G. European Beech Forests in Austria—Current Distribution and Possible Future Habitat. Forests 2023, 14, 2019. [Google Scholar] [CrossRef]
- BFW. Mischwälder- Weniger Risiko, Höhere Wertschöpfung: BFW Praxisinformation Nr 41; BFW: Vienna, Austria, 2016. [Google Scholar]
- Klein, D.; Schulz, C. Die Kohlenstoffbilanz der Bayerischen Forst- und Holzwirtschaft: Bayerische Landesanstalt für Wald und Forstwirtschaft; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2012. [Google Scholar]
- Karopka Manuel. Die Fichte—Baum des Jahres 2017 und Baum des Anstoßes; FVA-Einblick: Freiburg im Breisgau, Germany, 2017. [Google Scholar]
- Rehschuh, S.; Jonard, M.; Wiesmeier, M.; Rennenberg, H.; Dannenmann, M. Impact of European Beech Forest Diversification on Soil Organic Carbon and Total Nitrogen Stocks–A Meta-Analysis. Front. For. Glob. Change 2021, 4, 606669. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Schmidt, I.K.; Gundersen, P.; Vesterdal, L. Soil carbon accumulation and nitrogen retention traits of four tree species grown in common gardens. For. Ecol. Manag. 2013, 309, 47–57. [Google Scholar] [CrossRef]
- Grüneberg, E.; Ziche, D.; Wellbrock, N. Organic carbon stocks and sequestration rates of forest soils in Germany. Glob. Change Biol. 2014, 20, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Jílková, V. Soil respiration in temperate forests is increased by a shift from coniferous to deciduous trees but not by an increase in temperature. Appl. Soil Ecol. 2020, 154, 103635. [Google Scholar] [CrossRef]
- Prietzel, J. Humusveränderungen nach Einbringung von Buche und Eiche in Kiefernreinbestände. J. Plant Nutr. Soil Sci. 2004, 167, 428–438. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Kahl, T.; Arnstadt, T.; Baber, K.; Bässler, C.; Bauhus, J.; Borken, W.; Buscot, F.; Floren, A.; Heibl, C.; Hessenmöller, D.; et al. Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. For. Ecol. Manag. 2017, 391, 86–95. [Google Scholar] [CrossRef]
- BFW. Die Rotbuche: Bundesforschungs- und Ausbildungszentrum für Wald, Naturgefahren und Landschaft; BFW Praxisinformationen: Vienna, Austria, 2006. [Google Scholar]
- BFW. Die Fichte–Brotbaum oder Problemkind? BFW Praxisinformationen: Vienna, Austria, 2013. [Google Scholar]
- Weinfurter, P. Waldbau in Österreich auf Ökologischer Grundlage: Eine Orientierungshilfe für die Praxis; LFI Waldbau Berater: Vienna, Austria, 2013. [Google Scholar]
- Agestam, E.; Ekö, P.-M.; Nilsson, U.; Welander, N.T. The effects of shelterwood density and site preparation on natural regeneration of Fagus sylvatica in southern Sweden. For. Ecol. Manag. 2003, 176, 61–73. [Google Scholar] [CrossRef]
- Reh, M. Waldbaumerkblatt: Verjüngungsmethoden; Landwirtschaftskammer Oberösterreich: Linz, Austria, 2015. [Google Scholar]
- Barna, M. Natural Regeneration of Fagus sylvatica L.: A Review. Austrian J. For. Sci. 2011, 128, 71–91. [Google Scholar]
- Höllerl, S.; Neuner, M. Kohlenstoffbilanz des Wald- und Holzsektors bewirtschafteter und unbewirtschafteter Bergmischwälder der Bayerischen Alpen. Forstarchiv 2011, 82, 142–154. [Google Scholar]
- Reh, M.; Schuster, K.; Tomazej, M.; Zobl, A. Standortsgerechte Verjüngung des Waldes; Landwirtschaftskammer Oberösterreich: Linz, Austria, 2013. [Google Scholar]
- Mayer, M.; Sandén, H.; Rewald, B.; Godbold, D.L.; Katzensteiner, K. Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Funct. Ecol. 2017, 31, 1163–1172. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.W.; Roach, W.J.; Defrenne, C.E.; Pickles, B.J.; Snyder, E.N.; Robinson, A.; Lavkulich, L.M. Harvest Intensity Effects on Carbon Stocks and Biodiversity Are Dependent on Regional Climate in Douglas-Fir Forests of British Columbia. Front. For. Glob. Change 2020, 3, 88. [Google Scholar] [CrossRef]
- Mayer, M.; Matthews, B.; Rosinger, C.; Sandén, H.; Godbold, D.L.; Katzensteiner, K. Tree regeneration retards decomposition in a temperate mountain soil after forest gap disturbance. Soil Biol. Biochem. 2017, 115, 490–498. [Google Scholar] [CrossRef]
- Williams, C.A.; Vanderhoof, M.K.; Khomik, M.; Ghimire, B. Post-clearcut dynamics of carbon, water and energy exchanges in a midlatitude temperate, deciduous broadleaf forest environment. Glob. Change Biol. 2014, 20, 992–1007. [Google Scholar] [CrossRef]
- Ostrogović Sever, M.Z.; Alberti, G.; Delle Vedove, G.; Marjanović, H. Temporal Evolution of Carbon Stocks, Fluxes and Carbon Balance in Pedunculate Oak Chronosequence under Close-To-Nature Forest Management. Forests 2019, 10, 814. [Google Scholar] [CrossRef]
- Carrara, A.; Kowalski, A.S.; Neirynck, J.; Janssens, I.A.; Yuste, J.C.; Ceulemans, R. Net ecosystem CO2 exchange of mixed forest in Belgium over 5 years. Agric. For. Meteorol. 2003, 119, 209–227. [Google Scholar] [CrossRef]
- Granier, A.; Bréda, N.; Longdoz, B.; Gross, P.; Ngao, J. Ten years of fluxes and stand growth in a young beech forest at Hesse, North-eastern France. Ann. For. Sci. 2008, 65, 1. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Biogeosci. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- Wilkinson, M.; Crow, P.; Eaton, E.L.; Morison, J.I.L. Effects of management thinning on CO2 exchange by a plantation oak woodland in south-eastern England. Biogeosciences 2016, 13, 2367–2378. [Google Scholar] [CrossRef]
- Scott, N.A.; Rodrigues, C.A.; Hughes, H.; Lee, J.T.; Davidson, E.A.; Dail, D.B.; Malerba, P.; Hollinger, D.Y. Changes in carbon storage and net carbon exchange one year after an initial shelterwood harvest at Howland Forest, ME. Environ. Manag. 2004, 33, S9–S22. [Google Scholar] [CrossRef]
- Lindroth, A.; Holst, J.; Heliasz, M.; Vestin, P.; Lagergren, F.; Biermann, T.; Cai, Z.; Mölder, M. Effects of low thinning on carbon dioxide fluxes in a mixed hemiboreal forest. Agric. For. Meteorol. 2018, 262, 59–70. [Google Scholar] [CrossRef]
- Barna, M.; Sedmák, R.; Marusák, R. Response of European beech radial growth to shelterwood cutting. Folia Oecologica 2010, 37, 125. [Google Scholar]
- Boncina, A.; Kadunc, A.; Robic, D. Effects of selective thinning on growth and development of beech (Fagus sylvatica L.) forest stands in south-eastern Slovenia. Ann. For. Sci. 2007, 64, 47–57. [Google Scholar] [CrossRef]
- Bouriaud, O.; Don, A.; Janssens, I.A.; Marin, G.; Schulze, E.-D. Effects of forest management on biomass stocks in Romanian beech forests. For. Ecosyst. 2019, 6, 19. [Google Scholar] [CrossRef]
- Diaconu, D.; Kahle, H.-P.; Spiecker, H. Tree- and Stand-Level Thinning Effects on Growth of European Beech (Fagus sylvatica L.) on a Northeast- and a Southwest-Facing Slope in Southwest Germany. Forests 2015, 6, 3256–3277. [Google Scholar] [CrossRef]
- Leuschner, C.; Wulf, M.; Bäuchler, P.; Hertel, D. Forest continuity as a key determinant of soil carbon and nutrient storage in beech forests on sandy soils in Northern Germany. Ecosystems 2014, 17, 497–511. [Google Scholar] [CrossRef]
- Zingg, A. Dauerwald–ein neues altes Thema der Waldwachstumsforschung. Informationsblatt Forschungsbereich Wald. 2003, 15, 1–3. [Google Scholar]
- Zingg, A.; Frutig, F.; Bürgi, A.; Lemm, R.; Erni, V.; Bachofen, H. Ertragskundliche Leistung in den Plenterwald-Versuchsflächen der Schweiz | Yield performance in the plenter forest research plots in Switzerland. Schweiz. Z. Forstwes. 2009, 160, 162–174. [Google Scholar] [CrossRef]
- Uhl, E.; Hilmers, T.; Pretzsch, H. From Acid Rain to Low Precipitation: The Role Reversal of Norway Spruce, Silver Fir, and European Beech in a Selection Mountain Forest and Its Implications for Forest Management. Forests 2021, 12, 894. [Google Scholar] [CrossRef]
- Lenk, E.; Kenk, G. Langfristiges Wachstum Schwarzwälder Plenterwälder. AFZ-Wald 2007, 3, 132–135. [Google Scholar]
- Pötzelsberger, E.; Hasenauer, H. Soil change after 50years of converting Norway spruce dominated age class forests into single tree selection forests. For. Ecol. Manag. 2015, 338, 176–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Ammer, C. Diversity and forest productivity in a changing climate. New Phytol. 2019, 221, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Leuschner, C.; Thomas, F.M. Productivity of temperate broad-leaved forest stands differing in tree species diversity. Ann. For. Sci. 2010, 67, 503. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: Evidence on stand level and explanation on individual tree level. Eur. J. For. Res. 2009, 128, 183–204. [Google Scholar] [CrossRef]
- Pretzsch, H.; Block, J.; Dieler, J.; Dong, P.H.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zingg, A. Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Ann. For. Sci. 2010, 67, 712. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.-P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of mixed versus pure stands of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Pretzsch, H.; Del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef]
- Hilmers, T.; Avdagić, A.; Bartkowicz, L.; Bielak, K.; Binder, F.; Bončina, A.; Dobor, L.; Forrester, D.I.; Hobi, M.L.; Ibrahimspahić, A.; et al. The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. For. Int. J. For. Res. 2019, 92, 512–522. [Google Scholar] [CrossRef]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Weiss, P. Soil Organic Carbon Stocks in Mixed-Deciduous and Coniferous Forests in Austria. Front. For. Glob. Change 2021, 4, 688851. [Google Scholar] [CrossRef]
- Pretzsch, H.; Del Río, M.; Arcangeli, C.; Bielak, K.; Dudzinska, M.; Ian Forrester, D.; Kohnle, U.; Ledermann, T.; Matthews, R.; Nagel, R.; et al. Competition-based mortality and tree losses. An essential component of net primary productivity. For. Ecol. Manag. 2023, 544, 121204. [Google Scholar] [CrossRef]
- Penone, C.; Allan, E.; Soliveres, S.; Felipe-Lucia, M.R.; Gossner, M.M.; Seibold, S.; Simons, N.K.; Schall, P.; van der Plas, F.; Manning, P.; et al. Specialisation and diversity of multiple trophic groups are promoted by different forest features. Ecol. Lett. 2019, 22, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Lange, M.; Türke, M.; Pašalić, E.; Boch, S.; Hessenmöller, D.; Müller, J.; Prati, D.; Socher, S.A.; Fischer, M.; Weisser, W.W.; et al. Effects of forest management on ground-dwelling beetles (Coleoptera; Carabidae, Staphylinidae) in Central Europe are mainly mediated by changes in forest structure. For. Ecol. Manag. 2014, 329, 166–176. [Google Scholar] [CrossRef]
- Nolet, P.; Kneeshaw, D.; Messier, C.; Béland, M. Comparing the effects of even- and uneven-aged silviculture on ecological diversity and processes: A review. Ecol. Evol. 2018, 8, 1217–1226. [Google Scholar] [CrossRef]
- Vogel, S.; Gossner, M.M.; Mergner, U.; Müller, J.; Thorn, S. Optimizing enrichment of deadwood for biodiversity by varying sun exposure and tree species: An experimental approach. J. Appl. Ecol. 2020, 57, 2075–2085. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Odor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; de Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Winter, S.; Höfler, J.; Michel, A.K.; Böck, A.; Ankerst, D.P. Association of tree and plot characteristics with microhabitat formation in European beech and Douglas-fir forests. Eur. J. For. Res. 2015, 134, 335–347. [Google Scholar] [CrossRef]
- Bobiec, A.; van der Burgt, H.; Meijer, K.; Zuyderduyn, C.; Haga, J.; Vlaanderen, B. Rich deciduous forests in Białowieża as a dynamic mosaic of developmental phases: Premises for nature conservation and restoration management. For. Ecol. Manag. 2000, 130, 159–175. [Google Scholar] [CrossRef]
- Herbst, M.; Mund, M.; Tamrakar, R.; Knohl, A. Differences in carbon uptake and water use between a managed and an unmanaged beech forest in central Germany. For. Ecol. Manag. 2015, 355, 101–108. [Google Scholar] [CrossRef]
- Moning, C.; Müller, J. Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol. Indic. 2009, 9, 922–932. [Google Scholar] [CrossRef]
- McGarvey, J.C.; Thompson, J.R.; Epstein, H.E.; Shugart, H.H. Carbon storage in old-growth forests of the Mid-Atlantic: Toward better understanding the eastern forest carbon sink. Ecology 2015, 96, 311–317. [Google Scholar] [CrossRef]
- Glatthorn, J.; Feldmann, E.; Pichler, V.; Hauck, M.; Leuschner, C. Biomass Stock and Productivity of Primeval and Production Beech Forests: Greater Canopy Structural Diversity Promotes Productivity. Ecosystems 2018, 21, 704–722. [Google Scholar] [CrossRef]
- Meyer, P.; Nagel, R.; Feldmann, E. Limited sink but large storage: Biomass dynamics in naturally developing beech (Fagus sylvatica) and oak (Quercus robur, Quercus petraea) forests of north-western Germany. J. Ecol. 2021, 109, 3602–3616. [Google Scholar] [CrossRef]
- Leuschner, C.; Feldmann, E.; Pichler, V.; Glatthorn, J.; Hertel, D. Forest management impact on soil organic carbon: A paired-plot study in primeval and managed European beech forests. For. Ecol. Manag. 2022, 512, 120163. [Google Scholar] [CrossRef]
- Prevedello, J.A.; Vieira, M.V. Does the type of matrix matter? A quantitative review of the evidence. Biodivers. Conserv. 2010, 19, 1205–1223. [Google Scholar] [CrossRef]
- Mildrexler, D.J.; Berner, L.T.; Law, B.E.; Birdsey, R.A.; Moomaw, W.R. Large Trees Dominate Carbon Storage in Forests East of the Cascade Crest in the United States Pacific Northwest. Front. For. Glob. Change 2020, 3, 127. [Google Scholar] [CrossRef]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M.L.; et al. Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef]
- Staatsforsten, B. Grundsätze für die Bewirtschaftung von Buchen–und Buchenmischbeständen im Bayerischen Staatswald; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2011. [Google Scholar]
- Weller, A. Vergleich von Buchen-Durchforstungskonzepten bezüglich des dimensions-und altersabhängigen Rotkernrisikos basierend auf simulierten Z-Baum-Durchmessern. Forstarchiv 2016, 87, 107–120. [Google Scholar]
- Pretzsch, H. The course of tree growth. Theory and reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Vandekerkhove, K.; Vanhellemont, M.; Vrška, T.; Meyer, P.; Tabaku, V.; Thomaes, A.; Leyman, A.; de Keersmaeker, L.; Verheyen, K. Very large trees in a lowland old-growth beech (Fagus sylvatica L.) forest: Density, size, growth and spatial patterns in comparison to reference sites in Europe. For. Ecol. Manag. 2018, 417, 1–17. [Google Scholar] [CrossRef]
- Kobler, J.; Jandl, R.; Dirnböck, T.; Mirtl, M.; Schindlbacher, A. Effects of stand patchiness due to windthrow and bark beetle abatement measures on soil CO2 efflux and net ecosystem productivity of a managed temperate mountain forest. Eur. J. For. Res. 2015, 134, 683–692. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. Camb. Philos. Soc. 2006, 81, 117–142. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Fahrig, L.; Förster, B.; Heurich, M.; Hothorn, T.; Scheipl, F.; Thorn, S.; Müller, J. An experimental test of the habitat-amount hypothesis for saproxylic beetles in a forested region. Ecology 2017, 98, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Bußler, H.; Kneib, T. Saproxylic beetle assemblages related to silvicultural management intensity and stand structures in a beech forest in Southern Germany. J. Insect Conserv. 2008, 12, 107–124. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of dead-wood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304. [Google Scholar] [CrossRef]
- Vandekerkhove, K.; de Keersmaeker, L.; Menke, N.; Meyer, P.; Verschelde, P. When nature takes over from man: Dead wood accumulation in previously managed oak and beech woodlands in North-western and Central Europe. For. Ecol. Manag. 2009, 258, 425–435. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Müller, J. Current near-to-nature forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv. Biol. 2013, 27, 605–614. [Google Scholar] [CrossRef]
- Lachat, T.; Brang, P.; Bolliger, M.; Bollmann, K.; Brändli, U.-B.; Bütler, R.; Herrmann, S. Totholz im Wald Entstehung, Bedeutung und Förderung. Merkbl. Prax. 2019, 52, 1–12. [Google Scholar]
- Knohl, A.; Kolle, O.; Minayeva, T.Y.; Milyukova, I.M.; Vygodskaya, N.N.; Foken, T.; Schulze, E.D. Carbon dioxide exchange of a Russian boreal forest after disturbance by wind throw. Glob. Change Biol. 2002, 8, 231–246. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. In Advances in Ecological Research: Classic Papers; Elsevier: Amsterdam, The Netherlands, 2004; pp. 59–234. ISBN 9780120139347. [Google Scholar]
- Harmon, M.E.; Bond-Lamberty, B.; Tang, J.; Vargas, R. Heterotrophic respiration in disturbed forests: A review with examples from North America. J. Geophys. Res. Biogeosci. 2011, 116, G00K04. [Google Scholar] [CrossRef]
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodall, C.W. Release of coarse woody detritus-related carbon: A synthesis across forest biomes. Carbon Balance Manag. 2020, 15, 1. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Piaszczyk, W. Dissolved carbon and nitrogen release from deadwood of different tree species in various stages of decomposition. Soil Sci. Plant Nutr. 2019, 65, 100–107. [Google Scholar] [CrossRef]
- Shannon, V.L.; Vanguelova, E.I.; Morison, J.I.L.; Shaw, L.J.; Clark, J.M. The contribution of deadwood to soil carbon dynamics in contrasting temperate forest ecosystems. Eur. J. For. Res. 2021, 141, 241–252. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Tullus, A.; Lutter, R.; Ostonen, I. Impact of deadwood decomposition on soil organic carbon sequestration in Estonian and Polish forests. Ann. For. Sci. 2019, 76, 1–14. [Google Scholar] [CrossRef]
- Pramreiter, M.; Grabner, M. The Utilization of European Beech Wood (Fagus sylvatica L.) in Europe. Forests 2023, 14, 1419. [Google Scholar] [CrossRef]
- Profft, I.; Mund, M.; Weber, G.-E.; Weller, E.; Schulze, E.-D. Forest management and carbon sequestration in wood products. Eur. J. For. Res. 2009, 128, 399–413. [Google Scholar] [CrossRef]
- Lenk, E. Wachstum und Qualität bei Buche-Lichtwuchsdurchforstung; FVA-Einblick: Freiburg im Breisgau, Germany, 2007; Volume 11. [Google Scholar]
- Klädtke, J. Wachstum großkroniger Buchen und waldbauliche Konsequenzen. Forstarchiv 2002, 73, 211–217. [Google Scholar]
- Klädtke, J. Konzepte zur Buchen-Lichtwuchsdurchforstung. AFZ-Wald 2001, 56, 1047–1050. [Google Scholar]
- Pollanschütz, J. Formzahlfunktionen der Hauptbaumarten Österreichs. Informationsdienst Forstl. Bundesversuchsanstalt 1974, 153, 341–343. [Google Scholar]
- Ledermann, T.; Neumann, M. Biomass equations from data of old long-term experimental plots. Cent. Gesamte Forstwes. 2006, 123, 47–64. [Google Scholar]
- Wutzler, T.; Wirth, C.; Schumacher, J. Generic biomass functions for Common beech (Fagus sylvatica) in Central Europe: Predictions and components of uncertainty. Can. J. For. Res. 2008, 38, 1661–1675. [Google Scholar] [CrossRef]
- Eckmüllner, O. Allometric relations to estimate needle and branch mass of Norway spruce and Scots pine in Austria. Cent. Gesamte Forstwes. 2006, 123, 7–16. [Google Scholar]
- Wirth, C.; Schumacher, J.; Schulze, E.-D. Generic biomass functions for Norway spruce in Central Europe—A meta-analysis approach toward prediction and uncertainty estimation. Tree Physiol. 2004, 24, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine root biomass in forest ecosystems. For. Ecol. Manag. 2011, 261, 265–277. [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.-S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots Pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Vilén, T.; Meyer, J.; Thürig, E.; Lindner, M.; Green, T. Improved Regional and National Level Estimates of the Carbon Stock and Stock Change of Tree Biomass for Six European Countries (Deliverable 6.1) Improved National Estimates of the Carbon Stock and Stock Change of the Forest Soils for Six European Countries (Deliverable 6.2); CarboInvent_WP6-D6.1_D6.2-EFI_final; European Forest Institute: Helsinki, Finland, 2005. [Google Scholar]
- Liski, J.; Perruchoud, D.; Karjalainen, T. Increasing carbon stocks in the forest soils of western Europe. For. Ecol. Manag. 2002, 169, 159–175. [Google Scholar] [CrossRef]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2013, 362, 357–372. [Google Scholar] [CrossRef]
- Müller-Haubold, H.; Hertel, D.; Seidel, D.; Knutzen, F.; Leuschner, C. Climate Responses of Aboveground Productivity and Allocation in Fagus sylvatica: A Transect Study in Mature Forests. Ecosystems 2013, 16, 1498–1516. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C.; Hertel, D. Nutrient return with leaf litter fall in Fagus sylvatica forests across a soil fertility gradient. Plant Ecol. 2005, 177, 99–112. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Variation of soil and biomass carbon pools in beech forests across a precipitation gradient. Glob. Change Biol. 2010, 16, 1035–1045. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Schmid, I.; Koch, O.; Muhs, A.; Hölscher, D. Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant Soil 2004, 258, 43–56. [Google Scholar] [CrossRef]
- Leuschner, C.; Voß, S.; Foetzki, A.; Clases, Y. Variation in leaf area index and stand leaf mass of European beech across gradients of soil acidity and precipitation. Plant Ecol. 2006, 182, 247–258. [Google Scholar] [CrossRef]
- Etzold, S.; Ruehr, N.; Zweifel, R.; Dobbertin, M.; Zingg, A.; Pluess, P.; Häsler, R.; Eugster, W.; Buchmann, N. The Carbon Balance of Two Contrasting Mountain Forest Ecosystems in Switzerland: Similar Annual Trends, but Seasonal Differences. Ecosystems 2011, 14, 1289–1309. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, P.; Shen, H.; Fang, J. Global patterns of ecosystem carbon flux in forests: A biometric data-based synthesis. Glob. Biogeochem. Cycles 2014, 28, 962–973. [Google Scholar] [CrossRef]
- Jacobsen, C.; Rademacher, P.; Meesenburg, H.; Meiwes, K.J. Gehalte Chemischer Elemente in den Baumkompartimenten–Literaturstudie und Datensammlung; Berichte des Forschungszentrums Waldökosysteme der Universität Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Perruchoud, D.; Kienast, F.; Kaufmann, E.; Bräker, O.U. 20th Century Carbon Budget of Forest Soils in the Alps. Ecosystems 1999, 2, 320–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).