The Effect of the Vaccinium myrtillus L. Rhizosphere on the Maturity Index for Predatory Mites (Mesostigmata: Gamasina) in Assessing Anthropogenic Pollution of Forest Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collections
2.3. Soil Properties
2.4. Maturity Index
2.5. Assessment of Heavy (Trace) Metal Pollution
2.5.1. Contamination Factor and Pollution Load Index
2.5.2. Potential Ecological Risk Index
2.6. Statistical Analysis
3. Results
3.1. Analysis of the Soil Parameters
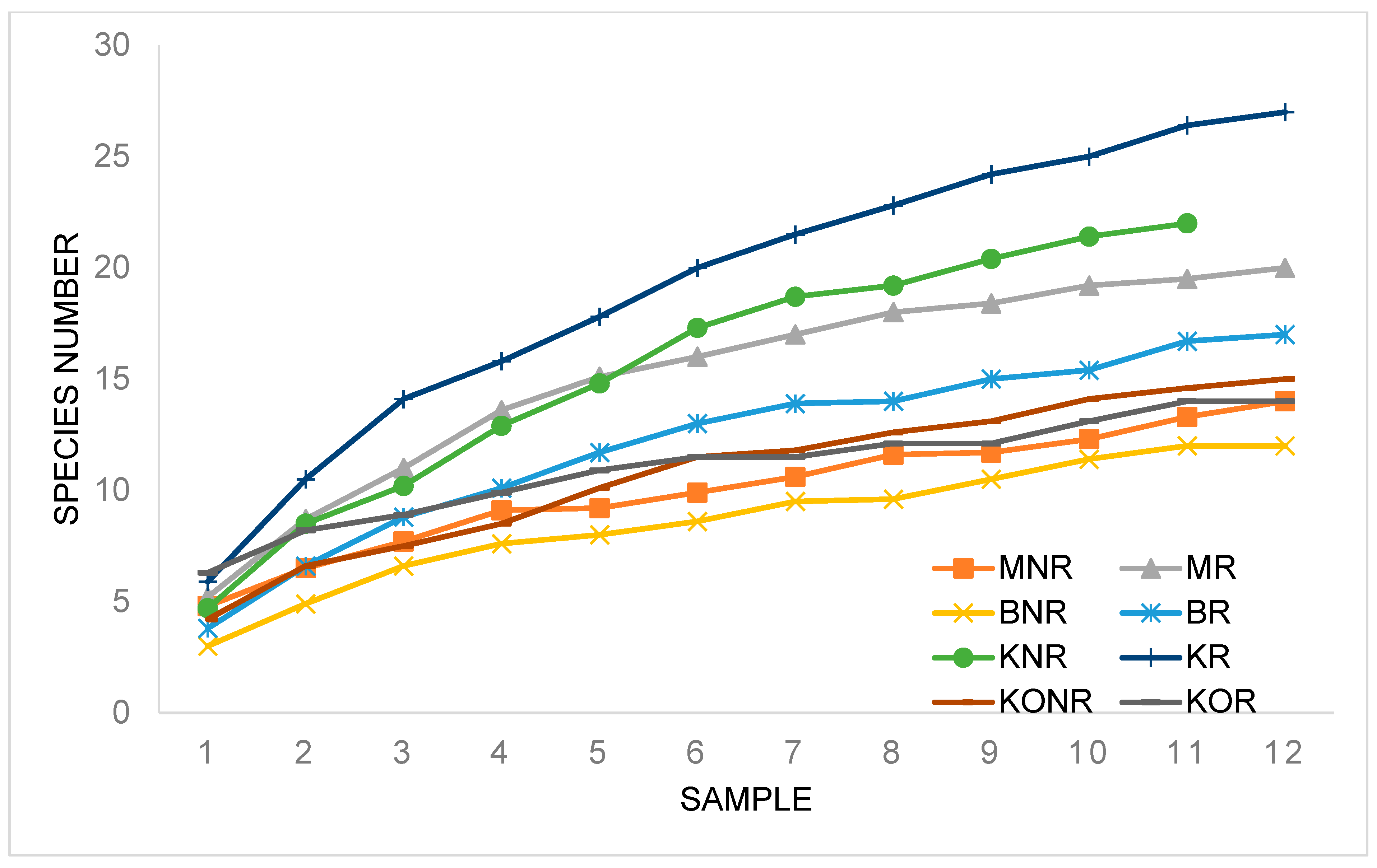
3.2. Predatory Mite Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mazurek, R.; Kowalska, J.; Gąsiorek, M.; Zadrożny, P.; Józefowska, A.; Zaleski, T.; Kępka, W.; Tymczuk, M.; Orłowska, K. Assessment of Heavy Metals Contamination in Surface Layers of Roztocze National Park Forest Soils (SE Poland) by Indices of Pollution. Chemosphere 2017, 168, 839–850. [Google Scholar] [CrossRef]
- Galli, L.; Capurro, M.; Menta, C.; Rellini, I. Is the QBS-Ar Index a Good Tool to Detect the Soil Quality in Mediterranean Areas? A Cork Tree Quercus suber L. (Fagaceae) Wood as a Case of Study. Ital. J. Zool. 2014, 81, 126–135. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Nadgórska-Socha, A.; Barczyk, G. The Influence of Heavy Metals on Biological Soil Quality Assessments in the Vaccinium myrtillus L. Rhizosphere under Different Field Conditions. Ecotoxicology 2021, 30, 292–310. [Google Scholar] [CrossRef]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of Heavy Metal Pollution and Human Health Risk in Urban Soils of Steel Industrial City (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kandziora-Ciupa, M.; Gospodarek, J.; Nadgórska-Socha, A. Pollution and Ecological Risk Assessment of Heavy Metals in Forest Soils with Changes in the Leaf Traits and Membrane Integrity of Vaccinium myrtillus L. Eur. J. For. Res. 2022, 141, 409–419. [Google Scholar] [CrossRef]
- Liu, M.; Li, Z.; Li, S.; Liang, F.; Huang, C.; Pan, H.; Wu, J.; Liu, S.; Pu, L.; Christie, P.; et al. The Effects of Pollution by Multiple Metals Derived from Long-Term Smelting Activities on Soil Mite Communities in Arable Soils under Different Land Use Types in East China. Environ. Sci. Pollut. Res. Int. 2023, 30, 47182–47208. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control. A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- George, P.B.; Keith, A.M.; Creer, S.; Barrett, G.L.; Lebron, I.; Emmett, B.A.; Robinson, D.A.; Jones, D.L. Evaluation of Mesofauna Communities as Soil Quality Indicators in a National-Level Monitoring Programme. Soil Biol. Biochem. 2017, 115, 537–546. [Google Scholar] [CrossRef]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod Communities as a Tool to Assess Soil Quality and Biodiversity: A New Approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Ruf, A. A Maturity Index for Predatory Soil Mites (Mesostigmata: Gamasina) as an Indicator of Environmental Impacts of Pollution on Forest Soils. Appl. Soil Ecol. 1998, 9, 447–452. [Google Scholar] [CrossRef]
- Madej, G.; Kozub, M. Possibilities of Using Soil Microarthropods, with Emphasis on Mites (Arachnida, Acari, Mesostigmata), in Assessment of Successional Stages in a Reclaimed Coal Mine Dump (Pszów, S Poland). Biol. Lett. 2014, 51, 19–36. [Google Scholar] [CrossRef]
- Yan, S.; Singh, A.N.; Fu, S.; Liao, C.; Wang, S.; Li, Y.; Cui, Y.; Hu, L. A Soil Fauna Index for Assessing Soil Quality. Soil Biol. Biochem. 2012, 47, 158–165. [Google Scholar] [CrossRef]
- Čoja, T.; Bruckner, A. The Maturity Index Applied to Soil Gamasine Mites from Five Natural Forests in Austria. Appl. Soil Ecol. 2006, 34, 1–9. [Google Scholar] [CrossRef]
- Ruf, A.; Beck, L. The Use of Predatory Soil Mites in Ecological Soil Classification and Assessment Concepts, with Perspectives for Oribatid Mites. Ecotoxicol. Environ. Saf. 2005, 62, 290–299. [Google Scholar] [CrossRef]
- Mróz, L.; Demczuk, M. Contents of Phenolics and Chemical Elements in Bilberry (Vaccinium myrtillus L.) Leaves from Copper Smelter Area (SW Poland). Pol. J. Ecol. 2010, 58, 475–486. [Google Scholar]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere Fauna: The Functional and Structural Diversity of Intimate Interactions of Soil Fauna with Plant Roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Xiao, S.; You, H.; You, W.; Liu, J.; Cai, C.; Wu, J.; Ji, Z.; Zhan, S.; Hu, Z.; Zhang, Z.; et al. Rhizosphere and Bulk Soil Enzyme Activities in a Nothotsuga Longibracteata Forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J. For. Res. 2017, 28, 521–528. [Google Scholar] [CrossRef]
- Baudoin, E.; Benizri, E.; Guckert, A. Impact of Growth Stage on the Bacterial Community Structure along Maize Roots, as Determined by Metabolic and Genetic Fingerprinting. Appl. Soil Ecol. 2002, 19, 135–145. [Google Scholar] [CrossRef]
- Garcia, C.; Roldan, A.; Hernandez, T. Ability of Different Plant Species to Promote Microbiological Processes in Semiarid Soil. Geoderma 2005, 124, 193–202. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, C.; Jiang, Y.; Yue, C.; Jiang, Q.; Min, H.; Fan, H.; Zeng, Q.; Chang, J. Soil Microbial Abundances and Enzyme Activities in Different Rhizospheres in an Integrated Vertical Flow Constructed Wetland. CLEAN Soil Air Water 2011, 39, 206–211. [Google Scholar] [CrossRef]
- Manu, M. The Influence of Some Environmental Factors on the Species Diversity of the Predator Mites (Acari: Mesostigmata) from Natural Forest Ecosystems of Bucegi Massif (Romania). Trav. Du Muséum Natl. D’histoire Nat. Grigore Antipa 2011, 54, 9–20. [Google Scholar] [CrossRef]
- Ugland, K.I.; Gray, J.S.; Ellingsen, K.E. The Species—Accumulation Curve and Estimation of Species Richness. J. Anim. Ecol. 2003, 72, 888–897. [Google Scholar] [CrossRef]
- Crossley Jr, D.A.; Blair, J.M. A High-Efficiency, “Low-Technology” Tullgren-Type Extractor for Soil Microarthropods. Agric. Ecosyst. Environ. 1991, 34, 187–192. [Google Scholar] [CrossRef]
- Manu, M.; Onete, M.; Băncilă, R.I. The Effect of Heavy Metals on Mite Communities (Acari: Gamasina) from Urban Parks-Bucharest, Romania. Environ. Eng. Manag. J. EEMJ 2018, 17, 2071–2081. [Google Scholar] [CrossRef]
- Błaszak, C. Zerconidae (Acari, Mesostigmata) Polski; Państwowe Wydawnictwo Naukowe Warszawa: Warszawa, Poland, 1974. [Google Scholar]
- Ghilyarov, M.S.; Bregetova, N.G. A Key to the Soil-Inhabiting Mites (Mesostigmata); Zoological Institute, USSR Academy of Sciences: Leningrad, Russia, 1977. (In Russian) [Google Scholar]
- Gwiazdowicz, D.J. Ascid Mites (Acari, Mesostigmata) from Selected Forest Ecosystems and Microhabitats in Poland.; Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego: Poznań, Poland, 2007. [Google Scholar]
- Karg, W. Cohors Gamasina Leach, Raubmilben; Fischer: Frankfurt am Main, Germany, 1993. [Google Scholar]
- Micherdziński, W. Die Familie Parasitidae Oudemans 1901 (Acarina, Mesostigmata); Państwowe Wydawnictwo Naukowe Warszawa: Warszawa, Poland, 1969. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Evaluation of Soil Properties and Plant–Catalogue; Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- Rutkowski, P.; Diatta, J.; Konatowska, M.; Andrzejewska, A.; Tyburski, Ł.; Przybylski, P. Geochemical Referencing of Natural Forest Contamination in Poland. Forests 2020, 11, 157. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A Proposal of “Core Enzyme” Bioindicator in Long-Term Pb-Zn Ore Pollution Areas Based on Topsoil Property Analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Y.; Tian, H.; Chen, H.; Wang, Y.; Huang, M. Proper Land Use for Heavy Metal-Polluted Soil Based on Enzyme Activity Analysis around a Pb-Zn Mine in Feng County, China. Environ. Sci. Pollut. Res. 2017, 24, 28152–28164. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.A.; Parvez, L.; Islam, M.A.; Dampare, S.B.; Suzuki, S. Heavy Metal Pollution of Coal Mine-Affected Agricultural Soils in the Northern Part of Bangladesh. J. Hazard. Mater. 2010, 173, 384–392. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Masunaga, S. Potential Ecological Risk of Hazardous Elements in Different Land-Use Urban Soils of Bangladesh. Sci. Total Environ. 2015, 512, 94–102. [Google Scholar] [CrossRef]
- Kolawole, T.O.; Olatunji, A.S.; Jimoh, M.T.; Fajemila, O.T. Heavy Metal Contamination and Ecological Risk Assessment in Soils and Sediments of an Industrial Area in Southwestern Nigeria. J. Health Pollut. 2018, 8, 180906. [Google Scholar] [CrossRef]
- Regulation on Soil Quality Standards. | UNEP Law and Environment Assistance Platform. Available online: https://leap.unep.org/en/countries/pl/national-legislation/regulation-soil-quality-standards (accessed on 18 October 2024).
- Mehmood, K.; Ahmad, H.R.; Abbas, R.; Saifullah; Murtaza, G. Heavy Metals in Urban and Peri-Urban Soils of a Heavily-Populated and Industrialized City: Assessment of Ecological Risks and Human Health Repercussions. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1705–1722. [Google Scholar] [CrossRef]
- Manu, M.; Iordache, V.; Băncilă, R.I.; Bodescu, F.; Onete, M. The Influence of Environmental Variables on Soil Mite Communities (Acari: Mesostigmata) from Overgrazed Grassland Ecosystems—Romania. Ital. J. Zool. 2016, 83, 89–97. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Bîrsan, C.C.; Mountford, O.; Onete, M. Soil Mite Communities (Acari: Mesostigmata) as Indicators of Urban Ecosystems in Bucharest, Romania. Sci. Rep. 2021, 11, 3794. [Google Scholar] [CrossRef]
- Ruf, A.; Beck, L.; Dreher, P.; Hund-Rinke, K.; Römbke, J.; Spelda, J. A Biological Classification Concept for the Assessment of Soil Quality: “Biological Soil Classification Scheme” (BBSK). Agric. Ecosyst. Environ. 2003, 98, 263–271. [Google Scholar] [CrossRef]
- Wissuwa, J.; Salamon, J.-A.; Frank, T. Effects of Habitat Age and Plant Species on Predatory Mites (Acari, Mesostigmata) in Grassy Arable Fallows in Eastern Austria. Soil Biol. Biochem. 2012, 50, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, G.; Kamczyc, J.; Bierza, W.; Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Jagodziński, A.M. Functional Ecosystem Parameters: Soil Respiration and Diversity of Mite (Acari, Mesostigmata) Communities after Disturbance in a Late Cambrian Bedrock Environment. Land Degrad. Dev. 2022, 33, 3343–3357. [Google Scholar] [CrossRef]
- Skubała, P.; Kafel, A. Oribatid Mite Communities and Metal Bioaccumulation in Oribatid Species (Acari, Oribatida) along the Heavy Metal Gradient in Forest Ecosystems. Environ. Pollut. 2004, 132, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rusek, J.; Marshall, V.G. Impacts of Airborne Pollutants on Soil Fauna. Annu. Rev. Ecol. Syst. 2000, 31, 395–423. [Google Scholar] [CrossRef]
- Santorufo, L.; Van Gestel, C.A.M.; Rocco, A.; Maisto, G. Soil Invertebrates as Bioindicators of Urban Soil Quality. Environ. Pollut. 2012, 161, 57–63. [Google Scholar] [CrossRef]
- Bora, P.; Gogoi, S.; Deshpande, M.V.; Garg, P.; Bhuyan, R.P.; Altaf, N.; Saha, N.; Borah, S.M.; Phukon, M.; Tanti, N.; et al. Rhizospheric Bacillus Spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea. Microorganisms 2023, 11, 2691. [Google Scholar] [CrossRef]
- Hosseini, A.; Hosseini, M.; Schausberger, P. Plant Growth-Promoting Rhizobacteria Enhance Defense of Strawberry Plants Against Spider Mites. Front. Plant Sci. 2022, 12, 783578. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality Index (QBS-Ar): 15 Years of Application at Global Scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Prach, K.; Tolvanen, A. How Can We Restore Biodiversity and Ecosystem Services in Mining and Industrial Sites? Environ. Sci. Pollut. Res. 2016, 23, 13587–13590. [Google Scholar] [CrossRef]

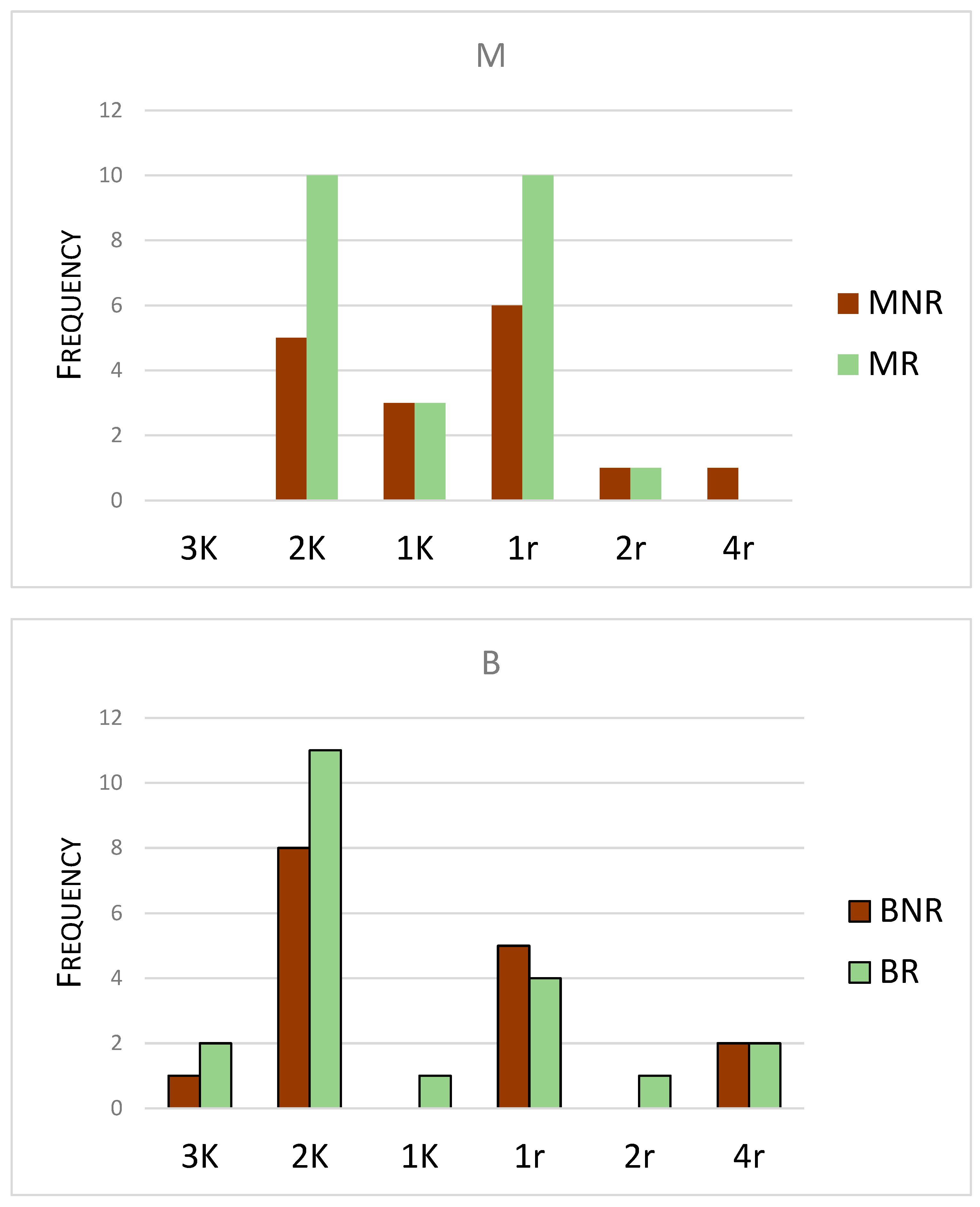
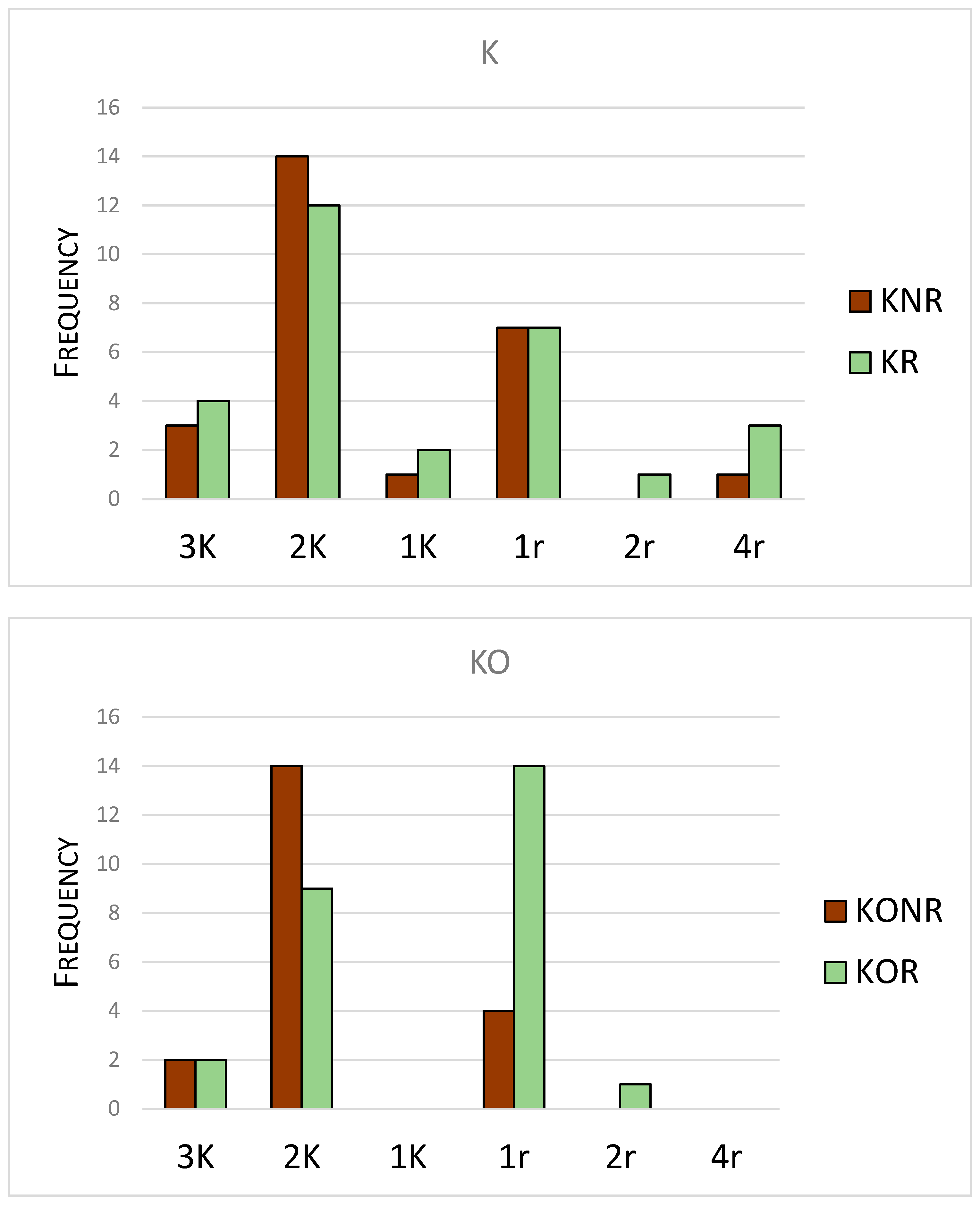
| Taxon | K | r |
|---|---|---|
| Eviphidae (Alliphis) | 4 | |
| Ascidae | 1 | |
| Digamasellidae | 2 | |
| Laelapidae | 1 | |
| Pachylaelapidae | 1 | |
| Parasitidae | 4 | |
| Pergamasidae | 2 | |
| Phytoseiidae | 2 | |
| Rhodacaridae | 2 | |
| Veigaiidae | 2 | |
| Zerconidae | 3 | |
| Hypoaspididae | 1 |
| PLI | PLI Grade | ERI Value | EIR Grade of Single Metal | Grade Symbol | PERI Value | Environmental PERI Grade |
|---|---|---|---|---|---|---|
| PLI < 1 | Unpolluted (U) | ERI < 40 | low ecological risk | L | PERI < 150 | low ecological risk (L) |
| 1 ≤ PLI < 2 | unpolluted to moderately polluted (UM) | 40 ≤ ERI < 80 | moderate ecological risk | M | 150 ≤ PERI < 300 | moderate ecological risk (M) |
| 2 ≤ PLIr < 3 | moderately polluted (M) | 80 ≤ ERI< 160 | considerable ecological risk | C | 300 ≤ PERI < 600 | high ecological risk (H) |
| 3 ≤ PLI < 4 | moderately to highly polluted (MH) | 160 ≤ ERI < 320 | high ecological risk | H | PERI ≥ 600 | very high ecological risk (VH) |
| 4 ≤ PLI < 5 | highly polluted (H) | ERI ≥ 320 | very high ecological risk | VH | ||
| PLI ≥ 5 | very highly polluted (VH) |
| Sample | pH | OM% | Ntotal% | Ctotal% | C/N Ratio | |
|---|---|---|---|---|---|---|
| M | NR | 4.65 ± 0.08 d,5 | 9.00 ± 2.89 c,1 | 0.26 ± 0.06 b,1 | 5.56 ± 1.24 b,1 | 21.48 ± 0.8 a,1 |
| R | 5.23 ± 0.19 d,3 | 17.65 ± 1.8 c,1 | 0.5 ± 0.04 b,1,2 | 9.58 ± 0.7 b,1,2 | 19.26 ± 0.62 a,3 | |
| B | NR | 5.14 ± 0.13 c,3 | 19.1 ± 5.59 b,1 | 0.41 ± 0.13 a,1,2 | 9.62 ± 3.16 a,1,2 | 23.35 ± 0.34 c,1 |
| R | 4.28 ± 0.15 c,4 | 57.05 ± 8.84 b,2 | 0.8 ± 0.18 a,3 | 22.68 ± 4.75 a,3 | 28.28 ± 0.2 c,2 | |
| K | NR | 4.00 ± 0.24 b,2 | 17.55 ± 0.75 a,1 | 0.47 ± 0.11 a,1,2 | 9.85 ± 2.27 a,1,2,4 | 21.16 ± 1.23 b,1 |
| R | 3.98 ± 0.06 b,2 | 43.25 ± 5.03 a,3 | 0.92 ± 0.05 a,3 | 21.62 ± 1.09 a,3,4 | 23.52 ± 0.25 b,1 | |
| KO | NR | 3.65 ± 0.11 a,1 | 17.85 ± 10.84 a,1 | 1.17 ± 0.58 a,1,2,3 | 30.75 ± 15.15 a,2,3,4 | 26.21 ± 1.73 d,2 |
| R | 3.56 ± 0.09 a,1 | 57.85 ± 12.51 a,2 | 0.85 ± 0.15 a,2,3 | 24.77 ± 4.44 a,3 | 29.24 ± 0.54 d,2 | |
| Site | F/ŋ2 | 224.99 */0.94 * | 34.63 */0.72 * | 7.01 */0.34 * | 10.94 */0.45 * | 164.19 */0.92 * |
| Sphere | F/ŋ2 | 5.2 */0.12 * | 202.2 */0.83 * | 25.64 */0.39 * | 28.29 */0.41 * | 12.59 */0.24 * |
| Site ∗ sphere | F/ŋ2 | 50.9 */0.79 * | 13.31 */0.50 * | 2.23/0.14 | 3.3 */0.20 * | 34.4 */0.72 * |
| Site | Sphere | Cd | Cu | Zn | Pb | Ni | PERI | GRADE | PLI | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|
| M | NR | 8.32 ± 2.64 c,2 | 14.61 ± 3.15 c,3 | 480.27 ± 180.26 c,2 | 619.38 ± 62.27 c,3 | 1.35 ± 0.27 b,1,2 | 709.60 | VH | 6 | VH |
| R | 31.03 ± 3.75 c,3 | 49.24 ± 7.39 c,5 | 1552.29 ± 298.03 c,3 | 1576.67 ± 278.89 c,4 | 2.86 ± 0.24 b,4,5 | 2116.02 | VH | 18 | VH | |
| B | NR | 6.55 ± 1.33 b,2 | 13.31 ± 4.13 b,2,3 | 621.6 ± 119.01 b,2 | 333.54 ± 61.67 b,2 | 2.93 ± 0.58 b,5 | 452.34 | H | 6 | VH |
| R | 3.01 ± 0.46 b,1 | 24.2 ± 7.60 b,4 | 459.11 ± 64.13 b,2 | 204.79 ± 29.49 b,1,2 | 1.95 ± 0.32 b,2,3 | 263.84 | Mo | 5 | H | |
| K | NR | 0.21 ± 0.11 a,1 | 7.08 ± 2.00 a,1,2,3 | 35.35 ± 13.53 a,1 | 84.23 ± 24.47 a,1 | 2.39 ± 0.69 b,3,4,5 | 74.29 | L | 1 | UM |
| R | 0.39 ± 0.09 a,1 | 7.46 ± 1.32 a,1,2,3 | 40.85 ± 7.31 a,1 | 62.99 ± 11.15 a,1 | 2.12 ± 0.41 b,2,3,4 | 64.96 | L | 1 | UM | |
| KO | NR | 0.07 ± 0.08 a,1 | 3.18 ± 0.76 a,1 | 23.1 ± 12.93 a,1 | 51.45 ± 27.44 a,1 | 0.97 ± 0.44 a,1 | 43.95 | L | 0.1 | U |
| R | 0.41 ± 0.09 a,1 | 5.49 ± 2.31 a,1,2 | 36.23 ± 5.63 a,1 | 36.75 ± 10.46 a,1 | 0.92 ± 0.25 a,1 | 44.43 | L | 0.1 | U | |
| Site | F/ŋ2 | 352.98 */0.96 * | 616.85 */0.88 * | 152.53 */0.92 * | 267.26 */0.95 * | 29.94 */0.69 * | ||||
| Sphere | F/ŋ2 | 101.15 */0.72 * | 100.26 */0.70 * | 36.91 */0.48 * | 42.88 */0.52 * | 0.16/0.00 | ||||
| Site ∗ sphere | F/ŋ2 | 150.02 */0.92 * | 39.35 */0.75 * | 54.87 */0.80 * | 70.67 */0.84 * | 18.03 */0.57 * |
| Sample | Abundance | Number of Species | MI | |
|---|---|---|---|---|
| M | NR | 12.27 ± 11.33 a,1 | 14 | 0.52 |
| R | 31 ± 16.92 a,1 | 20 | 0.66 | |
| B | NR | 17.64 ± 23.63 a,1 | 12 | 0.59 |
| R | 33.91 ± 19.53 a,1 | 17 | 0.67 | |
| K | NR | 48.64 ± 68.9 a,1 | 22 | 0.78 |
| R | 54.18 ± 35.97 a,1 | 27 | 0.64 | |
| KO | NR | 61.64 ± 48.71 b,2 | 15 | 0.89 |
| R | 115.18 ± 58.77 b,2 | 14 | 0.60 | |
| Site | F/ŋ2 | 11.76 */0.60 * | ||
| Sphere | F/ŋ2 | 8.34 */0.31 * | ||
| Site ∗ sphere | F/ŋ2 | 1.4/0.09 * |
| Equation | R2 | F | p |
|---|---|---|---|
| NR very highly polluted sites (M and B) | |||
| MI = 0.35 * − 0.05 Pb + 0.28 Ni * − 0.29 Cd * + 0.42 pH * + 0.32 OM * − 0.09 C/N Ratio | 1.00 | 321.05 | 0.00 |
| NR unpolluted to moderately polluted sites (K and KO) | |||
| MI = 0.09 + 1.02 C/N Ratio * + 0.38 OM * | 0.89 | 37.88 | 0.00 |
| R very highly to highly polluted sites (M and B) | |||
| MI = 0.63 * + 0.57 C/N Ratio * + 0.05 Pb − 0.12 Ni * + 0.46 OM * + 0.37 pH * − 0.38 Cd * + 0.11 Cu * | 1.00 | 4394.67 | 0.00 |
| R unpolluted to moderately polluted sites (K and KO) | |||
| MI = 0.67 * − 0.49 C/N Ratio − 0.015 OM + 0.14 Cu − 0.14 Cd * + 0.17 pH + 0.18 Ni | 1.00 | 317.42 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barczyk, G.; Nadgórska-Socha, A.; Kandziora-Ciupa, M. The Effect of the Vaccinium myrtillus L. Rhizosphere on the Maturity Index for Predatory Mites (Mesostigmata: Gamasina) in Assessing Anthropogenic Pollution of Forest Soils. Forests 2024, 15, 2245. https://doi.org/10.3390/f15122245
Barczyk G, Nadgórska-Socha A, Kandziora-Ciupa M. The Effect of the Vaccinium myrtillus L. Rhizosphere on the Maturity Index for Predatory Mites (Mesostigmata: Gamasina) in Assessing Anthropogenic Pollution of Forest Soils. Forests. 2024; 15(12):2245. https://doi.org/10.3390/f15122245
Chicago/Turabian StyleBarczyk, Gabriela, Aleksandra Nadgórska-Socha, and Marta Kandziora-Ciupa. 2024. "The Effect of the Vaccinium myrtillus L. Rhizosphere on the Maturity Index for Predatory Mites (Mesostigmata: Gamasina) in Assessing Anthropogenic Pollution of Forest Soils" Forests 15, no. 12: 2245. https://doi.org/10.3390/f15122245
APA StyleBarczyk, G., Nadgórska-Socha, A., & Kandziora-Ciupa, M. (2024). The Effect of the Vaccinium myrtillus L. Rhizosphere on the Maturity Index for Predatory Mites (Mesostigmata: Gamasina) in Assessing Anthropogenic Pollution of Forest Soils. Forests, 15(12), 2245. https://doi.org/10.3390/f15122245





