Phytosociological and Abiotic Factors Influencing the Coverage and Morphological Traits of the Invasive Alien Potentilla indica (Rosaceae) in Riparian Forests and Other Urban Habitats: A Case Study from Kraków, Southern Poland
Abstract
1. Introduction
- (1)
- How the type of vegetation, species diversity, and height of the herbaceous layer affect the coverage and morphological features of P. indica;
- (2)
- How the coverage and morphological characteristics of P. indica depend on light intensity and soil conditions (moisture, electrical conductivity, compactness, pH, and content of nutrients);
- (3)
- Whether the increasing cover of P. indica enhances its morphological features.
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Phytosociological Analysis
2.4. Measurement of Abiotic Factors
2.5. Morphometric Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Plant Communities
3.2. Characteristics of Abiotic Conditions
3.3. Morphological Variability of P. indica in Various Plant Communities
3.4. The Effect of Environmental Factors on Coverage and Morphological Features of P. indica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aronson, M.F.J.; LaSorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, L.J.; Cadotte, M.W. The application of selected invasion frameworks to urban ecosystems. NeoBiota 2020, 62, 365–386. [Google Scholar] [CrossRef]
- Ruas, R.B.; Costa, L.M.S.; Bered, F. Urbanization driving changes in plant species and communities—A global view. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar] [CrossRef]
- Pyšek, P. Alien and native species Central European urban floras: A quantitative comparison. J. Biogeogr. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Säumel, I.; Kowarik, I. Cities a sdrivers for biological invasions—The role of urban climate and traffic. Die Erde 2005, 136, 123–143. [Google Scholar]
- Gaertner, M.; Larson, B.M.H.; Irlich, U.M.; Holmes, P.M.; Stafford, L.; van Wilgen, B.W.; Richardson, D.M. Managing invasive species in cities: A framework from Cape Town, South Africa. Landsc. Urban Plan. 2016, 151, 1–9. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Tichýl, L.; Danihelka, J.; Fajmon, K.; Hájek, O.; Kintrová, K.; Kühn, I.; Láníková, D.; Otýpková, Z.; et al. Native and alien floras in urban habitats: A comparison across 32 cities of central Europe. Glob. Ecol. Biogeogr. 2012, 21, 545–555. [Google Scholar] [CrossRef]
- Boscutti, F.; Lami, F.; Pellegrini, E.; Buccheri, M.; Busato, F.; Martini, F.; Sibella, R.; Sigura, M.; Marini, L. Urban sprawl facilitates invasions of exotic plants a cross multiple spatial scales. Biol. Invasions 2022, 24, 1497–1510. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Bertram, C.; Rehdanz, K. The role of urban green space for human well-being. Ecol. Econ. 2015, 120, 139–152. [Google Scholar] [CrossRef]
- Jabbar, M.; Yusoff, M.M.; Shafie, A. Assessing the role of urban green spaces for human well-being: A systematic review. GeoJournal 2022, 87, 4405–4423. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Šipek, M.; Šajna, N. Public opinions and perceptions of peri-urban plant invasion: The role of garden waste disposal in forest fragments. Manag. Biol. Invasions 2020, 11, 733–746. [Google Scholar] [CrossRef]
- Rusterholz, H.-P.; Wirz, D.; Baur, B. Garden waste deposits as a source for non-native plants in mixed deciduous forests. Appl. Veg. Sci. 2012, 15, 329–337. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Maxianová, A.; Winkler, J.; Adamcová, D.; Podlasek, A. Environmental consequences and the role of illegal waste dump sand their impact on land degradation. Land Use Policy 2019, 89, 104234. [Google Scholar] [CrossRef]
- Strgulc Krajšek, S.; Bahčič, E.; Čoko, U.; Dolenc Koce, J. Disposal methods for selected invasive plant species used as ornamental garden plants. Manag. Biol. Invasions 2020, 11, 293–305. [Google Scholar] [CrossRef]
- Flor aof China Volume 9. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=111019 (accessed on 3 October 2024).
- Ertter, B.; Reveal, J.L. Duchesnea. In Flora of North America; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2014; Volume 9, pp. 272–273. [Google Scholar]
- Randall, R.P. A Global Compendium of Weeds, 3rd ed.; RPRandall: Perth, WA, Australia, 2017. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org (accessed on 4 July 2024).
- Qian, Y.; Lu Chen, D.; Li, D. Physiological integration improves mock strawberry [Duchesnea indica (Andr.) Focke] uniformity under heterogeneous saline conditions. Sci. Hortic. 2022, 291, 110579. [Google Scholar] [CrossRef]
- Jackowiak, B. On the distribution of Duchesnea indica (Rosaceae) in Vienna. Fragm. Florist. Geobot. 1992, 37, 547–593. [Google Scholar]
- Liefländer, A.; Lauerer, M. Spontan vorkommen von Duchesnea indica: Ein Neophyt breitetsichinden letzten Jahrenverstärktaus. Berichte Bayer. Bot. Ges. 2007, 77, 187–200. [Google Scholar]
- Panek, M.; Piwowarczyk, R. Spontaniczne rozprzestrzenianie się Duchesnea indica (Rosaceae) na terenie Sandomierza. Fragm. Florist. Geobot. Polon. 2017, 24, 167–173. [Google Scholar]
- Mink, J.N.; Singhurst, J.R.; Holmes, W.C. Potentilla indica (Rosaceae) new to Utah, with discussion of dispersal by the American Robin. Phytoneuron 2019, 5, 1–4. [Google Scholar]
- Littschwager, J.; Lauerer, M.; Blagodatskaya, E.; Kuzyakov, Y. Nitrogen uptake and utilisation as a competition factor between invasive Duchesnea indica and native Fragaria vesca. Plant Soil 2010, 331, 105–114. [Google Scholar] [CrossRef]
- Invasive Species in Belgium. Duchesnea Indica. Available online: https://ias.biodiversity.be/species/show/107 (accessed on 3 October 2024).
- Eliášst, P. Spoločenstvo s Duchesnea indica v hlavnom meste SR Bratislave. Bull. Slov. Bot. Spoločn. 2020, 42, 187–204. [Google Scholar]
- Šipek, M.; Šajna, N.; Horvat, E. Factors driving invasion of alien Prunus serotina Her., Duchesnea indica (Andrews) Th. Wolfand Impatiens parviflora DC. In Tolow Land Forest Fragments in NE Slovenia, Proceedings of the 11th International Conference on Biological Invasions: The Human Role in Biological Invasions—A Case of Dr Jekyll and Mr Hyde? NEOBIOTA202015–18 September 2020, Vodice, Croatia, Book of Abstracts; Jelaska, S.D., Ed.; Croatian Ecological Society: Zagreb, Croatia, 2020; p. 103. [Google Scholar]
- Luo, X.; Dong, M. Architectural plasticity in response to soil moisture in the stoloniferous herb, Duchesnea indica. ActaBot. Sin. 2002, 44, 97–100. [Google Scholar]
- Castro-Díez, P.; Alonso, Á. Effects of non-native riparian plants in riparian and fluvial ecosystems: A review for the Iberian Peninsula. Limnetica 2017, 36, 525–541. [Google Scholar] [CrossRef]
- Chundi, C.; Shengjun, W.; Douglas, M.C.; Maohua, M.; Juanjuan, Z.; Mingquan, L.; Xiaoxiao, T. Effects of local and landscape factors on exotic vegetation in the riparian zone of a regulated river: Implications for reservoir conservation. Landsc. Urban Plan. 2017, 157, 45–55. [Google Scholar] [CrossRef]
- Czortek, P.; Dyderski, M.K.; Jagodziński, A.M. River regulation drives shifts in urban riparian vegetation over three decades. Urban For. Urban Green. 2020, 47, 126524. [Google Scholar] [CrossRef]
- Wang, M.-Z.; Li, H.-L.; Liu, C.-X.; Dong, B.-C.; Yu, F.-H. Adaptive plasticity in response to light and nutrient availability in the clonal plant Duchesnea indica. J. Plant Ecol. 2022, 15, 795–807. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of the Republic of Poland 2023; Statistics Poland: Warsaw, Poland, 2024.
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012.
- Zając, A.; Zając, M. (Eds.) Distribution atlas of Vascular Plants in Poland: Appendix; Institute of Botany, Jagiellonian University: Kraków, Poland, 2019. [Google Scholar]
- Trzcińska-Tacik, H. Flora Synantropijna Krakowa; Rozprawy Habilitacyjne; Uniwersytet Jagielloński: Kraków, Poland, 1979; Volume 32, pp. 1–278. [Google Scholar]
- Guzik, J. Flora roślin naczyniowych Krakowa, jej stan współczesny, zróżnicowanie i walory. Cz.2. Flora synantropijna. Wszechświat 2006, 107, 90–96. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie; Springer: Wien, Austria, 1964. [Google Scholar]
- Dzwonko, Z. Przewodnik do Badań Fitosocjologicznych; Sorus: Poznań-Kraków, Poland, 2007. [Google Scholar]
- Podani, J. SYN-TAX 2000. Computer Programs for Data Analysis in Ecology and Systematics; Scientia Publishing: Budapest, Hungary, 2001. [Google Scholar]
- Vander Maarel, E. Transformation of cover-abundance values in phytosociology and its effect on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2005. [Google Scholar]
- Pielou, E.C. Population and Community Ecology: Principles and Methods; CRC Press: NewYork, NY, USA, 1974. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley and Sons: NewYork, NY, USA, 1975. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen: In Ökologischer, Dynamischer und Historischer Sicht; Eugen Ulmer UTB: Stuttgart, Germany, 2010. [Google Scholar]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Zare, H.; Ramezani Kakroudi, E.; Amini, T. A record of Duchesnea indica (Rosaceae) in Iran, its western most distributional limit in Asia. Iran. J. Bot. 2007, 13, 93–94. [Google Scholar]
- Zhang, M.; Liu, Z.; Yang, Z.; Shen, H.; Wang, J.; Wu, X. Altitudinal variation in species diversity, distribution, and regeneration status of a secondary Picea forest in Guandi Mountain, Northern China. Forests 2024, 15, 771. [Google Scholar] [CrossRef]
- Dar, M.E.U.I.; Gillani, N.; Shaheen, H.; Firdous, S.S.; Ahmad, S.; Khan, M.Q.; Hussain, M.A.; Habib, T.; Malik, N.Z.; Ullah, T.S.; et al. Comparative analysis of vegetation from eroded and non-eroded areas, a case study from Kashmir Himalayas, Pakistan. Appl. Ecol. Environ. Res. 2018, 16, 1725–1737. [Google Scholar] [CrossRef]
- Mucina, L. Conspectus of classes of European vegetation. Folia Geobot. 1997, 32, 117–172. [Google Scholar] [CrossRef]
- Nemoto, M.; Ohtsuka, T. Effect of short-growing indigenous plants growing in surroundings of crop fields on the suppression of weed colonization. J. WeedSci. Technol. 1998, 43, 26–34. [Google Scholar] [CrossRef][Green Version]
- Ali, K.W.; Shinwari, M.I.; Khan, A.S. Screening of 196 medicinal plant species leaf litter for allelopathic potential. Pak. J. Bot. 2019, 51, 2169–2177. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Volo, T.J.; Vivoni, E.R.; Martin, C.A.; Earl, S.; Ruddell, B.L. Modelling soil moisture, water partitioning, and plant water stress under irrigated conditions in desert urban areas. Ecohydrology 2014, 7, 1297–1313. [Google Scholar] [CrossRef]
- Jiang, Y.; Weng, Q. Estimation of hourly and daily evapotranspiration and soil moisture using downscaled LST over various urban surfaces. GISci. Remote Sens. 2017, 54, 95–117. [Google Scholar] [CrossRef]
- Joimel, S.; Cortet, J.; Jolivet, C.C.; Saby, N.P.A.; Chenot, E.-D.; Branchu, P.; Consalès, J.-N.; Lefort, C.; Morel, J.-L.; Schwartz, C. Physico-chemical characteristics of topsoil for contrasted forest, agricultural, urban and industrial land uses in France. Sci. Total Environ. 2016, 545–546, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Call, N.M. Fertilization and mowing on persistence of Indian mock strawberry (Duchesnea indica) and Common blue violet (Viola papilionacea) in a tall fescue (Festuca arundinacea) lawn. Weed Sci. 1993, 41, 548–550. [Google Scholar] [CrossRef]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef]
- Hector, A.; Dobson, K.; Minns, A.; Bazeley-White, E.; Lawton, J.H. Community diversity and invasion resistance: An experimental test in a grassland ecosystem and a review of comparable studies. Ecol. Res. 2001, 16, 819–831. [Google Scholar] [CrossRef]
- Chen, J.S.; Lei, N.F.; Yu, D.; Dong, M. Differential effects of clonal integration on performance in the stoloniferous herb Duchesnea indica, as growing at two sites with different altitude. Plant Ecol. 2006, 183, 147–156. [Google Scholar] [CrossRef]
- Quan, J.; Zhang, X.; Song, S.; Dang, H.; Chai, Y.; Yue, M.; Liu, X. Clonal plant Duchesnea indica Focke forms an effective survival strategy in different degrees of Pb-contaminated environments. Plant Ecol. 2018, 219, 1315–1327. [Google Scholar] [CrossRef]
- Baranová, B.; Troščáková-Kerpčárová, E.; Grul’ová, D. Survey of the Solidago canadensis L. morphological traits and essential oil production: Aboveground biomass growth and abundance of the invasive goldenrod appears to be reciprocally enhanced within the invaded stands. Plants 2022, 11, 535. [Google Scholar] [CrossRef]
- Jamil, A.; Yang, J.-Y.; Su, D.-F.; Tong, J.-Y.; Chen, S.-Y.; Luo, Z.-W.; Shen, X.-M.; Wei, S.-J.; Cui, X.-L. Rhizospheric soil fungal communitypatterns of Duchesnea indica in response to altitude gradient in Yunnan, southwest China. Can. J. Microbiol. 2020, 66, 359–367. [Google Scholar] [CrossRef]
- Wang, P.; Lei, J.-P.; Li, M.-H.; Yu, F.-H. Spatial heterogeneity in light supply affects intraspecific competition of a stoloniferous clonal plant. PLoS ONE 2012, 7, e39105. [Google Scholar] [CrossRef]
- Aerts, R. Interspecific competition in natural plant communities: Mechanisms, trade-offs and plant-soil feedbacks. J. Exp. Bot. 1999, 50, 29–37. [Google Scholar] [CrossRef]
- Heywood, V.H.; Brunel, S. Code of Conducton Horticulture and Invasive AlienPlants; Council of Europe Publishing: Strasbourg, France, 2009. [Google Scholar]
- Heywood, V.H.; Sharrock, S. European Code of Conduct for Botanic Gardens on Invasive Alien Species; Council of Europe: Strasbourg, France; Botanic Gardens Conservation International: Richmond, VA, USA, 2013. [Google Scholar]


| Group | Number of Species | Shannon-Wiener Index [H’] | Evenness Index [J’] | Simpson Index [SIMP] | Ellenberg’s Indicator Values | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light | Temperature | Continentality | Moisture | Reaction | Nutrients | |||||
| 1 | 10.00 (±3.26) | 1.77 (±0.40) | 0.78 (±0.10) | 0.75 (±0.11) | 5.35 (±0.38) | 5.44 (±0.23) | 4.03 (±0.51) | 5.90 (±0.32) | 7.02 (±0.27) | 7.18 (±0.33) |
| 2 | 17.20 (±3.01) | 2.14 (±0.32) | 0.76 (±0.10) | 0.79 (±0.09) | 6.03 (±0.31) | 5.62 (±0.20) | 4.17 (±0.50) | 6.00 (±0.31) | 7.16 (±0.32) | 7.37 (±0.39) |
| 3 | 12.95 (±3.09) | 1.84 (±0.45) | 0.72 (±0.15) | 0.73 (±0.15) | 5.07 (±0.37) | 5.37 (±0.27) | 3.82 (±0.47) | 5.74 (±0.43) | 6.91 (±0.13) | 6.92 (±0.48) |
| 4 | 16.00 (±1.56) | 2.21 (±0.32) | 0.80 (±0.11) | 0.82 (±0.09) | 4.71 (±0.30) | 5.40 (±0.22) | 3.96 (±0.42) | 5.63 (±0.32) | 6.63 (±0.29) | 6.42 (±0.24) |
| 5 | 12.70 (±5.95) | 1.66 (±0.48) | 0.67 (±0.09) | 0.69 (±0.12) | 5.44 (±0.29) | 5.22 (±0.17) | 3.13 (±0.13) | 6.02 (±0.30) | 6.57 (±0.48) | 6.95 (±0.57) |
| 6 | 22.80 (±3.79) | 2.57 (±0.24) | 0.83 (±0.09) | 0.85 (±0.06) | 5.32 (±0.81) | 5.46 (±0.13) | 3.48 (±0.14) | 5.64 (±0.26) | 5.94 (±0.38) | 5.99 (±0.47) |
| 7 | 13.50 (±1.72) | 2.01 (±0.26) | 0.77 (±0.07) | 0.79 (±0.07) | 7.03 (±0.31) | 5.60 (±0.24) | 3.16 (±0.19) | 5.27 (±0.11) | 6.39 (±0.55) | 6.54 (±0.26) |
| 8 | 21.30 (±5.19) | 2.40 (±0.28) | 0.79 (±0.06) | 0.82 (±0.06) | 6.41 (±0.29) | 5.58 (±0.22) | 3.28 (±0.20) | 5.48 (±0.14) | 5.92 (±0.64) | 6.31 (±0.60) |
| Kruskal–Wallis H test | 59.15 * | 38.67 * | 13.31 ns | 18.97 ns | 67.31 * | 22.70 ns | 55.04 * | 40.52 * | 62.83 * | 51.43 * |
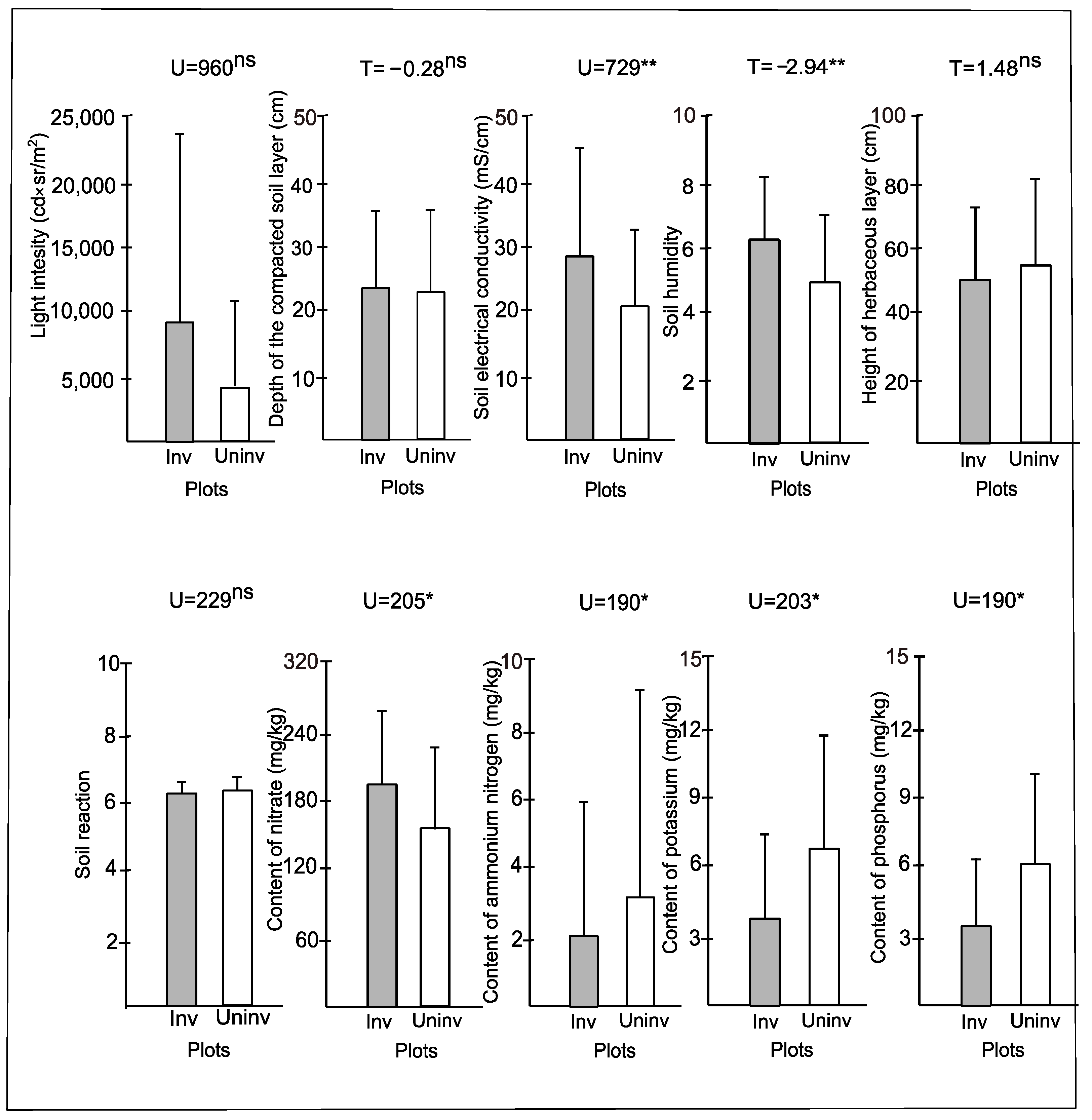
| Group | Coverage of P. indica | Light Intensity [cd × sr/m2] | Depth of Compact Soil Layer [cm] | Soil Electrical Conductivity [mS/cm] | Soil Moisture [0–10] | Height of Herbaceous Layer [cm] | Soil pH | Content of Nitrate [mg/kg] | Content of Amonium Nitrogen [mg/kg] | Content of Potassium [mg/kg] | Content of Phosphorus [mg/kg] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.57 (±1.77) | 5339.08 (±9311.79) | 28.69 (±8.94) | 0.41 (±0.19) | 7.47 (±2.07) | 67.85 (±23.07) | 6.24 (±0.24) | 190.00 (±39.44) | 1.00 (±2.11) | 2.56 (±3.29) | 2.40 (±2.67) |
| 2 | 0.40 (±0.84) | 2622.58 (±1853.81) | 12.42 (±6.47) | 0.08 (±0.02) | 2.44 (±0.87) | 69.83 (±14.25) | 6.38 (±0.16) | 170.00 (±67.08) | 3.00 (±4.47) | 12.50 (±4.69) | 8.80 (±5.22) |
| 3 | 0.93 (±1.23) | 4998.65 (±7658.86) | 36.58 (±13.22) | 0.29 (±0.11) | 6.70 (±1.10) | 57.64 (±9.41) | 6.15 (±0.34) | 202.50 (±78.57) | 3.00 (±4.83) | 7.42 (±3.51) | 2.80 (±2.66) |
| 4 | 0.40 (±0.84) | 4055.18 (±3349.25) | 30.88 (±7.70) | 0.22 (±0.06) | 4.96 (±1.17) | 59.56 (±24.67) | 6.50 (±0.00) | 130.00 (±67.08) | 0.00 (±0.00) | 0.90 (±1.24) | 7.00 (±3.16) |
| 5 | 0.83 (±0.76) | 8021.12 (±13,398.74) | 20.61 (±9.17) | 0.25 (±0.09) | 5.73 (±1.58) | 49.89 (±13.39) | 6.40 (±0.22) | 200.00 (±70.71) | 0.00 (±0.00) | 2.70 (±3.11) | 5.40 (±0.89) |
| 6 | 0.92 (±1.08) | 11,607.84 (±10,272.79) | 15.57 (±9.33) | 0.36 (±0.13) | 6.37 (±1.64) | 43.07 (±27.46) | 6.50 (±0.00) | 160.00 (±82.16) | 0.00 (±0.00) | 4.38 (±1.63) | 5.80 (±1.79) |
| 7 | 0.24 (±0.40) | 24,152.14 (±26,253.31) | 11.03 (±2.21) | 0.24 (±0.06) | 6.01 (±0.97) | 19.50 (±4.01) | 6.30 (±0.45) | 220.00 (±75.83) | 3.00 (±4.47) | 3.28 (±1.34) | 7.00 (±1.41) |
| 8 | 1.02 (±0.92) | 7282.02 (±5937.08) | 14.61 (±7.38) | 0.08 (±0.03) | 3.59 (±1.10) | 27.70 (±9.18) | 6.00 (±0.00) | 130.00 (±44.72) | 11.00 (±8.94) | 6.58 (±4.65) | 1.40 (±0.55) |
| Kruskal–Wallis H test | 27.73 * | 15.44 ns | 54.30 * | 50.04 * | 52.97 * | 49.30 * | 18.40 ns | 10.33 ns | 16.04 ns | 26.91 * | 25.48 ns |
| Group | N | Length of Pedicel [cm] | Length of Petiole [cm] | Width of Leaf [cm] | Length of Leaf [cm] | Number of Stolons | Length of the Longest Stolon | Number of Daughter Ramtes | N | Length of Fruit [cm] | Width of Fruit [cm] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 152 | 11.72 (±3.57) | 16.88 (±4.01) | 7.19 (±1.39) | 6.34 (±1.38) | 2.78 (±1.48) | 79.65 (±29.88) | 6.88 (±1.73) | 152 | 1.16 (±0.23) | 1.40 (±0.30) |
| 2 | 20 | 10.33 (±3.09) | 15.34 (±3.38) | 8.13 (±1.41) | 7.39 (±1.24) | 3.70 (±1.26) | 78.60 (±23.97) | 8.25 (±1.37) | 20 | 1.18 (±0.09) | 1.58 (±0.17) |
| 3 | 103 | 9.97 (±4.30) | 16.69 (±4.82) | 7.59 (±1.77) | 6.74 (±1.62) | 2.89 (±1.28) | 89.80 (±41.78) | 8.66 (±2.45) | 37 | 1.00 (±0.16) | 1.14 (±0.21) |
| 4 | 20 | 6.13 (±1.81) | 8.96 (±2.47) | 5.74 (±1.07) | 5.00 (±0.96) | 2.25 (±1.02) | 43.51 (±20.98) | 6.60 (±1.96) | 20 | 1.08 (±0.12) | 1.34 (±0.16) |
| 5 | 90 | 9.01 (±3.89) | 13.67 (±5.24) | 5.84 (±1.54) | 5.35 (±1.35) | 2.08 (±0.96) | 65.67 (±34.29) | 7.26 (±1.86) | 90 | 1.17 (±0.18) | 1.45 (±0.28) |
| 6 | 63 | 5.10 (±1.41) | 10.33 (±3.56) | 5.72 (±1.14) | 5.13 (±1.01) | 1.33 (±0.62) | 68.36 (±29.84) | 8.70 (±2.23) | 47 | 0.97 (±0.16) | 1.15 (±0.19) |
| 7 | 29 | 4.03 (±1.70) | 4.40 (±1.68) | 3.75 (±0.58) | 3.52 (±0.49) | 2.79 (±1.37) | 23.51 (±14.31) | 5.59 (±1.38) | 5 | 0.86 (±0.21) | 1.04 (±0.17) |
| 8 | 80 | 4.81 (±1.71) | 7.52 (±1.92) | 5.38 (±1.13) | 4.86 (±0.96) | 2.28 (±1.18) | 48.85 (±25.94) | 7.54 (±2.29) | 40 | 1.02 (±0.15) | 1.29 (±0.24) |
| Kruskal–Wallis H test | 288.89 * | 297.70 * | 214.71 * | 200.25 * | 115.54 * | 135.70 * | 83.02 * | - | 82.82 * | 89.66 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pliszko, A.; Wójcik, T.; Kostrakiewicz-Gierałt, K. Phytosociological and Abiotic Factors Influencing the Coverage and Morphological Traits of the Invasive Alien Potentilla indica (Rosaceae) in Riparian Forests and Other Urban Habitats: A Case Study from Kraków, Southern Poland. Forests 2024, 15, 2229. https://doi.org/10.3390/f15122229
Pliszko A, Wójcik T, Kostrakiewicz-Gierałt K. Phytosociological and Abiotic Factors Influencing the Coverage and Morphological Traits of the Invasive Alien Potentilla indica (Rosaceae) in Riparian Forests and Other Urban Habitats: A Case Study from Kraków, Southern Poland. Forests. 2024; 15(12):2229. https://doi.org/10.3390/f15122229
Chicago/Turabian StylePliszko, Artur, Tomasz Wójcik, and Kinga Kostrakiewicz-Gierałt. 2024. "Phytosociological and Abiotic Factors Influencing the Coverage and Morphological Traits of the Invasive Alien Potentilla indica (Rosaceae) in Riparian Forests and Other Urban Habitats: A Case Study from Kraków, Southern Poland" Forests 15, no. 12: 2229. https://doi.org/10.3390/f15122229
APA StylePliszko, A., Wójcik, T., & Kostrakiewicz-Gierałt, K. (2024). Phytosociological and Abiotic Factors Influencing the Coverage and Morphological Traits of the Invasive Alien Potentilla indica (Rosaceae) in Riparian Forests and Other Urban Habitats: A Case Study from Kraków, Southern Poland. Forests, 15(12), 2229. https://doi.org/10.3390/f15122229







