Effects of Downed Log Decomposition on Soil Properties and Enzyme Activities in Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Investigation
2.3. Soil Nutrient Content Measurement
2.3.1. Total Nitrogen and Phosphorus Contents (TN and TP)
2.3.2. Organic Carbon Content of the Soil (SOC)
2.4. Soil Enzyme Activity Measurement
2.4.1. Measurement of Soil Oxidase Activity
2.4.2. Measurement of Soil Hydrolase Activity
2.5. Data Processing
3. Results
3.1. Significance and Effect Sizes of Downed Log Species, Soil Depth, and Their Interactions on Soil Physicochemical Properties and Enzyme Activities
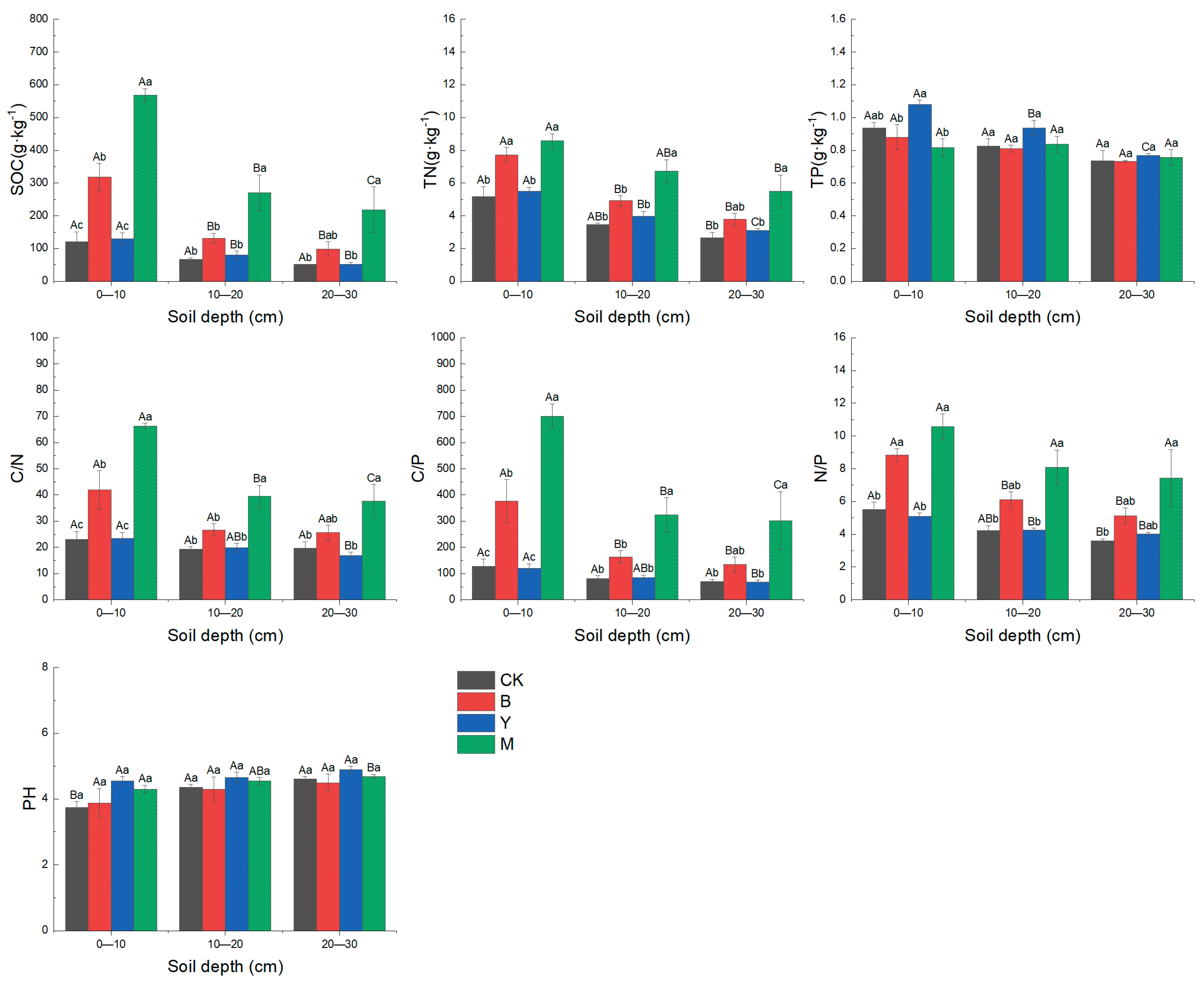
3.2. Effects of Downed Log Decomposition on Soil Physicochemical Properties and Enzyme Activities
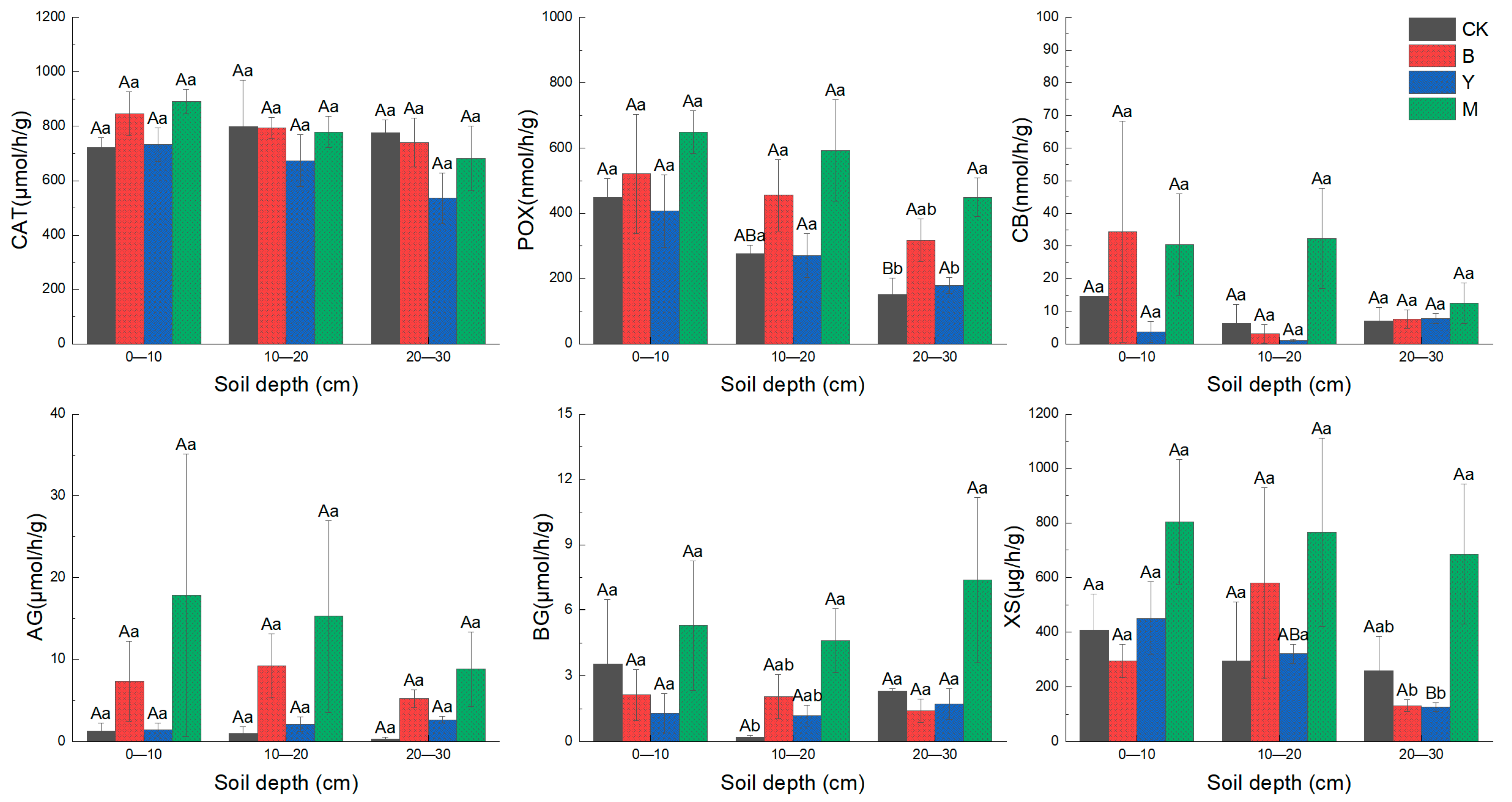
3.3. Effects of Downed Log Decomposition on Soil Enzyme Activity
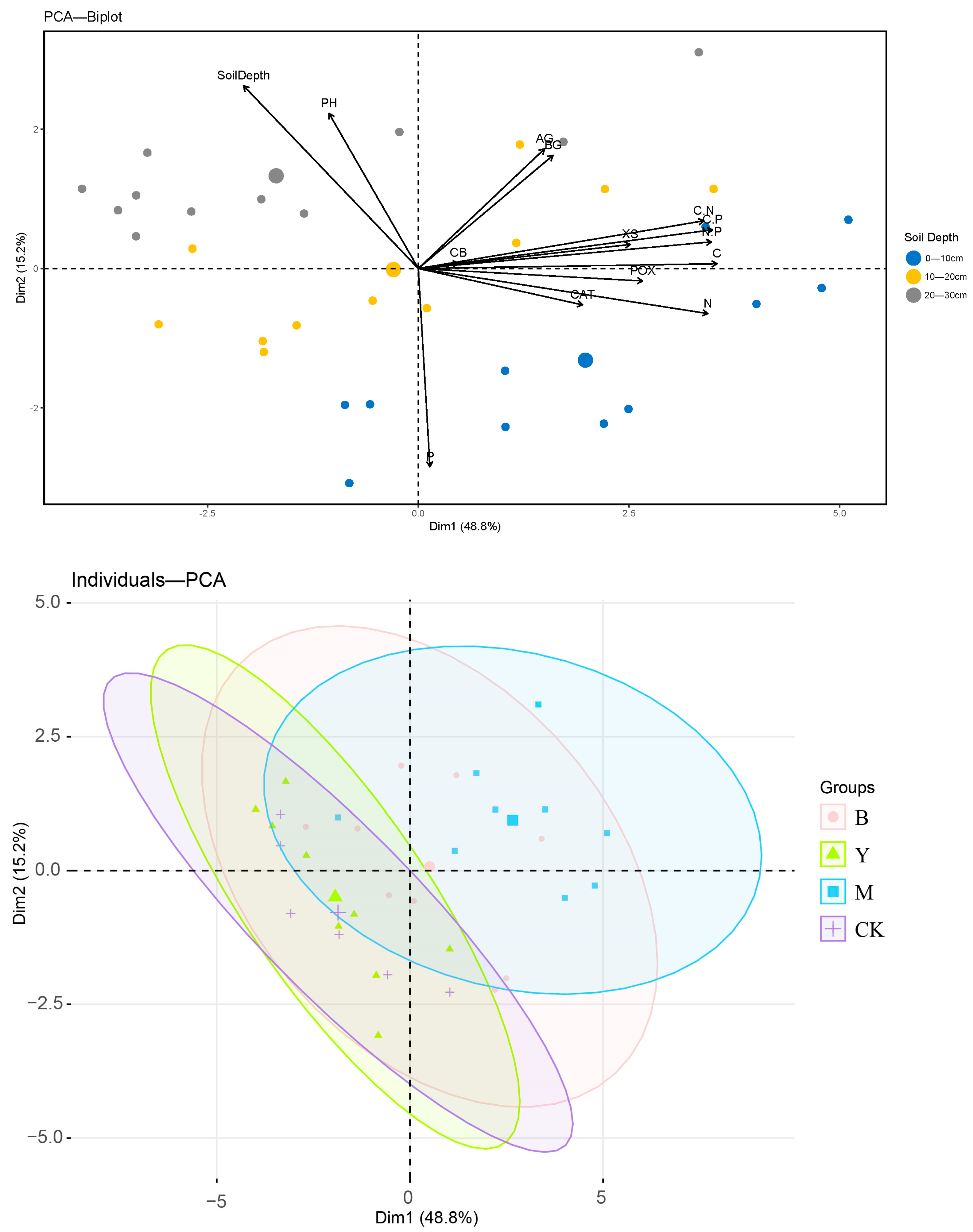
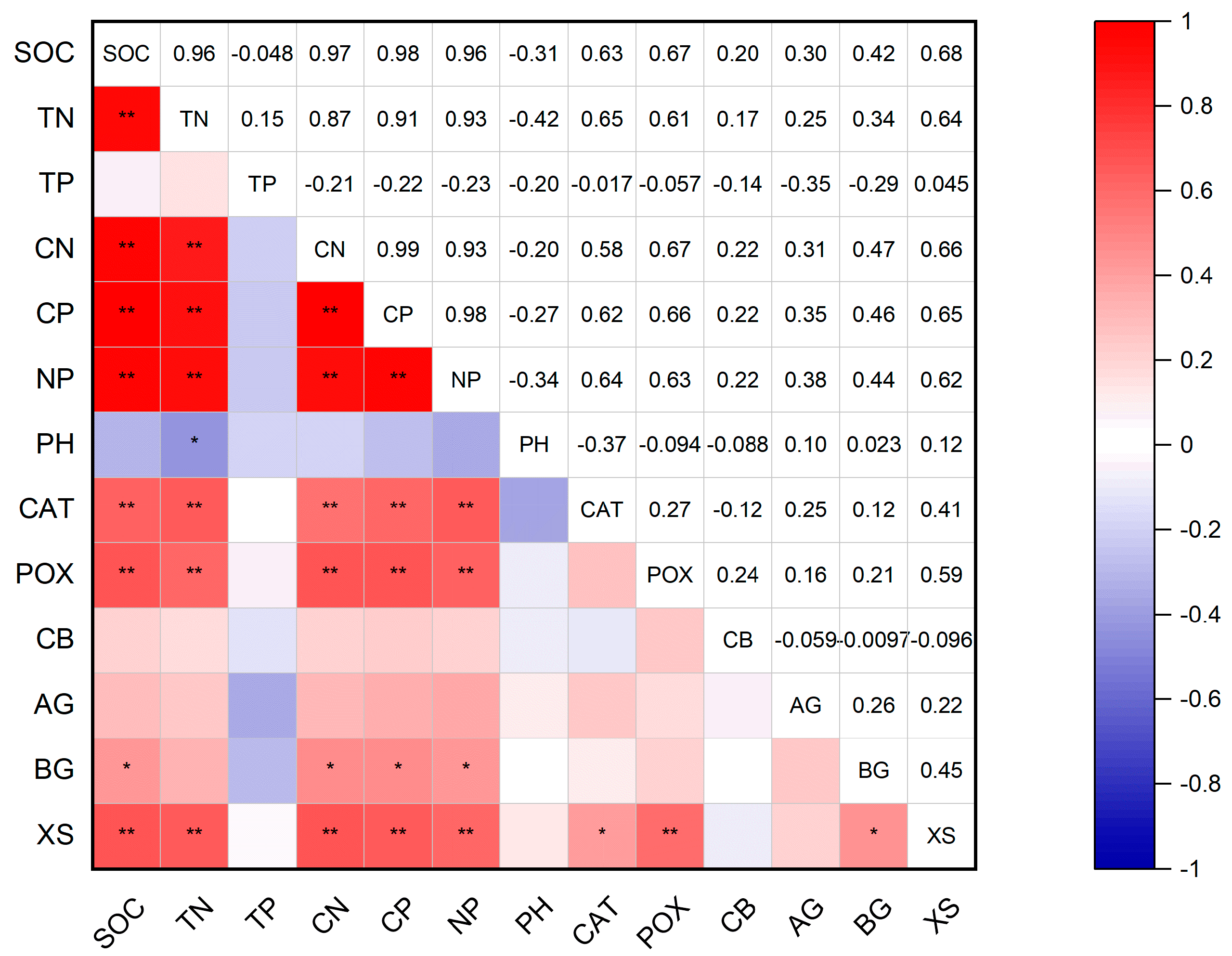
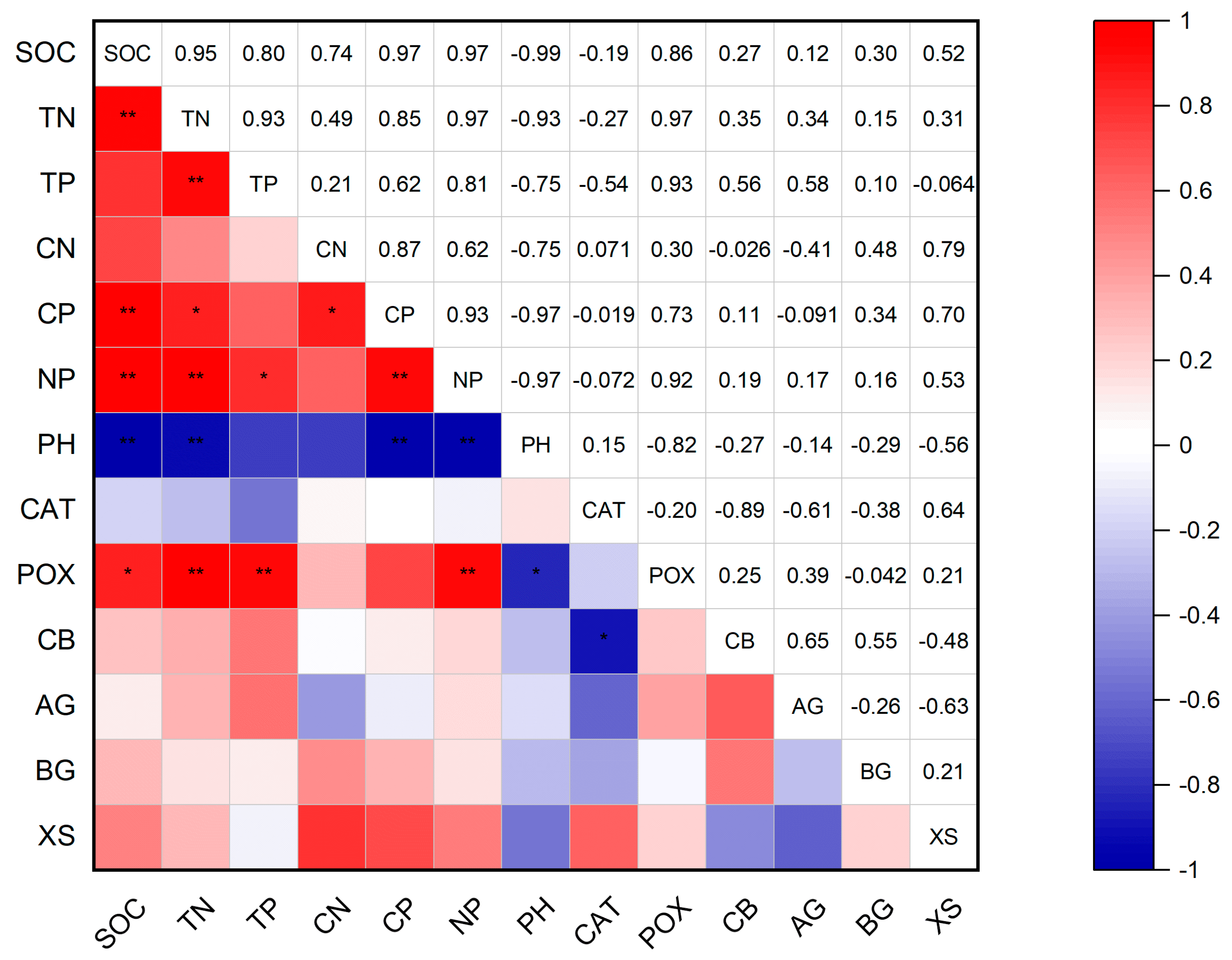
3.4. Correlation Between Soil Physicochemical Properties and Enzyme Activities Under Downed Logs and Control Plots
4. Discussion
4.1. Effects of Downed Logs on Soil Physicochemical Properties
4.2. Effects of Downed Logs on Soil Enzyme Activities
4.3. Discussion of the Correlation Analysis Between Downed Log Cover and CK
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, W.H. Biogeochemical Cycles. (Book Reviews: Biogeochemistry. An Analysis of Global Change.). Science 1991, 253, 686–687. [Google Scholar]
- Seibold, S.; Rammer, W.; Hothorn, T.; Seidl, R.; Ulyshen, M.D.; Lorz, J.; Cadotte, M.W.; Lindenmayer, D.B.; Adhikari, Y.P.; Aragon, R.; et al. The contribution of insects to global forest deadwood decomposition. Nature 2021, 597, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Błońska, E.; Ważny, R.; Górski, A.; Lasota, J. Decomposing benefits: Examining the impact of beech deadwood on soil properties and microbial diversity. Sci. Total Environ. 2024, 930, 172774. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart Grandy, A.; Liang, C.; Phillips, R.P. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef]
- Lyu, M.; Homyak, P.M.; Xie, J.; Peñuelas, J.; Ryan, M.G.; Xiong, X.; Sardans, J.; Lin, W.; Wang, M.; Chen, G.; et al. Litter quality controls tradeoffs in soil carbon decomposition and replenishment in a subtropical forest. J. Ecol. 2023, 111, 2181–2193. [Google Scholar] [CrossRef]
- Stutz, K.P.; Dann, D.; Wambsganss, J.; Scherer-Lorenzen, M.; Lang, F. Phenolic matter from deadwood can impact forest soil properties. Geoderma 2017, 288, 204–212. [Google Scholar] [CrossRef]
- Mackensen, J.; Bauhus, J. Density loss and respiration rates in coarse woody debris of Pinus radiata, Eucalyptus regnans and Eucalyptus maculata. Soil Biol. Biochem. 2003, 35, 177–186. [Google Scholar] [CrossRef]
- Moghimian, N.; Jalali, S.G.; Kooch, Y.; Rey, A. Downed logs improve soil properties in old-growth temperate forests of northern Iran. Pedosphere 2020, 30, 378–389. [Google Scholar] [CrossRef]
- Minnich, C.; Peršoh, D.; Poll, C.; Borken, W. Changes in Chemical and Microbial Soil Parameters Following 8 Years of Deadwood Decay: An Experiment with Logs of 13 Tree Species in 30 Forests. Ecosystems 2020, 24, 955–967. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Puletti, N.; Canullo, R.; Mattioli, W.; Gawryś, R.; Corona, P.; Czerepko, J. A dataset of forest volume deadwood estimates for Europe. Ann. For. Sci. 2019, 76, 68. [Google Scholar] [CrossRef]
- Campbell, J.L.; Green, M.B.; Yanai, R.D.; Woodall, C.W.; Fraver, S.; Harmon, M.E.; Hatfield, M.A.; Barnett, C.J.; See, C.R.; Domke, G.M. Estimating uncertainty in the volume and carbon storage of downed coarse woody debris. Ecol. Appl. 2019, 29, e01844. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.-w.; Li, W.-z. Spatial patterns and storage composition of woody debris in a natural secondary forest dominated by Pinus tabulaeformis on Loess Plateau, China. J. Mt. Sci. 2017, 14, 1839–1851. [Google Scholar] [CrossRef]
- Pearson, M.; Laiho, R.; Penttilä, T. Decay of Scots pine coarse woody debris in boreal peatland forests: Mass loss and nutrient dynamics. For. Ecol. Manag. 2017, 401, 304–318. [Google Scholar] [CrossRef]
- Qi, L.; Yuan, J.; Zhang, W.; Liu, H.; Li, Z.; Bol, R.; Zhang, S. Metagenomics reveals the underestimated role of bacteria in the decomposition of downed logs in forest ecosystems. Soil Biol. Biochem. 2023, 187, 109185. [Google Scholar] [CrossRef]
- Stutz, K.P.; Kaiser, K.; Wambsganss, J.; Santos, F.; Berhe, A.A.; Lang, F. Lignin from white-rotted European beech deadwood and soil functions. Biogeochemistry 2019, 145, 81–105. [Google Scholar] [CrossRef]
- Harmon, M.E. The role of woody detritus in biogeochemical cycles: Past, present, and future. Biogeochemistry 2021, 154, 349–369. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Maynard, D.S.; Bradford, M.A.; Lindner, D.L.; Oberle, B.; Zanne, A.E.; Crowther, T.W. A trait-based understanding of wood decomposition by fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 11551–11558. [Google Scholar] [CrossRef]
- Dossa, G.G.O.; Paudel, E.; Schaefer, D.; Zhang, J.-L.; Cao, K.-F.; Xu, J.-C.; Harrison, R.D. Quantifying the factors affecting wood decomposition across a tropical forest disturbance gradient. For. Ecol. Manag. 2020, 468, 118166, Corrected in For. Ecol. Manag. 2021, 491, 119170. [Google Scholar] [CrossRef]
- Mieszkin, S.; Richet, P.; Bach, C.; Lambrot, C.; Augusto, L.; Buée, M.; Uroz, S. Oak decaying wood harbors taxonomically and functionally different bacterial communities in sapwood and heartwood. Soil Biol. Biochem. 2021, 155, 108160. [Google Scholar] [CrossRef]
- Khan, K. Nutrient Dynamics Assessment of Coarse Wood Debris and Its Effect on Soil Properties Subjected to Successional Decay Levels of Three Forests Types in Northeast, China. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Yang, L.P.; Liu, W.Y.; Yang, G.P.; Ma, W.Z.; Li, D.W. Composition and carbon storage of woody debris in the moist evergreen broadleaf forest and secondary forests in Ailao Mountains. Chin. J. Appl. Ecol. 2007, 18, 2153–2159. [Google Scholar]
- Sollins, P. Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington. Can. J. For. Res. Rev. Can. Rech. For. 1982, 12, 757–763. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Qi, K.; Yang, B.; Bao, W.; Pang, X. Distinct effects of N and P addition on soil enzyme activities and C distribution in aggregates in a subalpine spruce plantation. Biogeochemistry 2018, 141, 199–212. [Google Scholar] [CrossRef]
- Wall, A. Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. For. Ecol. Manag. 2008, 256, 1372–1383. [Google Scholar] [CrossRef]
- Seongjun, K.; Guanlin, L.; Hyun, H.S.; Hanna, C.; Hyun-Jun, K.; Yowhan, S.J.F. Differential Effects of Coarse Woody Debris on Microbial and Soil Properties in Pinus densiflora Sieb. et Zucc. Forests. Forests 2017, 8, 292. [Google Scholar] [CrossRef]
- Lu, L.; Hu, M.; Wang, J.; Xu, X.; Gui, H.; Yan, X.; Miao, Y.; Wang, W.; Han, S. Impact of Downed Logs of Masson Pine (Pinus massoniana Lamb.) on Soil Microbial Community in a Climate Transitional Forest of Central China. Forests 2023, 14, 955. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Sha, L.; Song, Q.; Tan, Z.; Zhou, W.; Wu, C. Variation and influencing factors of soil water content in subtropical evergreen broadleaf forests in Ailao Mountains. Chin. J. Ecol. 2013, 32, 332–336. [Google Scholar] [CrossRef]
- Kong, L.L.X.; Li, Y. Effects of different tree species on the spatial heterogeneity of soluble organic carbon in forest soils. J. Agric. 2023, 13, 46–55. [Google Scholar]
- Piaszczyk, W.; Błońska, E.; Lasota, J.; Lukac, M. A comparison of C:N:P stoichiometry in soil and deadwood at an advanced decomposition stage. Catena 2019, 179, 1–5. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Fernandez-Souto, A.; Austin, A. Coarse Woody Debris Stimulates Soil Enzymatic Activity and Litter Decomposition in an Old-Growth Temperate Forest of Patagonia, Argentina. Ecosystems 2013, 16, 1025–1038. [Google Scholar] [CrossRef]
- Meyer, N.; Xu, Y.; Karjalainen, K.; Adamczyk, S.; Biasi, C.; van Delden, L.; Martin, A.; Mganga, K.; Myller, K.; Sietiö, O.M.; et al. Living, dead, and absent trees—How do moth outbreaks shape small-scale patterns of soil organic matter stocks and dynamics at the Subarctic mountain birch treeline? Glob. Chang. Biol. 2021, 28, 441–462. [Google Scholar] [CrossRef]
- Kahl, T.; Mund, M.; Bauhus, J.; Schulze, E.D. Dissolved organic carbon from European beech logs: Patterns of input to and retention by surface soil. Coence 2012, 19, 364–373. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer Science + Business Media, LLC: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lombardi, F.; Cherubini, P.; Tognetti, R.; Cocozza, C.; Lasserre, B. Investigating biochemical processes to assess deadwood decay of beech and silver fir in Mediterranean mountain forests. Ann. For. Sci. 2013, 70, 101–111. [Google Scholar] [CrossRef]
- Kang, H.; Gao, H.; Yu, W.; Yi, Y.; Wang, Y.; Ning, M. Changes in soil microbial community structure and function after afforestation depend on species and age: Case study in a subtropical alluvial island. Sci. Total Environ. 2018, 625, 1423–1432. [Google Scholar] [CrossRef]
- Banerjee, S.; Bora, S.; Thrall, P.H.; Richardson, A.E. Soil C and N as causal factors of spatial variation in extracellular enzyme activity across grassland-woodland ecotones. Appl. Soil Ecol. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Junninen, K.; Komonen, A. Conservation ecology of boreal polypores: A review. Biol. Conserv. 2011, 144, 11–20, Correction in Biol. Conserv. 2011, 144, 1779. [Google Scholar] [CrossRef]
- Bernal, B.; McKinley, D.C.; Megonigal, P.J.; Hungate, B.A.; White, P.M. Limits to soil carbon stability; Deep, ancient soil carbon decomposition stimulated by new labile organic inputs. Soil Biol. Biochem. 2016, 98, 85–94. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; Kandeler, E.; de Arano, I.M.; Arias-González, E. Soil microbial functional activity is governed by a combination of tree species composition and soil properties in temperate forests. Appl. Soil Ecol. 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Piotrowska-Dlugosz, A.; Dlugosz, J.; Frac, M.; Gryta, A.; Breza-Boruta, B. Enzymatic activity and functional diversity of soil microorganisms along the soil profile—A matter of soil depth and soil-forming processes. Geoderma Int. J. Soil Sci. 2022, 416, 115779. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Lasota, J.; Bońska, E. Effect of Organic Matter Released from Deadwood at Different Decomposition Stages on Physical Properties of Forest Soil. Forests 2020, 11, 24. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 2010, 53, 87–98. [Google Scholar] [CrossRef]

| Parameters | Tree Species | Soil Depth | Tree Species × Soil Depth | |||
|---|---|---|---|---|---|---|
| F-Value | Partial Eta Squared | F-Value | Partial Eta Squared | F-Value | Partial Eta Squared | |
| SOC | 43.608 ** | 0.829 | 32.228 ** | 0.782 | 4.588 ** | 0.505 |
| TN | 22.213 ** | 0.712 | 30.263 ** | 0.771 | 0.691 | 0.133 |
| TP | 8.277 ** | 0.479 | 12.348 ** | 0.578 | 2.302 | 0.338 |
| C/N | 38.467 ** | 0.81 | 17.615 ** | 0.662 | 2.851 | 0.388 |
| C/P | 30.93 ** | 0.775 | 15.961 ** | 0.639 | 3.224 * | 0.417 |
| N/P | 22.429 ** | 0.714 | 9.469 ** | 0.513 | 0.847 | 0.158 |
| pH | 3.19 | 0.262 | 2.773 | 0.236 | 0.161 | 0.035 |
| CAT | 3.18 | 0.261 | 3.499 | 0.28 | 0.156 | 0.034 |
| POX | 5.254 * | 0.369 | 3.033 | 0.252 | 0.045 | 0.01 |
| CB | 1.751 | 0.163 | 0.812 | 0.083 | 0.704 | 0.135 |
| AG | 1.933 | 0.177 | 0.198 | 0.022 | 0.136 | 0.029 |
| BG | 5.263 * | 0.369 | 0.189 | 0.021 | 0.262 | 0.055 |
| XS | 4.459 * | 0.331 | 1.177 | 0.116 | 0.372 | 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Xu, C.; Lu, Z.; Wang, H.; Guo, H. Effects of Downed Log Decomposition on Soil Properties and Enzyme Activities in Southwest China. Forests 2024, 15, 2182. https://doi.org/10.3390/f15122182
Liu T, Xu C, Lu Z, Wang H, Guo H. Effects of Downed Log Decomposition on Soil Properties and Enzyme Activities in Southwest China. Forests. 2024; 15(12):2182. https://doi.org/10.3390/f15122182
Chicago/Turabian StyleLiu, Tingting, Chenzhan Xu, Zhiyun Lu, Hang Wang, and Huijun Guo. 2024. "Effects of Downed Log Decomposition on Soil Properties and Enzyme Activities in Southwest China" Forests 15, no. 12: 2182. https://doi.org/10.3390/f15122182
APA StyleLiu, T., Xu, C., Lu, Z., Wang, H., & Guo, H. (2024). Effects of Downed Log Decomposition on Soil Properties and Enzyme Activities in Southwest China. Forests, 15(12), 2182. https://doi.org/10.3390/f15122182






