Abstract
Dehydration is the principal conservation process for waterlogged archaeological wood (WAW), with the aim of preventing shrinkage and cracking. For well-preserved WAW, shrinkage mainly takes place when the moisture content is below the fiber saturation point. Here, we conduct a new trial using ionic liquid as a dimensional stabilizer to maintain a stable swollen state of WAW. Molecular dynamics simulation (MD), shrinkage measurement, Fourier transform infrared spectroscopy (FTIR), and dynamic vapor sorption (DVS) were adopted to investigate the interactions and effects of 1-Butyl-3-methylimidazolium chloride ([Bmim][Cl]) on WAW (Dipterocarpaceae Dipterocarpus sp. with a maximum moisture content of 80.3%) in comparison with the conventional material polyethylene glycol (PEG). The results show that [Bmim][Cl] and its water mixtures have a comparable or slightly greater ability to swell amorphous cellulose than does water at room temperature, while crystalline cellulose is left intact. The samples treated with [Bmim][Cl] show less shrinkage than the PEG 300- and PEG 2000-treated samples at all tested concentrations after air-drying. The best dimension control was achieved by 40 wt% [Bmim][Cl], with volumetric shrinkage reduced from 5.03% to 0.47%. DVS analysis reveals that [Bmim][Cl] reduces moisture contents at moderate and low relative humidity (<80%) when the concentration is at or below 20 wt%, which suggests that good dimensional stability was not achieved by simply preserving the moisture content but possibly through the interaction of the ionic liquid with the wood polymers.
1. Introduction
Wood is undoubtedly one of the most important materials in human history. Wooden crafts and constructions that have fortunately been preserved until the present are our valuable assets, witnessing our ancestors’ culture, history, and technology. Waterlogged archaeological wood (WAW) is one of those wooden remains that requires careful conservation actions to prevent shrinkage, cracking, and deformation during the dehydration process. Due to submerged burial environments, they have been subjected to limited microbial attacks and oxidation. Even so, deterioration of WAW varies from one sample to another, depending on the specific wood species, burial time, microbial community, and oxygen level. Under a bad scenario, bacterial decay causes significant loss of holocellulose and the formation of countless microcavities and tunnels within secondary walls [1,2]. As a result, shrinkage of WAW during dehydration could be tremendous if no measures were taken.
Consolidation is believed to be the principal procedure in the conservation process, normally achieved through impregnating WAW with materials that restore its structural integrity. Consolidants should be of low molecular weight during the impregnation process due to the limited penetrability of the wood cell wall and should later precipitate from the solution or solidify to provide mechanical supports for resisting drying stress. Water-soluble materials are much more preferred in practice, as they do not require additional solvent exchange processes. Polyethylene glycol (PEG) and sugar are the most common materials used in conservation practices owing to their high solubility in water [3]. They crystallize or precipitate in the pores of WAW as dehydration proceeds, which provides physical supports for the fragile cell walls. The PEG or sugar treatment is sometimes followed by freeze-drying to prevent the shrinkage induced by capillary forces, thus achieving better effects than using consolidants alone [4,5]. Alternatives like trehalose, mannitol, and xylitol have also been adopted as a new generation of consolidants because of their lower hygroscopicity and similar consolidation effects [6,7,8,9]. Hydrolyzed feather keratin also shows prominent anti-shrinkage efficiency for highly deteriorated WAW [10,11]. Apart from these water-soluble filling materials, in situ polymerization shows an even better dimensional control ability. Surface-initiated activator regenerated by electron transfer atom transfer radical polymerization (SI-ARGET ATRP) can effectively introduce polymethacrylate into cell walls and provide excellent and controllable anti-shrinkage efficiency through the densification of cell walls and the generation of expansion stress, which counteracts the drying stress during dehydration [12,13,14]. Organosiloxane is also a type of material that greatly increase dimensional stability through the condensation process and chemical linkage to both cellulose and lignin with selected functional groups [15,16,17]. A reversibly cross-linked polymer based on 2-formylphenylboric acid-terminated low-molecular-weight polydimethylsiloxane (PDMS-PBA) has been developed recently for the consolidation of WAW, and it yields an anti-shrinkage efficiency as high as 95.7%, partially owing to the cross-linking of PBA moieties [18]. Despite the availability of various consolidation materials and methods, consolidation is sometimes unnecessary for well-preserved WAW.
Well-preserved WAW, like fresh wood, usually shows shrinkage when the moisture content is below the fiber saturation point. It possesses adequate mechanical strength to prevent the collapse of cell structure caused by capillary force, and therefore, conventional consolidants do not take effect as usual. However, shrinkage also happens as a result of the decreased swelling of hemicellulose, amorphous cellulose, and lignin, thus causing warping and cracks when the moisture content drops below the fiber saturation point. The modification of WAW is not as simple as that for modern wood because of its waterlogged state and the lack of specialized equipment at archaeological sites. Therefore, water-soluble materials are preferred in practice. For example, phenylboronic acid (PBA) treatment was found to effectively reduce shrinkage of highly degraded WAW, together with freezing drying, through the inhibition of the intermolecular hydrogen bonds of cellulose [19]. However, PBA were not effective for well-preserved WAW, possibly due to the low accessibility of hydrogen bonds. For well-preserved WAW, the simplest way to prevent shrinkage is to maintain the swelling state. Apart from controlling the humidity of the surroundings, low-molecular-weight PEG is widely used to replace the water in WAW [20]. It is assumed that any pores of at least a few nanometers would be filled by PEG [21], preventing these pores from collapsing after dehydration. Meanwhile, experiments using small-angle neutron scattering show that PEG influences the packing of microfibrils and reduces both the microfibril bundle width and interfibrillar distance [21]. This indicates that microfibril bundles in PEG-impregnated WAW are less swollen than those in the original WAW, and PEG may not be a perfect agent to control these dimensions. Moreover, WAW impregnated with PEG is also susceptible to microbial degradation and generates organic acids like formic acid, although this did not occur to a large extent in the samples analyzed [22,23,24]. Therefore, we would like to explore other compounds for maintaining moisture or a stable swollen state of WAW, which should be more stable, not volatile, and capable of swelling WAW in the same manner as water molecules. A compound meeting these requirements would act as a permanent swelling agent that is unlikely to evaporate like water and is not greatly influenced by relative humidity. Ionic liquids (IL) are salt compounds normally melting below 100 °C and composed of organic cations and various anions. They have been mainly used as solvents and plasticizers due to their ability to effectively break the polymer hydrogen bond network [25]. Ionic liquids like [Emim][OAc] and [Bmim][Cl] can be used as cellulose solvents at high temperatures, which is probably enough to slightly swell WAW (as does water) at room temperature. Meanwhile, ionic liquids are miscible with water and hygroscopic to potentially maintain the moisture content for WAW. Research also suggests that water suppresses the solubility of cellulose in ionic liquids [26,27,28]. It is therefore inferred that a proper dose of ionic liquids might partially fill the role of water and help maintain the stable swollen state of WAW to prevent cracks when relative humidity decreases. As more thermally stable compounds than PEG with various combination of cations and anions, ionic liquids might display better application prospects. Concerns regarding the long-term effects of ILs on cellulose should be addressed only when ILs is confirmed to be effective to improve the dimensional stability of WAW. This paper aims to investigate the possibility and mechanisms of ionic liquid as a dimensional stabilizer for well-preserved waterlogged archaeological wood by means of molecular dynamics, shrinkage measurement, and dynamic vapor sorption analysis.
2. Materials and Methods
2.1. Materials
The WAW sample was a piece of wood plank (Dipterocarpaceae Dipterocarpus sp.; microsections are shown in Figure 1) collected from the Xiaobaijiao No. 1 shipwreck [29] of the Qing Dynasty in Ningbo City, Zhejiang Province, China. The maximum moisture content (defined as the mass of water divided by the mass of dry wood) is 80.3% ± 9.7%, and the basic density (defined as the mass of dry wood divided by the waterlogged volume) is 578 ± 32 kg/m3. The basic density of contemporary wood of Dipterocarpus tonkinensis from Yunnan Province, China, is 535 kg/m3 [30], which suggests the WAW is quite well preserved. The slightly higher basic density is probably due to different species or growing condition. 1-Butyl-3-methylimidazolium chloride ([Bmim][Cl]) was purchased from Shanghai D&B Biotechnology Co., Ltd. (Shanghai, China), and polyethylene glycol (PEG) 300 and 2000 were purchased from Beijing Tongguang Fine Chemcials Co., Ltd. (Beijing, China). The reagents were all of analytical grade and were used directly.
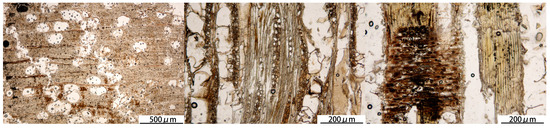
Figure 1.
Anatomical structure of the WAW sample.
2.2. Molecular Dynamics (MD) Simulation
Cellulose in wood is comprised of both crystalline cellulose (CC) and amorphous cellulose (AC). In this study, it is essential to evaluate the interactions between IL [Bmim][Cl] and cellulose of both forms in the presence of water. The CC configuration was generated according to the methods of Ref. [31]. CC consists of 36 chains in Iβ form, and each chain is composed of 5 cellobiose units. A total of 25 AC chains, with a polymerization degree of 5 cellobiose units, are introduced in each simulation system. Due to very limited interactions between CC with water or IL, the amount of water and IL are introduced as 0, 12.5, or 25% with reference to the weight of the AC.
The MD simulation was performed in Gromacs 2022.1 software [32] with an Amber14sb force field under constant temperature, constant pressure, and periodic boundary conditions. The model molecules were placed into a box of 8.00 nm × 8.00 nm × 5.19 nm. A GLYCAM all-atom force field was adopted for CC and AC. The GAFF all-atom force field was adopted for [Bmim][Cl]. A TIP3P water configuration was adopted for the water molecules [33]. The steepest descent method is used to minimize the energy of the initial structure for 50,000 steps. The NPT (constant particles, constant pressure, and constant temperature) ensemble was used in the simulation, and the leap-frog method was used to integrate the equations. The long-range electrostatic interactions use the PME (particle-mesh Ewald) method [34]. The truncation distance of the van der Waals interaction and the Coulomb interaction is 12 Å, updated every 10 steps, and the Lincs algorithm [35] is used to constrain all bond lengths, with parameters of “lincs_iter = 1 and lincs_order = 4”.The V-rescale temperature coupler [36] was used to heat the system temperature from 0 K to 298 K. The Parrinello–Rahman method [37] was adopted to control the pressure constant of the system at 1 bar and to maintain the pressure isotropy. The non-bond interaction is calculated using the neighborhood grid cutting scheme, and the short and long cutoff distances are 9 Å and 14 Å, respectively. The standard for hydrogen bond formation is that the donor–acceptor angle is less than 30°, and the distance between the donor–acceptor atoms is less than 0.35 nm. All simulations were randomly assigned an initial speed using the Maxwell–Boltzmann distribution, with a total simulation of 50 million steps, a time step size of 2 fs, a total simulation time of 100 ns, and a total of 10,000 conformations. The visualization of the simulation results was completed using the Gromacs embedded program and VMD 1.9.4 (Visual Molecular Dynamics) software.
2.3. Impregnation of Ionic Liquid and Polyethylene Glycol
WAW blocks were prepared in the size of approximate 10 mm (longitudinal) × 5 mm (radial) × 5 mm (tangential). They were impregnated with 5 wt%, 20 wt%, or 40 wt% [Bmim][Cl] or PEG 300, or 40 wt% PEG 2000 in a water solution for 7 days. Three replicates were used in each set, and three untreated blocks were used as the control. Thereafter, they were air-dried at 25 ± 3 °C and 65 ± 5%RH.
2.4. Shrinkage Measurement
The effectiveness of the treatment was evaluated by calculating the volumetric shrinkage and shrinkage in the radial and tangential directions according to the following equation:
where V0—the volume of a sample in a waterlogged state; Vt—the volume of a sample after air-drying. Shrinkage in the radial or tangential direction was calculated in the same way, and mean values of three block samples under each condition were calculated.
2.5. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
In order to determine whether IL evidently influences the wood components, the samples were directly analyzed by ATR-FTIR (Thermo Fisher Nicolet IS5, America) from 4000 to 400 cm−1 with a spectral resolution of 4 cm−1 and 16 scans.
2.6. Dynamic Vapor Sorption (DVS)
The equilibrium moisture content under different relative humidity (RH) conditions was measured using a high-throughput dynamic moisture-adsorption tester (SPSx-1μ, ProUmid, Ulm, Germany). Approximate 20–30 mg wood slices were prepared for each test by hand-cutting them from the treated samples. Prior to the test, the samples were air-dried at 103 °C for more than 24 h until their weights no longer decreased. The humidity ranged from 0 to 95%RH, at a gradient of 10%RH and a stable temperature of 25 °C. The default measurement frequency was every 10 min. The time required for every gradient fell between a minimum of 60 min and a maximum of 600 min. The default weight limit had an upper cap of 1000%. The equilibrium condition was defined as dm/dt ≤ 0.01.
3. Results and Discussion
3.1. Theoretical Investigation of the Swelling of Cellulose by IL–Water Mixtures
During the dehydration of WAW, the loss of absorbed water below the fiber saturation point usually causes the shrinkage of wood polymers, including cellulose. The key concept of this paper is to partially replace water with ionic liquid (IL) to maintain the stable swollen state of WAW. Amorphous cellulose (AC) is a principal swelling component, and swelling of crystalline cellulose (CC) should be avoided in WAW. The systems, each consisting of 25 AC chains, one CC crystal, and certain amount of water and/or IL (with reference to the weight of AC), were simulated at 298 K, and the end views are shown in Figure 2. The reference system in Figure 2a, consisting of 25 wt% water, shows excess water molecules at the corners that do not directly interact with AC or CC, which suggests a maximum swollen state of AC by water. In the remaining systems, all the [Bmim][Cl] and water molecules fully interact with cellulose through hydrogen bonds and other intermolecular forces. The crystalline structure of CC in all the systems is still well arranged and is not disturbed by [Bmim][Cl]. This can be quantitatively illustrated by the radius of gyration (Rg) and the number of hydrogen bonds within the CC. As shown in Figure 3a, the radius of gyration of CC only increases by 0.003–0.004 nm after [Bmim][Cl] is introduced into the systems, which suggests that IL or the IL–water mixtures does not further swell CC and change its volume or conformation at room temperature. The number of hydrogen bond within the CC is higher in the system containing 25 wt% [Bmim][Cl] in comparison with that in the system containing 25 wt% water (as shown in Figure 3c), while it decrease very slightly when both [Bmim][Cl] and water are present. The above observation suggests that IL [Bmim][Cl], as well as its water mixture, basically do not swell CC at room temperature, and thus it is safe in terms of preventing the unwanted swelling of CC in WAW. Nonetheless, AC seems to be more swelled by IL than by water. The Rg of AC with 25 wt% IL is larger than that with 25 wt% water, and an even higher Rg of AC can be achieved by IL–water mixtures (Figure 3b). The intermolecular hydrogen bonds between the AC chains are almost the same in the systems consisting of 25 wt% water or 25 wt% IL (shown in Figure 3d), revealing a comparable capability to break the AC’s intermolecular hydrogen bonds. As IL is hygroscopic, the IL–water mixture is more capable of breaking these hydrogen bonds when compared with IL or water alone, making it a stronger swelling agent for WAW. These simulation results suggest that, theoretically, IL has the ability to maintain the swollen state of AC, regardless of the amount of water or relative humidity in the surroundings.
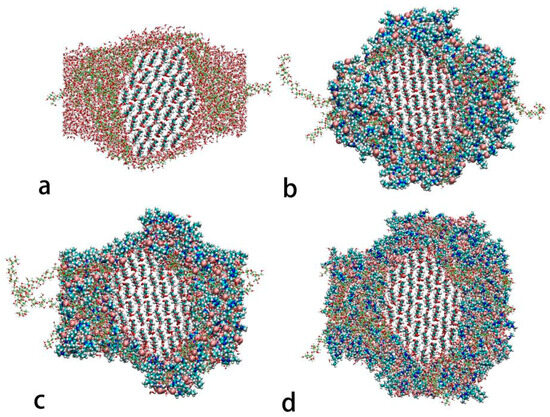
Figure 2.
End view of MD systems of CC and AC with (a) 25 wt% water; (b) 25 wt% IL; (c) 25 wt% IL and 12.5 wt% water; (d) 25 wt% IL and 25 wt% water. Well-arranged CC is in the center of each system, surrounded by AC with green carbon atoms; water molecules with small red atoms; and IL molecules with dark blue N atoms, light blue C atoms, and large red Cl atoms.
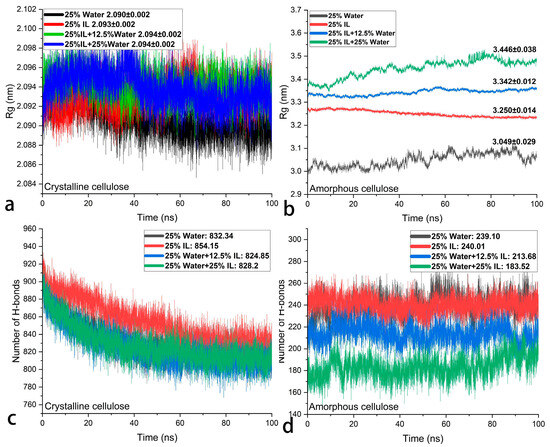
Figure 3.
Radius of gyration of CC (a), AC (b), and the number of hydrogen bonds within CC (c), and AC (d).
Moreover, water and [Bmim][Cl] are evenly matched in terms of their ability to form hydrogen bonds with AC. Shown in Figure 4a, the number of hydrogen bonds between AC and water drops from 1037.42 in the system containing 25 wt% water to 562.33 in the system containing an additional 25 wt% IL. Meanwhile, the number of hydrogen bonds between AC and IL drops from 54.09 in the system containing 25 wt% IL to 30.72 in the system containing an additional 25 wt% water (in Figure 4b). Last but not least, the interaction energy (IE) of AC with IL is slightly affected by the addition of water (shown in Figure 4d), while the IE of AC with water can be increased much more by the addition of IL (shown in Figure 4c). This suggests that [Bmim][Cl] has a greater affinity for AC than for water due to other intermolecular forces such as induction force, laying the foundation for keeping AC swollen. Although here, the conventional agent PEG was not compared under the same conditions, the previously reported MD calculation and experiments have already shown its strong interaction with cellulose [31,38]. Moisture-induced swelling in the transverse direction slightly increased by increasing the amount of PEG 200 up to 25% [31], which also theoretically suggests that PEG is helpful in achieving dimensional stability by increasing the swollen state. A quantitative comparison may not be very significant because the swelling of cellulose or WAW can be additionally adjusted by modifying the concentration, types of ionic liquids, or molecular weight of PEG, and extensive swelling is unwanted in the conservation of WAW. But if we look to other benefits from ILs, they provide better thermal stability, anti-microbial attributes [39,40], and fire-retardant properties [41,42], making it a better candidate for swelling WAW.
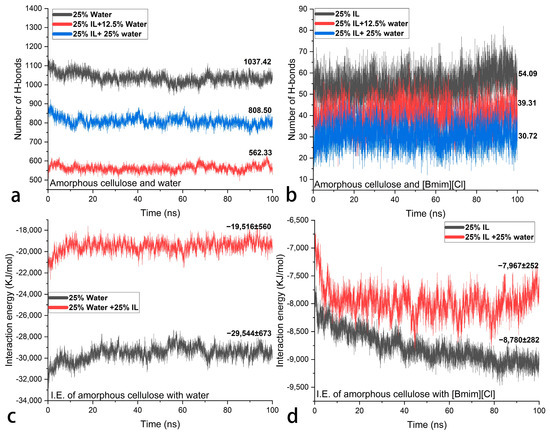
Figure 4.
Number of hydrogen bonds between AC and water (a) and between AC and [Bmim][Cl] (b); the interaction energy of AC with water (c) and of AC with [Bmim][Cl] (d).
3.2. Dimensional Stability of Well-Preserved WAW Treated with Stabilizing Agents
After confirming the ability of IL to swell AC, trials are made to evaluate the practical results in controlling the dimensional stability in comparison with the results for the conventional stabilizing agents PEG 300 and PEG 2000, which are shown in Figure 5. The volumetric shrinkage of the untreated air-dried WAW sample is 5.03%, which shows that it is generally well-preserved. However, 3.66% tangential shrinkage still leads to cracking in the wood planks preserved at the China Port Museum after air-drying. After treating the samples with stabilizing agents and air-drying them at 65 ± 5%RH, 25 °C, both IL [Bmim][Cl] and PEG yield positive and comparable results. The lowest volumetric shrinkage of 0.47% was achieved by 40 wt% IL, closely followed by 0.69% with 40 wt% PEG 300. PEG 2000, of higher molecular weight, is not as effective as PEG 300, probably due to its larger molecular size. As well-preserved WAW does not need PEG to provide mechanical reinforcement, a smaller molecular size would be advantageous for entering pores and gaps of a wider size range, which may be superior for keeping microfibrils or their bundles from getting close to each other. When used in lower concentrations, such as 5 wt% or 20 wt%, PEG 300 is evidently less effective than IL. For instance, 5 wt% PEG 300 treatment reduced volumetric shrinkage to 4.91%, while 5 wt% IL effectively reduce volumetric shrinkage to 3.18%. These observations validate the simulation results showing that IL can swell amorphous cellulose and possibly hemicellulose and lignin like water and the possibility of using IL to maintain dimensional stability.
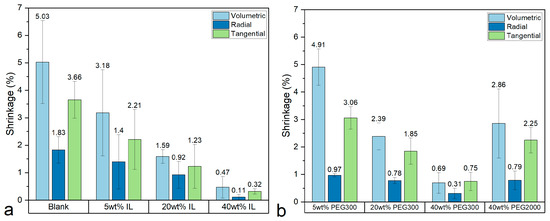
Figure 5.
Shrinkage of WAW treated with IL (a) and PEG (b).
3.3. FTIR Analysis
It is also important to know whether IL affects the wood components. As shown in Figure 6, the absorption bands at 897, 1031, 1508, and 1736 cm−1 are very representative bands of C–H bending in cellulose, C–O stretching in both hemicellulose and cellulose, aromatic ring stretching in lignin, and acetyl groups in hemicellulose, respectively [43,44]. Although the band at 897 cm−1 for cellulose was unfortunately overlapped by another band from IL, the presence of cellulose can also be suggested by the band at 1031 cm−1, and presumably, it is much stable than is hemicellulose. The remainder of the bands are not overlapped by the absorption bands of IL, and their relative intensities are basically unchanged, which suggests that the main components of the wood were not dissolved nor degraded during the treatment and drying process.
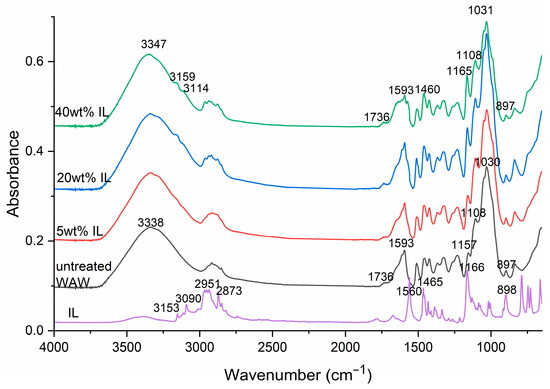
Figure 6.
FTIR spectra of untreated WAW and WAW treated with IL.
3.4. Hygroscopicity of WAW Treated by IL
The isotherms of the equilibrium moisture contents (EMCs) of the blank sample and the samples treated with IL or PEG are shown in Figure 7. We would like to focus on the MCs at a high relative humidity (95%) and those at 60%RH where the wooden artefact or remains were normally preserved. Due to the hygroscopic nature of IL and PEG, samples treated with them (Figure 7b–h) all show elevated EMCs at 95%RH in comparison with the results for the untreated sample (Figure 7a). Overall, the samples treated with 40 wt% IL (76.7%) are more hygroscopic than those treated with PEG, but details for the EMCs are complicated. The MC (18.0%) results at 95%RH of the sample treated with 5 wt% is lower than that (22.0%) for the sample treated with 5 wt% PEG 300. However, the EMCs of the samples treated with IL surge with the increase in treatment concentration. It is speculated that IL [Bmim][Cl] is more affinitive to wood polymers than is PEG, but excess [Bmim][Cl] molecules, which do not directly interact with wood polymers, are more prone to absorb moisture from the air. At 60%RH, all the samples treated with IL and PEG showed a lower moisture content compared with that of the untreated sample, except for that treated with 40 wt% IL, which suggests that both IL and PEG have good affinity with wood components and partially inhibit water sorption at moderate and low relative humidity. It is also inferred that the good dimensional stability of the air-dried samples was not achieved by them simply retaining moisture but possibly through the direct interaction of the stabilizing agents with the wood polymers. Moreover, the hysteresis of the samples treated with stabilizing agents are all reduced, indicating that fewer irreversible changes occur during the wetting–drying cycle.
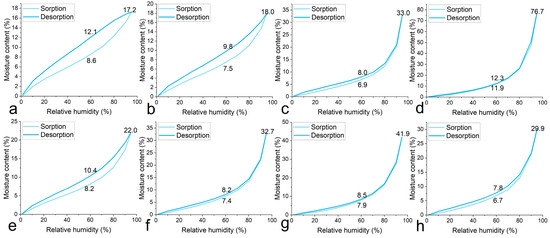
Figure 7.
Isotherms of equilibrium moisture contents of untreated WAW sample (a), WAW samples treated by 5 wt% IL (b), 20 wt% IL (c), 40 wt% IL (d), 5 wt% PEG 300 (e), 20 wt% PEG 300 (f), 40 wt% PEG 300 (g), and 40 wt% PEG 2000 (h).
Although 40 wt% IL treatment provides very satisfying dimension control, hygroscopicity should be taken into consideration if the relative humidity cannot be maintained below 80%, at which point the MC starts to rise rapidly.
4. Conclusions
For well-preserved waterlogged archaeological wood (WAW), consolidation is sometimes not necessary nor effective in reducing shrinkage and cracking. An alternative method to improve dimensional stability is to maintain a stable swollen state. Here, we propose ionic liquids as a new generation of dimensional stabilizers for WAW. Molecular dynamics simulation suggests that [Bmim][Cl] and its water mixtures can effectively swell amorphous cellulose as does water, without affecting crystalline cellulose. The volumetric shrinkage of WAW samples (Dipterocarpaceae Dipterocarpus sp. with a maximum moisture content of 80.3%) after air-drying can be effectively reduced by using a 40 wt% [Bmim][Cl] treatment from 5.03% to 0.47%, which is comparable to that of samples treated by the conventional material polyethylene glycol. When using lower concentrations, such as 5 wt% and 20 wt%, [Bmim][Cl] treatment is more effective than PEG treatment for dimensional control. Dynamic vapor sorption analysis shows that [Bmim][Cl] reduces moisture contents at moderate and low relative humidity, which further indicates that the observed improved dimensional stability is possibly achieved by the direct interaction of [Bmim][Cl] with wood polymers instead of by maintaining a high moisture content. We hope that this paper will provide a new solution for improving the dimensional stability of well-preserved WAW and other lignocellulosic materials. As some ionic liquids exhibit excellent anti-microbial capabilities, fire resistance, and thermal stability, a multifunctional dimensional stabilizer based on ILs is believed to be more beneficial to WAW than is PEG.
Author Contributions
Conceptualization, Y.Z. and J.W.; methodology, Y.Z.; formal analysis, Y.Z. and K.W.; investigation, Y.Z. and M.W.; resources, Z.Z., T.J., Y.F. and J.W.; writing—original draft preparation, Y.Z.; writing—review and editing, Z.Z., M.W., L.H. and X.H.; visualization, Y.Z.; supervision, Z.Z. and J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a technical service project of the National Center for Archaeology of China, grant number 2022-H-KJ-003.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Björdal, C.G.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The vasa experience with polyethylene glycol: A conservator’s perspective. J. Cult. Herit. 2013, 13, S175–S182. [Google Scholar] [CrossRef]
- Kılıç, N.; Kılıç, A.G. Evaluation of the Polyethylene Glycol Impregnation and Vacuum Freeze-Drying Method for Waterlogged Archaeological Wood: Conservation of the Yenikapı 1 Shipwreck. Stud. Conserv. 2023, 69, 336–347. [Google Scholar] [CrossRef]
- Liu, X.; Tu, X.; Ma, W.; Zhang, C.; Huang, H.; Varodi, A.M. Consolidation and Dehydration of Waterlogged Archaeological Wood from Site Huaguangjiao No.1. Forests 2022, 13, 1919. [Google Scholar] [CrossRef]
- Babiński, L. Dimensional changes of waterlogged archaeological hardwoods pre-treated with aqueous mixtures of lactitol/trehalose and mannitol/trehalose before freeze-drying. J. Cult. Herit. 2015, 16, 876–882. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhang, B.; Hu, Y. A comparative study of reinforcement materials for waterlogged wood relics in laboratory. J. Cult. Herit. 2018, 36, 94–102. [Google Scholar] [CrossRef]
- Tahira, A.; Howard, W.; Pennington, E.R.; Kennedy, A. Mechanical strength studies on degraded waterlogged wood treated with sugars. Stud. Conserv. 2017, 62, 223–228. [Google Scholar] [CrossRef]
- Kennedy, A.; Pennington, E.R. Conservation of chemically degraded waterlogged wood with sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Endo, R.; Sugiyama, J. New attempts with the keratin-metal/magnesium process for the conservation of archaeological waterlogged wood. J. Cult. Herit. 2022, 54, 53–58. [Google Scholar] [CrossRef]
- Endo, R.; Kamei, K.; Iida, I.; Yokoyama, M.; Kawahara, Y. Physical and mechanical properties of waterlogged wood treated with hydrolyzed feather keratin. J. Archaeol. Sci. 2010, 37, 1311–1316. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Hu, D. An aqueous approach to functionalize waterlogged archaeological wood followed by improved surface-initiated ARGET ATRP for maintaining dimensional stability. Cellulose 2021, 28, 2433–2443. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Hu, D. Surface-initiated arget atrp for maintaining the dimension of waterlogged archaeological wood (pinus massoniana): Polymer distribution behaviors and anti-shrinkage mechanism. Wood Sci. Technol. 2022, 56, 205–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, K.; Hu, D. Well-controlled SI-ARGET ATRP of EGDMA for maintaining the dimensions of waterlogged archaeological wood. Wood Sci. Technol. 2023, 57, 523–535. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B. Application of methyltrimethoxysilane to increase dimensional stability of waterlogged wood. J. Cult. Herit. 2017, 25, 149–156. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B.; Dutkiewicz, A. Organosilicon compounds with various active groups as consolidants for the preservation of waterlogged archaeological wood. J. Cult. Herit. 2019, 35, 123–128. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J. Reactivity of Waterlogged Archaeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H-13C Solution-State NMR Studies. Molecules 2022, 27, 3407. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Y.; Zhou, L.; Feng, N.; Li, C.; Sheng, L.; Li, Y.; Sun, J. Consolidation of waterlogged archaeological woods by reversibly cross-linked polymers. J. Archaeol. Sci. Rep. 2024, 57, 104675. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Kan, L.; Wang, Y.; Wang, K.; Hu, D. Aqueous modification of waterlogged archaeological wood by phenylboronic acid to reduce hygroscopicity and improve the dimensional stability. Wood Sci. Technol. 2024, 58, 941–957. [Google Scholar] [CrossRef]
- Collett, H.; Bouville, F.; Giuliani, F.; Schofield, E. Structural Monitoring of a Large Archaeological Wooden Structure in Real Time, Post PEG Treatment. Forests 2021, 12, 1788. [Google Scholar] [CrossRef]
- Penttilä, P.A.; Altgen, M.; Awais, M.; Österberg, M.; Rautkari, L.; Schweins, R. Bundling of cellulose microfibrils in native and polyethylene glycol-containing wood cell walls revealed by small-angle neutron scattering. Sci. Rep. 2020, 10, 20844. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Gu, J.D. Characterization of aerobic bacteria involved in degrading polyethylene glycol (peg)-3400 obtained by plating and enrichment culture techniques. J. Polym. Environ. 2007, 15, 57–65. [Google Scholar] [CrossRef]
- Glastrup, J.; Shashoua, Y.; Egsgaard, H.; Mortensen, M.N. Degradation of peg in the warship vasa. Macromol. Symp. 2006, 238, 22–29. [Google Scholar] [CrossRef]
- Fincher, E.L.; Payne, W.J. Bacterial utilization of ether glycols. Appl. Microbiol. 1962, 10, 542–547. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review. Intern. J. Molecul. Sci. 2024, 25, 1720. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Z.; Wang, W. Cosolvent or Antisolvent? A Molecular View of the Interface between Ionic Liquids and Cellulose upon Addition of Another Molecular Solvent. J. Phys. Chem. B 2013, 117, 11780–11792. [Google Scholar] [CrossRef]
- Liu, H.; Sale, K.L.; Simmons, B.A.; Singh, S. Molecular Dynamics Study of Polysaccharides in Binary Solvent Mixtures of an Ionic Liquid and Water. J. Phys. Chem. B 2011, 115, 10251–10258. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, R.; Balamurugan, K.; Shi, J.; Subramanian, V.; Simmons, B.A.; Singh, S. Theoretical insights into the role of water in the dissolution of cellulose using IL/water mixed solvent systems. J. Phys. Chem. B 2015, 119, 14339–14349. [Google Scholar] [CrossRef]
- Han, L.; Guo, J.; Tian, X.; Jiang, X.; Yin, Y. Evaluation of peg and sugars consolidated fragile waterlogged archaeological wood using nanoindentation and atr-ftir imaging. Int. Biodeterior. Biodegrad. 2022, 170, 105390. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, J.; Liu, P. Timber Log of China; China Forestry Publishing House: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Liu, W.; Shomali, A.; Zhang, C.; Coasne, B.; Carmeliet, J.; Derome, D. Nanostructure and interfacial mechanical properties of PEG/cellulose nanocomposites studied with molecular dynamics. Carbohydr. Polym. 2024, 343, 122429. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Darden, T.A.; York, D.M.; Pedersen, L.G. Particle mesh Ewald-an N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 1992, 98, 10089–10092. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Chem. Theory Comput. 1997, 4, 1463–1472. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Martonák, R.; Laio, A.; Parrinello, M. Predicting Crystal Structures: The Parrinello-Rahman Method Revisited. Phys. Rev. Lett. 2003, 90, 075503. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Doan, C.; Giffard, M.; Hediger, S.; De Paëpe, G.; Trân, Q.-K. Dynamics property recovery of archaeological-wood fibers treated with polyethylene glycol demonstrated by high-resolution solid-state NMR. Cellulose 2012, 19, 1537–1545. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Liu, S.; Sun, C.; Li, J.; Sun, Y.; Zhang, C.; Sun, S.; Yu, Q.; Yu, B.; et al. Lubrication and antibacterial performance and mechanism of functionalized ionic liquids in glycerol media with different water contents. Tribol. Int. 2025, 202, 110307. [Google Scholar] [CrossRef]
- Gao, C.; Qu, X.; Fu, L.; Chen, J.; Chu, Y.; Qiu, H. Ionic liquid-derived carbon dots with enhanced antibacterial activity and antibiofilm capability for treatment of MRSA-infected wounds. Chem. Eng. J. 2024, 497, 154717. [Google Scholar] [CrossRef]
- Miyafuji, H.; Fujiwara, Y. Fire resistance of wood treated with various ionic liquids (ILs). Holzforschung 2013, 67, 787–793. [Google Scholar] [CrossRef]
- Kopač, L.; Scharf, A.; Lin, C.; Sandberg, D.; Medved, S.; Jones, D. Investigation into a bio-based adhesive in combination with fire retardants for particleboard manufacture. Wood Mater. Sci. Eng. 2024, in press.
- Zhou, Y.; Wang, K.; Chen, X.; Hu, D. Alterations in the anatomy and chemical structure of archaeological wood from a tomb of northern china due to different fungal rots. Wood Res. 2020, 65, 757–770. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).