Abstract
Soil biodiversity plays a critical role in supporting multiple ecosystem functions. As some of the most diverse and abundant metazoans on the Earth, soil nematode communities exhibit changes along environmental gradients, but the ways in which the abundance and diversity of nematode communities vary along elevational gradients remain poorly understood. Taking advantage of an investigation on Huangshan Mountain, Southeast China, with elevation ranging from 500 to 1200 m, we assessed the abundance and diversity of soil nematodes, as well as the soil physicochemical properties, across subtropical forest ecosystems. Nematode communities were analyzed at the genus level, and the α-diversity was calculated as the genus richness, while the β-diversity was based on the Bray–Curtis dissimilarity. The results showed that, among the top 20 nematode genera ranked by absolute abundance, most genera, such as Eucephalobus, Prismatolaimus, Filenchus, and Rotylenchulus, reached their peak abundance at the highest elevation (1000 m). Additionally, the abundances of Oriverutus, Tylenchus, Criconema, and Tripyla exhibited a positive correlation with the elevation. Moreover, the abundance and α-diversity of the total nematodes and each trophic group of nematodes increased linearly with the elevation, likely due to increased soil moisture at higher elevation. In contrast, the β-diversity of the total nematodes, bacterivores, and herbivores decreased with increasing elevation, indicating the importance of stochastic processes in shaping community assembly at high altitudes. This pattern suggests that as the elevation increases, the nematode communities become more homogeneous in structure. Taken together, our study’s findings demonstrate the divergent responses of nematodes’ α- and β-diversity to an elevation gradient, highlighting the importance of the soil nematode diversity in maintaining ecosystem functions such as nutrient cycling and food web stability in mountainous regions. These results emphasize the need to incorporate the below-ground biodiversity into conservation strategies, particularly in the face of environmental changes driven by climate and human activities.
1. Introduction
Soil biodiversity is essential in maintaining effective ecosystem functioning across various ecosystems [1,2]. Numerous studies, ranging from localized experiments to large-scale global investigations, have consistently demonstrated that declines in soil biodiversity, encompassing microbes, mesofauna, and macrofauna, adversely affect key ecosystem functions, such as organic matter decomposition, nutrient cycling, and plant productivity [3,4,5]. Understanding the biogeographic patterns of soil biodiversity is therefore vital in improving predictions of ecosystem functioning, particularly under changing environmental conditions [6,7]. Elevation gradients, in particular, offer valuable insights into how biodiversity is structured across different environmental conditions [8,9,10]. By examining these patterns, researchers can not only address fundamental questions in biogeography but also gain predictive power to assess how climate change will alter soil ecosystems. These insights are especially important as shifts in temperature and precipitation, driven by climate change, are expected to affect soil biodiversity, consequently affecting the ecosystem services that are vital to human societies.
A substantial body of literature has documented changes in local soil biodiversity (i.e., changes in α-diversity) across elevation gradients [8,11,12]. For example, linear, hump-shaped, and random models between soil biodiversity and elevation gradients have been reported [8,13,14]. These different elevation–diversity relationships are largely attributed to elevation-induced differences in the climate and soil properties, such as the temperature and pH. Across an altitudinal gradient from forests to alpine tundra on Changbai Mountain in Northeast China, Shen et al. [8] showed that the soil pH is a major driver in determining the diversity pattern of soil eukaryotic microbes. In contrast to the α-diversity, the impact of environmental gradients on compositional variations among local communities (i.e., β-diversity), a crucial aspect of biodiversity, remains less well understood. In theory, high β-diversity within a single habitat suggests greater differences in the taxonomic, functional, and phylogenetic composition of communities, which in turn provide more opportunities for species with varying functional traits to enhance ecosystem functions [15,16,17]. Moreover, β-diversity can be divided into various components to represent different ecological processes and reflect community assembly mechanisms, such as balanced variation and abundance gradients [9,18]. Balanced variation refers to the replacement of individuals from one species by an equal number of individuals from different species across sites, analogous to the turnover component [18]. An abundance gradient describes the loss of individuals between sites, comparable to nestedness patterns in incidence-based models [18]. Therefore, integrating the α- and β-diversity of soil communities can reflect the composition and function of biological communities from different dimensions. Furthermore, most studies have focused on the biogeographic patterns of a particular trophic level of soil biota (e.g., bacterial and fungal diversity) [6,19], and the ways in which elevation gradients affect the α- and β-diversity of soil biota across multiple trophic levels remain largely unexplored.
Soil nematodes are the most diverse and abundant metazoans on the Earth, occupying a range of trophic levels in the soil food web, including primary (herbivores), secondary (microbivores), and tertiary (predators) consumers [20,21]. Accordingly, soil nematodes are often considered as proxies for the evaluation of the dynamics of soil biota across multiple trophic levels [22,23,24]. Moreover, soil nematodes are known as important soil engineers in driving ecosystem functions. Nematodes graze on microbes to facilitate nutrient mineralization and cycling and influence the population sizes of pathogen communities [20,25,26]. They can also directly affect plant productivity by feeding roots [27,28]. Soil nematodes are sensitive to changes in soil physiochemical properties, such as the soil moisture and pH, which in turn vary with the elevational gradient [29,30,31]. Previous research indicates that nematodes exhibit higher activity and mobility in moist soil environments [32,33]. In this respect, the role of stochastic processes in nematode community assembly may be strengthened as the soil moisture increases. Moreover, microbial responses to elevational gradients can cascade up to affect nematode communities through soil food chains [34,35]. Therefore, elevational gradients could affect nematodes’ abundance and diversity directly through influencing the habitat conditions and indirectly via changing nematodes’ food resources (plants and microbes). Numerous studies have examined how soil nematode communities vary with elevation, suggesting a range of different patterns. For instance, the soil nematode diversity or abundance may increase with altitude [36,37], decline [9], peak at mid-elevation [38], or display no clear correlation with the altitude [39]. However, to our knowledge, there are few or no empirical studies simultaneously demonstrating how the abundance and α- and β-diversity of soil nematodes respond to elevation gradients.
Here, we studied how the abundance and diversity of soil nematodes responded to an elevation gradient in a subtropical mountain ecosystem. The vertical distribution of vegetation reflects the horizontal zonation of many typical vegetation types in the subtropical region, including evergreen and deciduous broadleaf forests. Given the important role of nematodes in driving soil nutrient cycling and plant production, assessing how the nematode diversity responds to elevation would provide insights for an understanding of plant health and landscape functionality in subtropical mountain ecosystems. This study aimed to answer two major questions. First, how do the abundance and diversity (α- and β-diversity) of soil nematode communities change along an elevational gradient? Second, which mechanisms underlie the responses of the abundance and diversity of soil nematodes to changes in elevation? We hypothesized that (1) the nematode abundance and α-diversity would increase with elevation due to enhanced soil water availability, which is known to favor nematode survival and reproduction, and (2) the nematode β-diversity would decrease with elevation as stochastic processes dominate community assembly in harsher, high-elevation environments.
2. Materials and Methods
2.1. Study Sites
This study was conducted in the Huangshan national positioning observation and research station of forest ecosystems (117°58′–118°04′ E and 30°04′–30°08′ N), Anhui Province, China. This region is characterized by a humid subtropical monsoon climate, which influences the distribution and seasonal patterns of its vegetation and wildlife. The monsoon climate leads to distinct seasonal changes, with hot summers and relatively mild winters. The study spanned a wide elevational gradient from 500 to 1200 m above sea level (a.s.l.), representing various vegetation zones. At lower elevation (500–800 m), subtropical evergreen broadleaf forests dominate (e.g., Quercus glauca, Phoebe sheareri, Quercus fabri), while, at higher elevation (1000–1200 m), the vegetation transitions to temperate forests with mixed coniferous and broadleaf species (Pinus massoniana, Fagus engleriana, Cornus controversa). Detailed information about the plant species at each sampling site can be found in Table S1. The average annual temperature in the region is 15.5 °C, which varies along the elevational gradient. Precipitation is abundant, with average annual rainfall of 1759 mm. The heaviest rainfall is concentrated during the summer months, particularly during the monsoon season, influencing the soil moisture, plant growth, and the availability of water resources for local wildlife.
2.2. Soil Sampling and Measurements
In October 2021, soil was sampled from six sites along an elevation gradient (500 m, 600 m, 700 m, 800 m, 900 m, 1000 m a.s.l.) on the south-facing slope. At each elevation, three plots (5 m × 5 m) were established in a 50 m × 50 m area, and the intervals between any adjacent plots were at least 10 m. To reduce variability due to slope-related environmental factors and ensure consistency across sampling sites, all sampling sites were carefully selected to minimize the influence of the slope steepness. Specifically, the surface slope at all sampling locations was controlled to be less than 15 degrees. In each plot, five random soil cores (0–20 cm in depth and 5 cm in diameter) were collected and pooled to form a composite sample. A total of 18 soil samples (6 elevation × 3 replicates) were gathered over two days of fieldwork, immediately stored on ice, and quickly delivered to the laboratory for subsequent analysis.
2.3. Measurements of Soil Properties
The soil physicochemical properties were measured according to standardized procedures, as described in our previous study [13] at the same site. Soil moisture was measured gravimetrically by drying soil samples in an oven at 105 °C to a constant mass. The soil pH was measured using a pH meter (soil-to-water ratio, 1:2.5). The total organic carbon (TOC) was measured using a total organic carbon analyzer (Analytik Jena AG, Multi N/C3100, Jena, Germany). Nematode populations were extracted from 100 g fresh soil using a sequential extraction method [30]. After the total numbers of nematodes were counted, 100 specimens per sample were randomly selected and identified to the genus level with a ×40 magnification microscope. If the total number was less than 100, all nematodes were identified. The nematodes were assigned to one of several trophic guilds—bacterivore, fungivore, herbivore, and omnivore–carnivore—according to Yeates et al. (1993) [21] and the Nemaplex database (http://nemaplex.ucdavis.edu/) (accessed on 8 August 2024).
2.4. Statistical Analysis
The α-diversity of the total nematode community and each trophic guild was assessed using the number of nematode genera as an indicator of the taxonomic richness. To evaluate the β-diversity, we employed the abundance-based Bray–Curtis dissimilarity index, which quantifies the compositional differences between replicate plots at the same elevation. This approach allowed for an examination of community turnover or variation across spatial scales within each elevation. Additionally, we calculated the standardized effect size of the β-diversity, referred to as the β-deviation. This metric was obtained by comparing the observed β-diversity values with those generated by null models, which randomized the species distribution patterns while maintaining the observed α-diversity and abundance. The β-deviation accounts for the potential influence of the α-diversity on the community composition, thereby providing a more robust measure of the β-diversity that isolates the effects of ecological processes [40]. Specifically, β-deviation values close to zero indicate that the observed β-diversity aligns with stochastic expectations, suggesting random community assembly. In contrast, values significantly above or below zero imply that deterministic processes, such as environmental filtering or biotic interactions, strongly influence the community structure.
To examine how the elevation gradient influenced the nematode abundance and diversity, bivariate Spearman regressions were conducted to evaluate the relationships between the elevation and nematode abundance, as well as the α- and β-diversity/deviation. To assess the effects of the soil physicochemical properties on the nematode community composition, distance-based redundancy analysis (dbRDA) using the Bray–Curtis dissimilarity was performed. A generalized additive model was employed to determine the significance of each predictor in multi-response regression models. The variation in the community composition explained by these predictors was systematically partitioned into unique and shared contributions. Additionally, to disentangle the roles of species replacement and abundance gradients in the overall beta-diversity, the Bray–Curtis beta-diversity was decomposed into balanced variation and abundance gradient components. Finally, linear mixed models and variance decomposition were applied to assess the relative contributions of the soil physicochemical properties to the nematode abundance and diversity (α- and β-deviation). The optimal linear mixed model was selected using the Akaike Information Criterion (AIC) and coefficients of determination, implemented through the R package lme4. All statistical analyses were performed in R 3.5.2.
3. Results
The soil pH was significantly higher at 600 m and 700 m compared to other elevation levels, but did not differ significantly among 500 m, 800 m, and 1000 m (Table 1). The soil moisture increased along the elevational gradient, ranging from 22% to 62% (Table 1). The soil TN and TOC content reached their highest values at the highest elevation and their lowest values at 700 m (Table 1).

Table 1.
Effects of elevation on soil physicochemical properties. Data are means and standard deviation (n = 3). Different letters indicate significant difference across elevation (Fisher’s LSD test, p < 0.05).
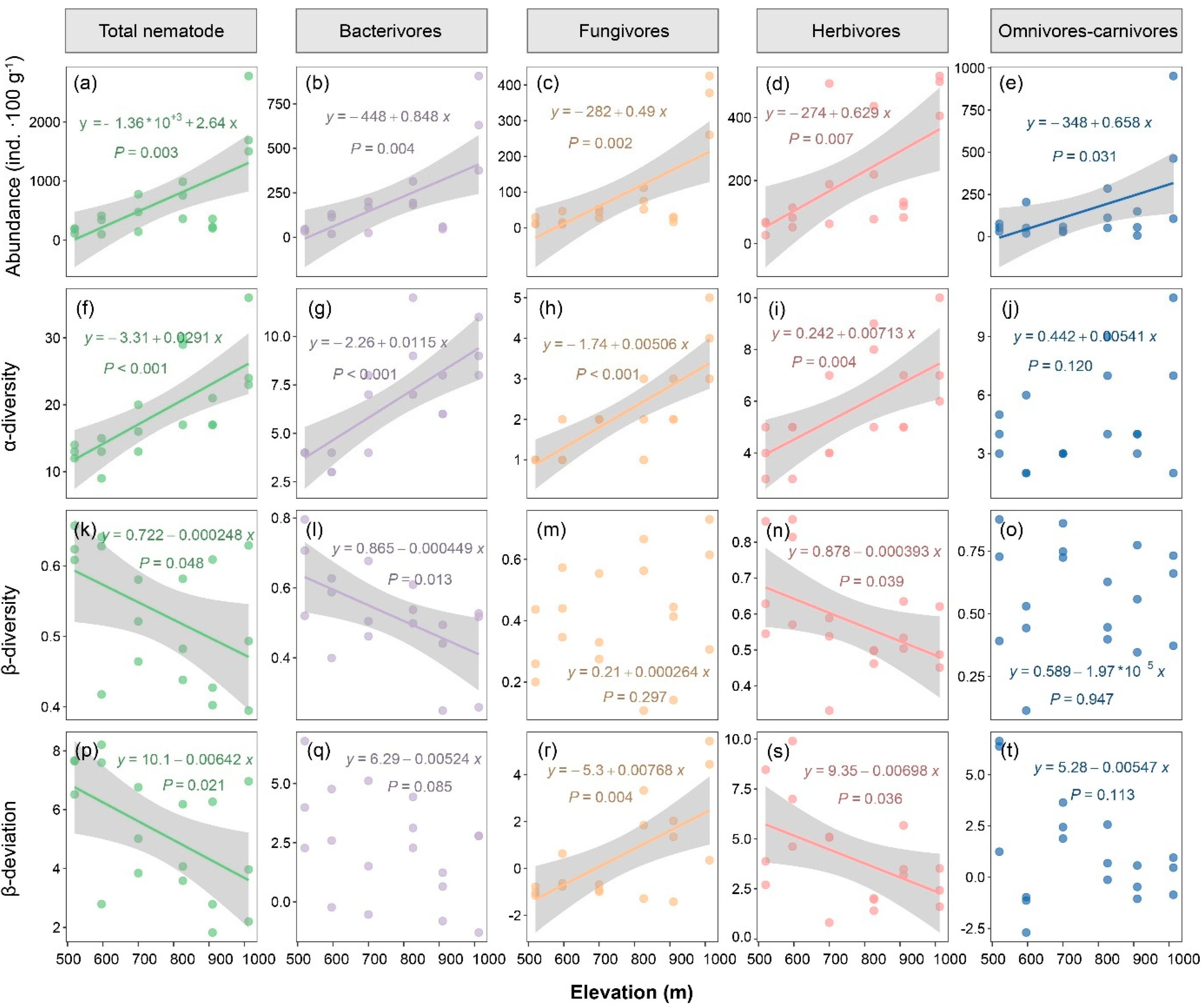
A total of 11614 individuals and 50 genera of soil nematodes were generated from the 18 soil samples. Among the top 20 nematode genera ranked by absolute abundance, the abundance of all genera, except for Protorhabditis, Acrobeloides, Criconema, and Prodorylamus, such as Eucephalobus, Prismatolaimus, Filenchus, and Rotylenchulus, reached the highest levels at the maximum elevation (1000 m) (Table 2). Additionally, the abundances of Oriverutus, Tylenchus, Criconema, and Tripyla showed a positive correlation with the elevation. The abundance of the total nematodes and each trophic group of soil nematodes was significantly and positively related to the elevation (r = 0.43–0.72; p < 0.05; Figure 1a–e).

Table 2.
The abundance of the top 20 nematode genera (ranked by absolute abundance) along the elevational gradient. Data are means and standard deviation (n = 3). Different letters indicate significant difference across elevation (Fisher’s LSD test, p < 0.05). BF: bacterivores; FF: fungivores; HF: herbivores; OP: omnivores–carnivores.
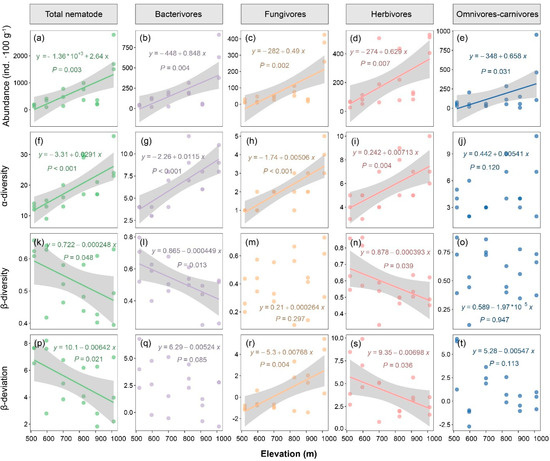
Figure 1.
Nematode abundance and diversity with increasing elevation. (a–e) abundance; (f–j) α-diversity; (k–o) β-diversity; (p–t) β-deviation. α-Diversity is measured by genera numbers of nematodes. β-Diversity is measured by Bray–Curtis dissimilarity. β-Deviation is the standardized effect size of the β-diversity, calculated by comparing the observed β-diversity to the null models. Significant regression lines are shown.
The α-diversity of the total nematodes, bacterivores, fungivores, and herbivores significantly increased with the elevation (r = 0.66–0.83; p < 0.05; Figure 1f–i). No significant elevational pattern was found for omnivores–carnivores (Figure 1j). However, the β-diversity of the total nematodes (r = −0.45, p < 0.05), bacterivores (r = −0.54, p < 0.05), and herbivores (r = −0.51, p < 0.05) decreased with increasing elevation (Figure 1k,l,n). Similarly, the β-deviation, which excluded the influences of α-diversity, of the total nematodes and herbivores was negatively related to the elevation (r = −0.57 and −0.44, respectively; p < 0.05; Figure 1p,s). In contrast, the β-diversity of fungivores was positively related to the elevation (r = 0.53, p < 0.05; Figure 1r).
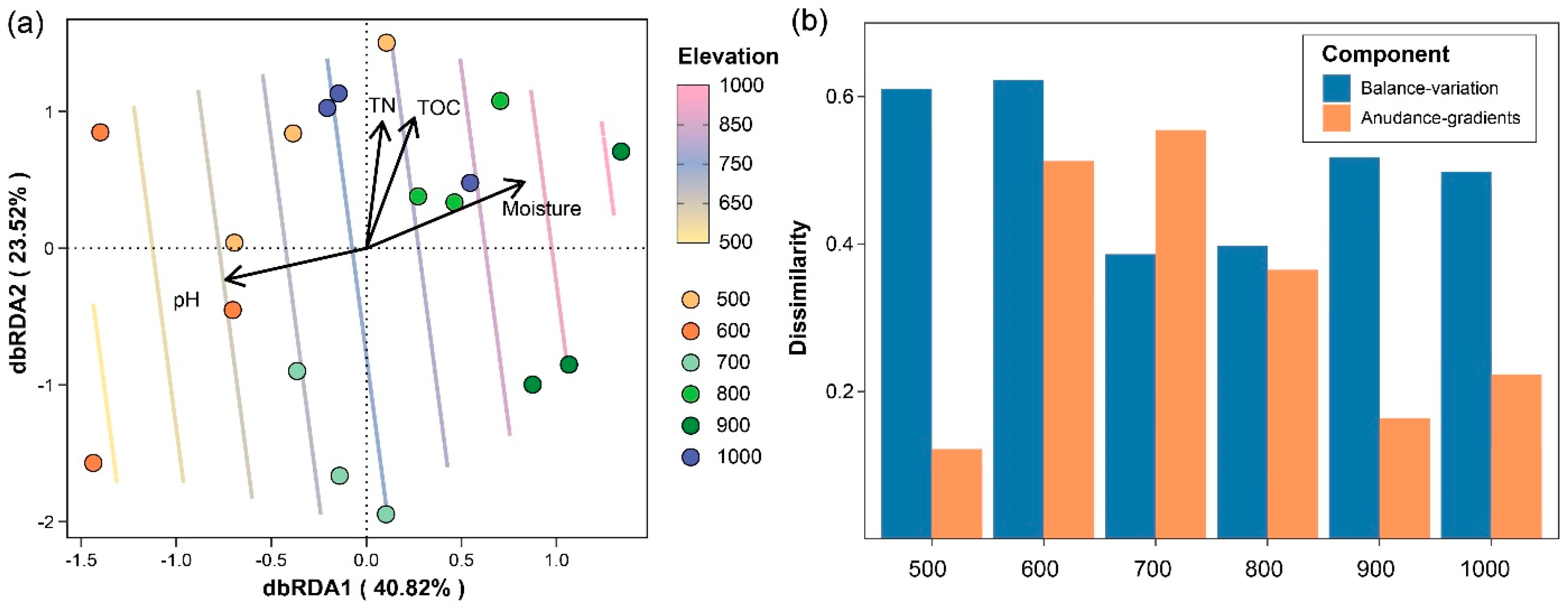
The distance-based redundancy analysis (dbRDA) plot (Figure 2a) showed the variation in the nematode community composition along the elevational gradient. The first constrained axis (dbRDA1) explained 40.82% of the total variation in the community composition, while the second constrained axis (dbRDA2) accounted for 23.52%. The soil physicochemical properties, including the total nitrogen (TN), total organic carbon (TOC), moisture content, and pH, were identified as significant drivers of the nematode community structure. The arrows indicated the direction and strength of the correlations between these variables and the dbRDA axes. The elevation, represented as colored contour lines, was passively fitted onto the ordination plot using the ordisurf function, demonstrating clear differentiation in the community composition across altitudes. Lower elevation (500–600 m) was associated with distinct nematode communities compared to higher elevation (800–1000 m). The decomposition of the Bray–Curtis β-diversity into balanced variation and abundance gradient components (Figure 2b) revealed distinct patterns across the elevational gradient. At lower elevation (500–600 m) and higher elevation (700–1000 m), balanced variation contributions dominated, suggesting species replacement as the main driver of community dissimilarity.
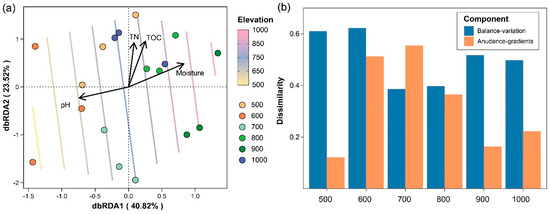
Figure 2.
Ordination plot of distance-based redundancy analysis (dbRDA) and decomposition of nematode β-diversity along elevational gradient. (a) dbRDA result illustrating the variation in nematode communities along elevational gradient. Percentage values in axis titles represent the explained community composition variation by each constrained axis. The contour lines in different colors indicate altitudes added passively using the ordisurf function from the vegan package with the default parameters. (b) Abundance-based Bray–Curtis dissimilarity was decomposed into balanced variation and abundance gradient components.
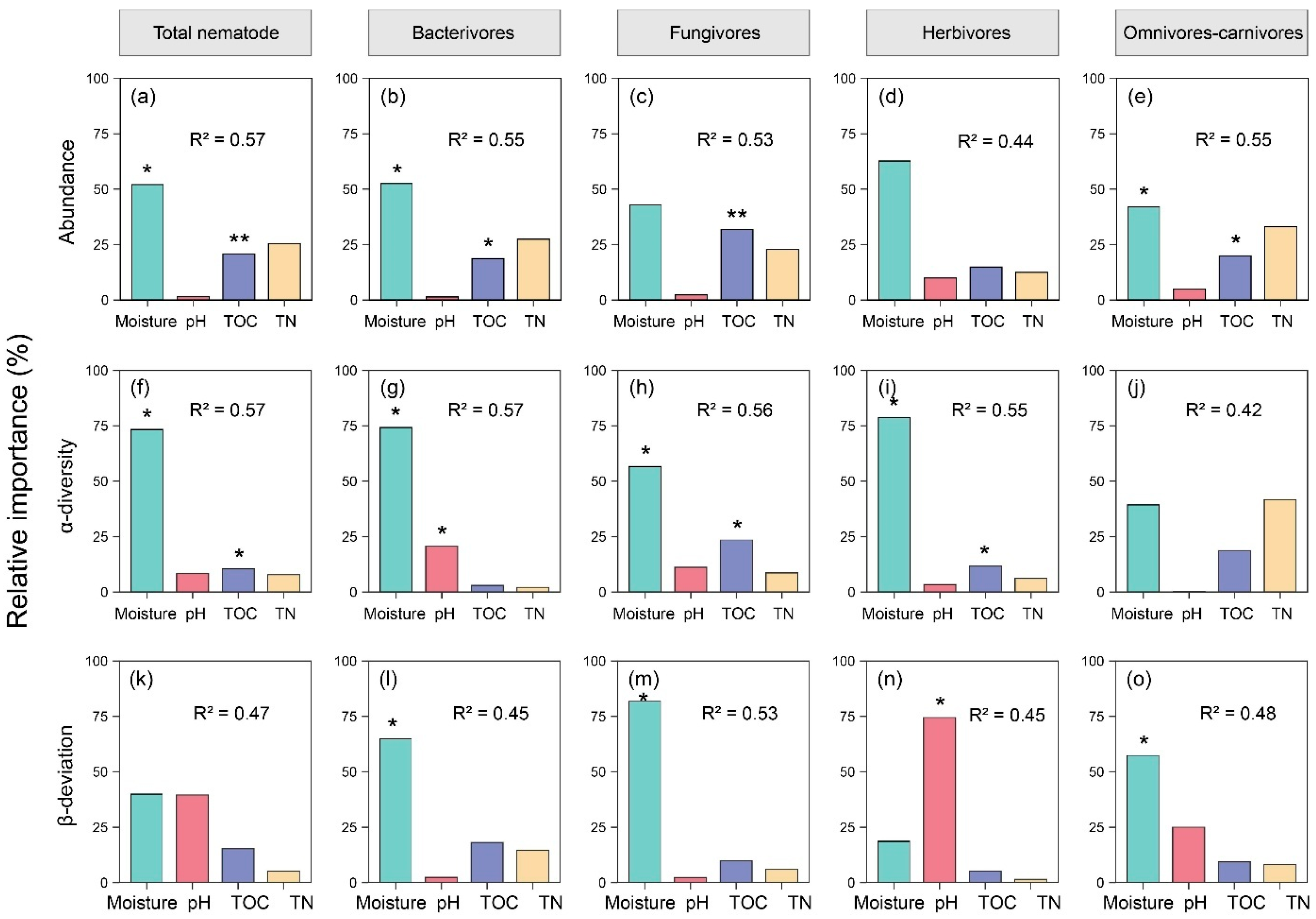
Linear mixed models and variance partitioning were used to identify the determinants of the soil nematode abundance and diversity. Soil moisture was the primary driver of the nematode abundance across all trophic groups (R2 ranging from 0.44 to 0.57; Figure 3a–e). Its relative importance exceeded 50% for the total nematodes, bacterivores, and herbivores (Figure 3a–e). Notably, the TOC significantly affected the abundance of total nematodes, bacterivores, fungivores, and omnivores–carnivores, while the pH showed a limited influence. The α-diversity of the nematode communities was predominantly regulated by the soil moisture (average relative importance >60%, R2 between 0.42 and 0.57, Figure 3f–j). For the total nematodes, fungivores, and herbivores, the soil TOC had a significant but secondary effect. Moisture remained the most influential factor in the β-deviation (R2 = 0.45–0.53; Figure 3k–o). Interestingly, the pH played a critical role in certain groups, such as herbivores (relative importance = 73.5%; Figure 3n).
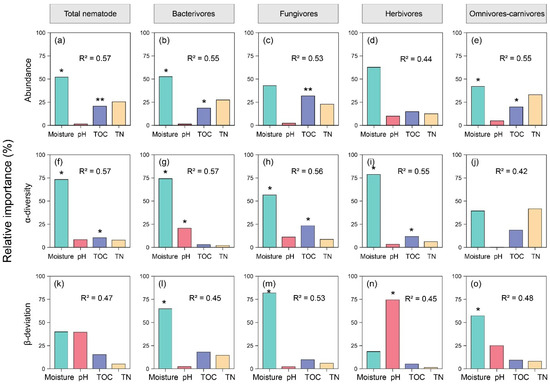
Figure 3.
Relative contributions of soil physicochemical properties to nematode abundance and diversity (α- and β-deviation). (a–e) abundance; (f–j) α-diversity; (k–o) β-deviation. The analysis was performed using linear mixed models and variance decomposition. The optimal model was selected based on the Akaike Information Criterion (AIC) and coefficients of determination. Significant differences at: *, p < 0.05; **, p < 0.01.
4. Discussion
Our study revealed previously overlooked and markedly distinct patterns in the responses of the soil nematode abundance, α-diversity, and β-diversity to the elevation gradient in subtropical forests. As the elevation increased, the nematode abundance and α-diversity increased, while the β-diversity decreased in our study mountain. These divergent results point to the differences in the mechanisms in regulating nematode communities across elevation gradients.
Most of the studies on elevation’s effects on soil biodiversity have focused on soil microbes and fauna, which have produced mixed results. For example, a study showed that the earthworm richness increased with increasing elevation in tropical rainforests due to variations in the soil pH and root length density [41]. However, a contrasting pattern was also reported [42]. Additionally, the abundance and richness of mites and springtails showed different patterns in response to elevation, even within a single ecosystem [10,43,44]. In our study site, the relationships between the elevation and plant or microbial α-diversity were nonlinear, with lower diversity being detected at moderate elevation [13]. In theory, the responses of plant and microbial diversity to elevation would cascade along the food web chain to soil nematodes. However, we showed that the nematode abundance and α-diversity of total nematodes, bacterivores, fungivores, and herbivores significantly increased with the elevation (Figure 1). This result aligns with some studies [39,45] but contradicts others [9] that focused on nematodes. These different responses among plants, microbes, and nematodes suggest that the changes in the nematode α-diversity with elevation were independent of their food resources. We further found that soil moisture was the major factor in shaping the nematode abundance and α-diversity (Figure 2). Soil nematodes live in the water films around soil particles, and their activities, such as movement, predation, and reproduction, rely on water availability in the soil pore space [20,30]. Our results, together with recent studies [32,46], indicate that changes in water availability may exert dominant control over the nematode α-diversity along elevation gradients. High soil moisture can directly increase the nematode diversity by stimulating nematodes’ movement and predation and indirectly by increasing the prey (e.g., microbes) abundance and diversity. Given that nematodes occupy multiple trophic levels in soil food webs, future studies should explore how the responses of nematodes to water availability affect ecosystem functions.
In contrast with the nematode α-diversity, we found that the β-diversity of total nematodes, bacterivores, and herbivores decreased with increasing elevation (Figure 1), indicating that these nematode communities become more similar in their structure at higher elevation. However, the β-deviation, which excluded the influences of the α-diversity, of fungivorous nematodes was positively related to the elevation (Figure 1). This result is thus consistent with a meta-analysis showing that the patterns of β-diversity components depend on the trophic level or feeding habits of the functional groups [47]. Increased soil moisture along elevation gradients promoted the movement and dispersal of bacterivorous and herbivorous nematodes, which in total accounted for about 50% of the total nematode abundance across all elevation levels (Figure S1), thus strengthening the importance of stochastic processes in shaping nematode community assembly. This could consequently increase the uniformity (i.e., reduce β-diversity) in the composition of the developing communities at higher elevation. Moreover, the dominance of balanced variation components in the total dissimilarity suggests that environmental filtering, alongside spatial and historical limitations, plays a role in driving community differences across plots [9,18]. Compared with bacterivorous ones, fungivorous nematodes may have lower mobility and shorter travel distances for the following two reasons. First, the dispersal of fungal spores is typically limited to much shorter distances than that of bacteria, increasing the difficulty in free dispersal for fungal communities [48,49]. Second, fungi tend to be more tolerant to harsh environments such as droughts than bacteria [50]. These characteristics of soil fungal communities may cascade to affect the community assembly of fungivorous nematodes [51,52]. Together, these results indicate that stochastic and deterministic processes play different roles in the community assembly of different soil biota at multiple functional groups and trophic levels.
The divergent responses of the nematode α- and β-diversity along elevation gradients have profound implications for ecosystem functioning and stability. The increase in α-diversity with elevation suggests enhanced potential for nutrient cycling and microbial grazing at higher elevation, driven by the greater taxonomic richness of nematode communities. For example, bacterivorous and fungivorous nematodes, which facilitate nutrient mineralization [20,25,26], may thrive in moist, high-elevation soils, thereby boosting ecosystem productivity and sustaining plant growth [5,27]. Conversely, the observed decline in β-diversity with increasing elevation indicates reduced compositional turnover among communities, potentially limiting functional redundancy—a key element in maintaining ecosystem stability under environmental disturbances [40]. The homogenization of the community composition at higher altitudes may render these ecosystems more vulnerable to perturbations, such as extreme weather events or changes in land use [53,54]. This reduced resilience could compromise the overall functionality of forest systems, including their capacity to buffer nutrient cycles and suppress pathogenic organisms. To address these challenges, future research could explore how the interplay between nematode functional traits and environmental gradients influences ecosystem functions. For instance, assessing how the dispersal limitations of specific trophic groups, such as herbivorous or omnivorous nematodes, affect soil food web dynamics may yield insights into ecosystem resilience [53]. Additionally, conservation strategies should prioritize preserving diverse nematode assemblages across elevation levels to ensure the stability and multifunctionality of mountain ecosystems under changing climatic conditions.
The conclusions of this study should be interpreted with caution. First, our research, focused on a subtropical mountain ecosystem and limited to six representative altitudes, may not fully capture the impact of the entire altitudinal gradient on the nematode diversity [55]. Future studies could expand the sampling range to cover more altitudinal zones, thereby providing a more comprehensive understanding of the diversity patterns. While the soil moisture was identified as a key driver of the nematode diversity and community composition (Figure 2), the considerable unexplained variation suggests that other undetected factors, such as the topography, microclimate, and evolutionary processes, may also play a significant role in mediating the relationships between the elevation and nematode diversity [9,20,46]. Incorporating high-resolution spatial data and functional trait analysis could help to elucidate these additional influences. Additionally, the absence of detailed data on nematode food sources, such as microbes and plants, limits our understanding of how biotic interactions within soil micro-food webs influence nematode diversity [56,57]. Leveraging advanced molecular tools, such as metagenomics and stable isotope probing, could provide deeper insights into these biotic interactions. This study also relied on data from a single time point, neglecting potential seasonal dynamics in nematode communities. Long-term, multi-seasonal studies could capture temporal variations and improve the robustness of biodiversity assessments. Lastly, as a case study in a specific mountainous region, our findings may not be fully generalized to other areas. Comparative analyses across multiple mountain systems under different climatic conditions would validate and broaden the applicability of these results.
5. Conclusions
In this study, we provide novel insights into how elevation influences soil nematode communities and their diversity within subtropical forest ecosystems. Our findings highlight the contrasting patterns of α- and β-diversity along the elevation gradient, with the nematode abundance generally increasing with the elevation. The responses of the β-diversity varied across different trophic groups, indicating that distinct ecological processes govern the structuring of nematode communities. These results suggest that multiple factors, including the soil moisture and habitat-specific conditions, play key roles in shaping the below-ground biodiversity. Our study underscores the importance of considering both the α- and β-diversity when assessing the ecological impacts of environmental gradients. Further research is needed to explore how these patterns influence ecosystem functions such as nutrient cycling and food web dynamics across different elevations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15122149/s1, Table S1. Main characteristics of the sampling sites along an elevational gradient on Huangshan Mountain. Figure S1. Relative abundance of soil nematode trophic groups at different elevation levels. Data are means and standard errors (n = 3). Different letters indicate significant differences across elevation (Fisher’s LSD test, p < 0.05).
Author Contributions
Conceptualization, C.M. and Z.H.; Methodology, C.M. and K.D.; Formal Analysis, K.D. and Z.H.; Investigation, C.M. and K.D.; Writing—Original Draft Preparation, Z.H. and K.D.; Writing—Review and Editing, C.M., Z.Q., S.C., R.S., H.F. and Z.Z.; Supervision, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by the National Natural Science Foundation of China [32071628, 32301434] and the Anhui Higher Institutions Natural Science Foundation [2023AH051012]. Z.H. acknowledges support from the China Postdoctoral Science Foundation [2022M711657] and the Jiangsu Funding Program for Excellent Postdoctoral Talent [2022ZB326].
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Bardgett, R.D.; Kelly, E. Biodiversity in the Dark. Nat. Geosci. 2010, 3, 297–298. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil Biodiversity and Soil Community Composition Determine Ecosystem Multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Chen, D.; Wang, S.; Wu, Y.; Wang, B.; Liu, S.; Yue, L.; Yu, J.; Bai, Y. Soil Biota Diversity and Plant Diversity Both Contributed to Ecosystem Stability in Grasslands. Ecol. Lett. 2023, 26, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B.H. Beyond Biogeographic Patterns: Processes Shaping the Microbial Landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef]
- Shen, C.; Liang, W.; Shi, Y.; Lin, X.; Zhang, H.; Wu, X.; Xie, G.; Chain, P.; Grogan, P.; Chu, H. Contrasting Elevational Diversity Patterns between Eukaryotic Soil Microbes and Plants. Ecology 2014, 95, 3190–3202. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhang, C.; Zheng, L.; Li, H. Altitudinal Variation in Soil Nematode Communities in an Alpine Mountain Region of the Eastern Tibetan Plateau. Eur. J. Soil Biol. 2024, 121, 103617. [Google Scholar] [CrossRef]
- Bokhorst, S.; (Ciska) Veen, G.F.; Sundqvist, M.; De Long, J.R.; Kardol, P.; Wardle, D.A. Contrasting Responses of Springtails and Mites to Elevation and Vegetation Type in the Sub-Arctic. Pedobiologia 2018, 67, 57–64. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on Mountainsides: Contrasting Elevational Patterns of Bacterial and Plant Diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Lu, Y.; Chen, L.; Zhang, Z.; Zhang, H.; Wang, X.; Shu, L.; Yang, Q.; Song, Q. When Microclimates Meet Soil Microbes: Temperature Controls Soil Microbial Diversity along an Elevational Gradient in Subtropical Forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Song, L.; Yang, T.; Xia, S.; Yin, Z.; Liu, X.; Li, S.; Sun, R.; Gao, H.; Chu, H.; Ma, C. Soil Depth Exerts Stronger Impact on Bacterial Community than Elevation in Subtropical Forests of Huangshan Mountain. Sci. Total Environ. 2022, 852, 158438. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Si, G.; Wang, J.; Luo, T.; Zhang, G. Bacterial Community in Alpine Grasslands along an Altitudinal Gradient on the Tibetan Plateau. FEMS Microbiol. Ecol. 2014, 87, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.S.; Isbell, F.; Seidl, R. β-Diversity, Community Assembly, and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Omidipour, R.; Tahmasebi, P.; Faal Faizabadi, M.; Faramarzi, M.; Ebrahimi, A. Does β Diversity Predict Ecosystem Productivity Better than Species Diversity? Ecol. Indic. 2021, 122, 107212. [Google Scholar] [CrossRef]
- Wang, S.; Loreau, M. Biodiversity and Ecosystem Stability across Scales in Metacommunities. Ecol. Lett. 2016, 19, 510–518. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning Abundance-based Multiple-site Dissimilarity into Components: Balanced Variation in Abundance and Abundance Gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Chen, Y.; Wilson, M.C.; Yang, X.; Ma, C.; Lu, J.; Chen, X.; Wu, J.; Shu, W.; et al. Island Biogeography of Soil Bacteria and Fungi: Similar Patterns, but Different Mechanisms. ISME J. 2020, 14, 1886–1896. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil Nematode Abundance and Functional Group Composition at a Global Scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera-An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; De Ruiter, P.C.; et al. Soil Food Web Properties Explain Ecosystem Services across European Land Use Systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Z.; Liu, T.; Kardol, P.; Ma, C.; Hu, Y.; Cui, Y.; Zhao, C.; Zhang, W.; Guo, D. Drivers of Nematode Diversity in Forest Soils across Climatic Zones. Proc. R. Soc. B 2023, 290, 20230107. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Li, H.; Geisen, S.; Hu, F.; Liu, M. Management Effects on Soil Nematode Abundance Differ among Functional Groups and Land-Use Types at a Global Scale. J. Anim. Ecol. 2022, 91, 1770–1780. [Google Scholar] [CrossRef]
- Griffiths, B.S. Microbial-Feeding Nematodes and Protozoa in Soil: Their Effectson Microbial Activity and Nitrogen Mineralization in Decomposition Hotspots and the Rhizosphere. Plant Soil 1994, 164, 25–33. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere Fauna: The Functional and Structural Diversity of Intimate Interactions of Soil Fauna with Plant Roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Topalović, O.; Geisen, S. Nematodes as Suppressors and Facilitators of Plant Performance. New Phytol. 2023, 238, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Cook, R.; Yeates, G.W.; Denton, C.S. The Influence of Nematodes on Below-Ground Processes in Grassland Ecosystems. Plant Soil 1999, 212, 23–33. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of Nitrogen Enrichment on Belowground Communities in Grassland: Relative Role of Soil Nitrogen Availability vs. Soil Acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Vandegehuchte, M.L.; Sylvain, Z.A.; Reichmann, L.G.; De Tomasel, C.M.; Nielsen, U.N.; Wall, D.H.; Sala, O.E. Responses of a Desert Nematode Community to Changes in Water Availability. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, B.; Lu, S.; Chen, D.; Wu, Y.; Zhu, Y.; Hu, S.; Bai, Y. Soil Acidification Reduces the Effects of Short-Term Nutrient Enrichment on Plant and Soil Biota and Their Interactions in Grasslands. Glob. Chang. Biol. 2020, 26, 4626–4637. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, J.; Chu, P.; Hu, S.; Xie, Y.; Tuvshintogtokh, I.; Bai, Y. Regional-Scale Patterns of Soil Microbes and Nematodes across Grasslands on the Mongolian Plateau: Relationships with Climate, Soil, and Plants. Ecography 2015, 38, 622–631. [Google Scholar] [CrossRef]
- Lu, L.; Li, G.; He, N.; Li, H.; Liu, T.; Li, X.; Whalen, J.K.; Geisen, S.; Liu, M. Drought Shifts Soil Nematodes to Smaller Size across Biological Scales. Soil Biol. Biochem. 2023, 184, 109099. [Google Scholar] [CrossRef]
- Steffan, S.A.; Chikaraishi, Y.; Currie, C.R.; Horn, H.; Gaines-Day, H.R.; Pauli, J.N.; Zalapa, J.E.; Ohkouchi, N. Microbes Are Trophic Analogs of Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 15119–15124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, W.-J.J.; Zhang, X.-K.K.; Liang, W.-J.J. Soil Nematode Abundance and Diversity in Different Forest Types at Changbai Mountain, China. Zool. Stud. 2012, 51, 619–626. [Google Scholar]
- Kergunteuil, A.; Campos-Herrera, R.; Sánchez-Moreno, S.; Vittoz, P.; Rasmann, S. The Abundance, Diversity, and Metabolic Footprint of Soil Nematodes Is Highest in High Elevation Alpine Grasslands. Front. Ecol. Evol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Kouser, Y.; Shah, A.A.; Rasmann, S. The Functional Role and Diversity of Soil Nematodes Are Stronger at High Elevation in the Lesser Himalayan Mountain Ranges. Ecol. Evol. 2021, 11, 13793–13804. [Google Scholar] [CrossRef]
- Devetter, M.; Háněl, L.; Řeháková, K.; Doležal, J. Diversity and Feeding Strategies of Soil Microfauna along Elevation Gradients in Himalayan Cold Deserts. PLoS ONE 2017, 12, e0187646. [Google Scholar] [CrossRef]
- Pitteloud, C.; Descombes, P.; Sànchez-Moreno, S.; Kergunteuil, A.; Ibanez, S.; Rasmann, S.; Pellissier, L. Contrasting Responses of Above- and below-Ground Herbivore Communities along Elevation. Oecologia 2020, 194, 515–528. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Yang, X.; Deng, M.; Wang, Z.; Wang, P.; Yang, S.; Li, P.; Peng, Z.; Yang, L.; et al. Long-Term Nitrogen Input Alters Plant and Soil Bacterial, but Not Fungal Beta Diversity in a Semiarid Grassland. Glob. Chang. Biol. 2021, 27, 3939–3950. [Google Scholar] [CrossRef]
- González, G.; García, E.; Cruz, V.; Borges, S.; Zalamea, M.; Rivera, M.M. Earthworm Communities along an Elevation Gradient in Northeastern Puerto Rico. Eur. J. Soil Biol. 2007, 43, S24–S32. [Google Scholar] [CrossRef]
- Decaëns, T. Macroecological Patterns in Soil Communities. Glob. Ecol. Biogeogr. 2010, 19, 287–302. [Google Scholar] [CrossRef]
- Liu, D.; Liu, D.; Yu, H.; Wu, H. Strong Variations and Shifting Mechanisms of Altitudal Diversity and Abundance Patterns in Soil Oribatid Mites (Acari: Oribatida) on the Changbai Mountain, China. Appl. Soil Ecol. 2023, 186, 104808. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, X.; Lux, J.; Chen, T.; Potapov, M.; Wu, D.; Scheu, S. Drivers of Collembola Assemblages along an Altitudinal Gradient in Northeast China. Ecol. Evol. 2022, 12, e8559. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Moroenyane, I.; Tripathi, B.; Kerfahi, D.; Takahashi, K.; Yamamoto, N.; An, C.; Cho, H.; Adams, J. Soil Nematodes Show a Mid-Elevation Diversity Maximum and Elevational Zonation on Mt. Norikura, Japan. Sci. Rep. 2017, 7, 3028. [Google Scholar] [CrossRef]
- Xiong, D.; Wei, C.Z.; Wubs, E.R.J.; Veen, G.F.; Liang, W.; Wang, X.; Li, Q.; Van der Putten, W.H.; Han, X. Nonlinear Responses of Soil Nematode Community Composition to Increasing Aridity. Glob. Ecol. Biogeogr. 2020, 29, 117–126. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Wang, J. A Meta-analysis of Nestedness and Turnover Components of Beta Diversity across Organisms and Ecosystems. Glob. Ecol. Biogeogr. 2018, 27, 96–109. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in Microbes: Fungi in Indoor Air Are Dominated by Outdoor Air and Show Dispersal Limitation at Short Distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Peay, K.G.; Garbelotto, M.; Bruns, T.D. Evidence of Dispersal Limitation in Soil Microorganisms: Isolation Reduces Species Richness on Mycorrhizal Tree Islands. Ecology 2010, 91, 3631–3640. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Yang, T.; Tedersoo, L.; Liu, X.; Gao, G.; Dong, K.; Adams, J.M.; Chu, H. Fungi Stabilize Multi-kingdom Community in a High Elevation Timberline Ecosystem. iMeta 2022, 1, e49. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wei, C.; Wang, X.; Xiaotao, L.; Fang, S.; Li, Y.; Wang, X.; Liang, W.; Han, X.; Bezemer, T.M.; et al. Spatial Patterns and Ecological Drivers of Soil Nematode β-Diversity in Natural Grasslands Vary among Vegetation Types and Trophic Position. J. Anim. Ecol. 2021, 90, 1367–1378. [Google Scholar] [CrossRef]
- de Vries, F.T.; Liiri, M.E.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land Use Alters the Resistance and Resilience of Soil Food Webs to Drought. Nat. Clim. Chang. 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, C.; Chen, X.; Yao, J.; Jiang, L.; Liu, M. Home-Field Advantage in Soil Respiration and Its Resilience to Drying and Rewetting Cycles. Sci. Total Environ. 2021, 750, 141736. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C. The Elevational Gradient of Species Richness: A Uniform Pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Thakur, M.P.; Geisen, S. Trophic Regulations of the Soil Microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef]
- Jiang, Y.; Luan, L.; Hu, K.; Liu, M.; Chen, Z.; Geisen, S.; Chen, X.; Li, H.; Xu, Q.; Bonkowski, M.; et al. Trophic Interactions as Determinants of the Arbuscular Mycorrhizal Fungal Community with Cascading Plant-Promoting Consequences. Microbiome 2020, 8, 142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).