Potential Global Distribution and Habitat Shift of Prunus subg. Amygdalus Under Current and Future Climate Change
Abstract
1. Introduction
2. Methods
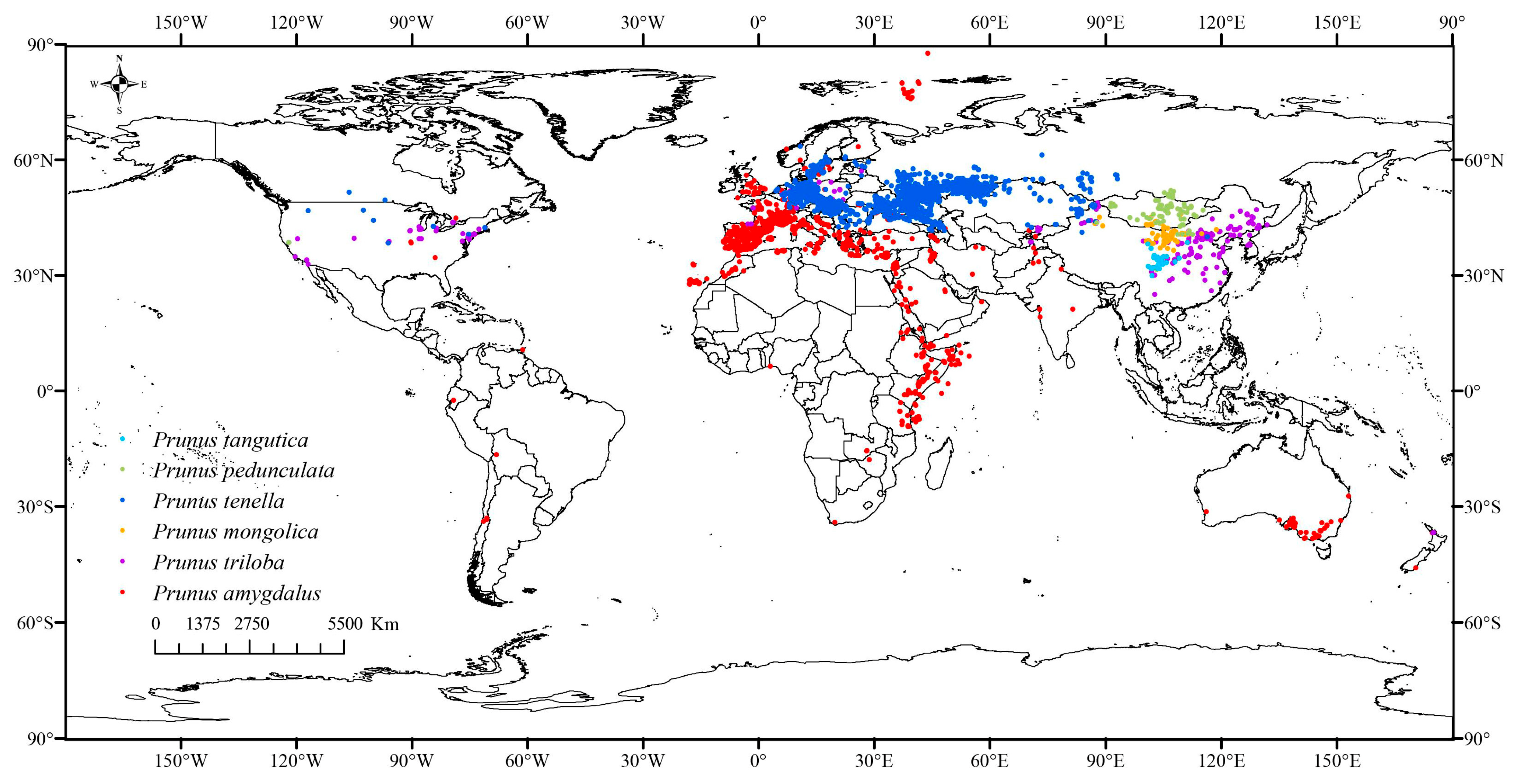
2.1. Species Occurrence Information
2.2. Environmental Parameters
2.3. Calibration, Construction, and Evaluation of the MaxEnt Model
2.4. Geospatial Analysis
3. Results
3.1. Model Evaluations
3.2. Important Environmental Variables Preference
3.3. Potential Distribution Areas Under Current Climate
3.4. Changes in the Suitable Habitat Areas of Prunus subg. Amygdalu in the Future
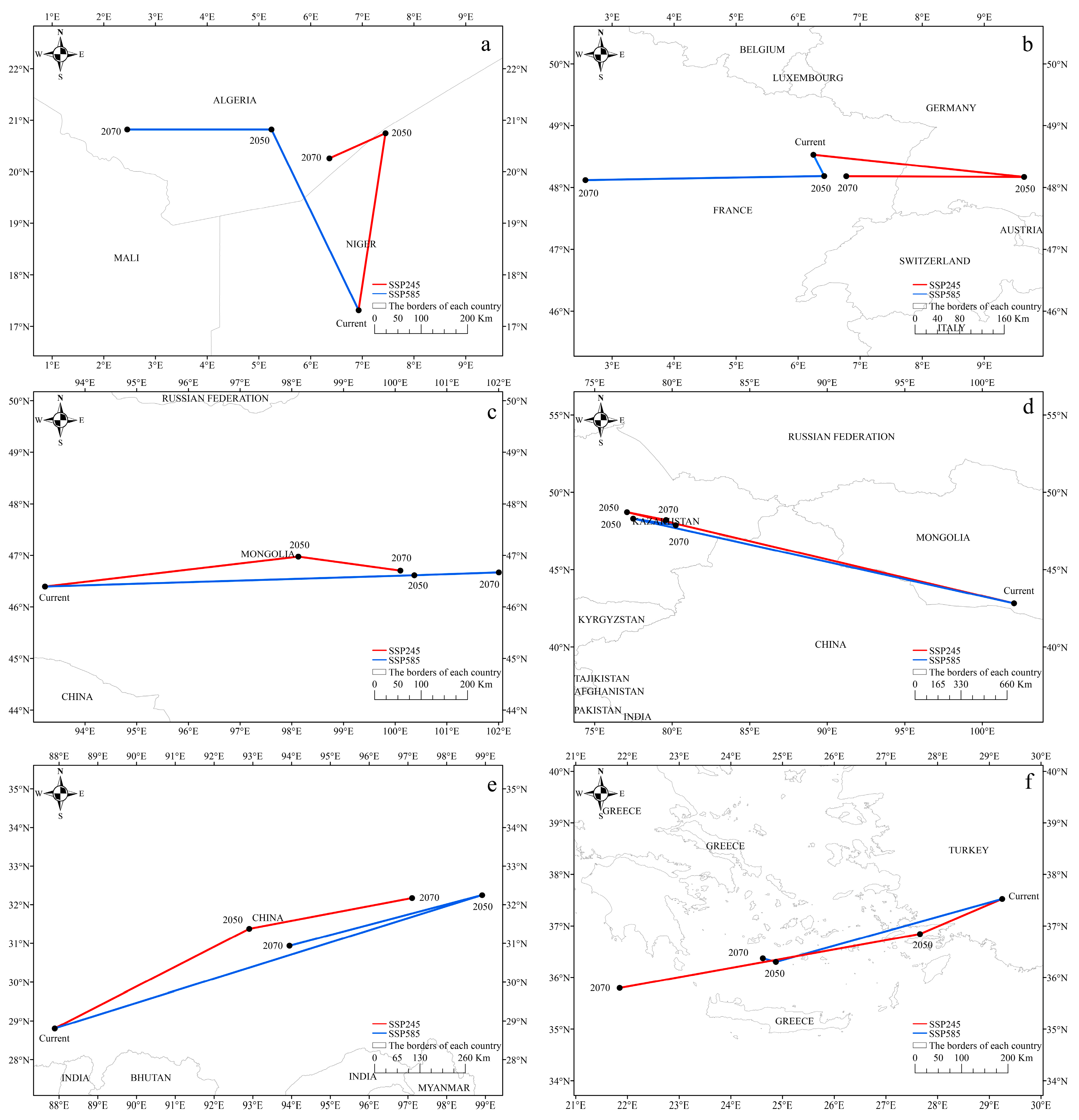
3.5. The Spatial Shift of Potential Habitats Centroid in the Future
4. Discussion
4.1. Environmental Effecting
4.2. Change of the Distribution Areas Under Future Climate Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cong, M.; Xu, Y.; Tang, L.; Yang, W.; Jian, M. Predicting the dynamic distribution of Sphagnum bogs in China under climate change since the last interglacial period. PLoS ONE 2020, 15, e0230969. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.P.; Arias, P.A.; Berger, S.; Canadell, J.G.; Cassou, C.; Chen, D.; Cherchi, A.; Connors, S.L.; Coppola, E.; Cruz, F.A.; et al. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change; Cambridge University Press: Cambridge, UK, 2023; pp. 3–32. [Google Scholar]
- Vorosmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Scholze, M.; Knorr, W.; Arnell, N.W.; Prentice, I.C. A climate-change risk analysis for world ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 13116–13120. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, X.P.; Zhang, Q.; Zhang, D.F.; Liu, X. Prediction for the potential distribution area of Codonopsis pilosula at global scale based on Maxent model. J. Appl. Ecol. 2017, 28, 992–1000. [Google Scholar]
- He, Y.; Ma, J.; Chen, G. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the maxent model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, K.; Duan, Y. Comparison of complete chloroplast genome sequences of Amygdalus pedunculata Pall. Nat. Biotechnol. 2020, 36, 2850–2859. [Google Scholar]
- Wang, W.; Yang, T.; Wang, H.L.; Li, Z.J.; Ni, J.W.; Su, S.; Xu, X.Q. Comparative and phylogenetic analyses of the complete chloroplast genomes of six almond species (Prunus spp. L.). Sci. Rep. 2020, 10, 10137. [Google Scholar] [CrossRef]
- Asgari, K.; Khadivi, A. Morphological and pomological characterizations of almond (Prunus amygdalus L.) genotypes to choose the late-blooming superiors. Euphytica 2021, 217, 42. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Yu, Z.; Zeng, B. Assembly and analysis of the complete mitochondrial genome of the Chinese wild dwarf almond (Prunus tenella). Front. Genet. 2024, 14, 1329060. [Google Scholar] [CrossRef]
- Peng, S.; Ting-Duan, Z.; Fu-Rong, L.; Wei, W.; Jian-Xun, L.; Chen, J. Superior Variety Selection and Overall Evaluations of Amygdalus tangutica in Western Sichuan. J. Sichuan For. Sci. 2017, 38, 79–84. [Google Scholar]
- Wang, X.Q.; Wang, J.X.; Ma, X.; Zhang, Y.Y.; Long, D.I. Effects of leaf extracts of Amorpha fruticosa on seed germination and seedling growth of Amygdalus pedunculata. J. Appl. Ecol. 2021, 32, 57–65. [Google Scholar]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S2), 19729–19736. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geog. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Lemmen, C.; Van Oosterom, P.; Bennett, R. The land administration domain model. Land Use Policy 2015, 49, 535–545. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? J. Ecol. 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hou, J.; Xiang, J.; Li, D.; Liu, X. Prediction of potential suitable distribution areas of Quasipaa spinosa in China based on MaxEnt optimization model. Biology 2023, 12, 366. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Hanley, J.A.; Mcneil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Raes, N.; ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Wittlinger, L.; Petrikovičová, L. Phytogeographical analysis and ecological factors of the distribution of Orchidaceae taxa in the Western Carpathians (Local study). Plants 2021, 10, 588. [Google Scholar] [CrossRef]
- Sun Lei, S.L.; Ding ChunRui, D.C. Nutritional components of Amygdalus communis L. and Amygdalus communis L. kernel oil in Xinjiang. China Oils Fats 2018, 43, 87–89. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2010, 29, 773–785. [Google Scholar] [CrossRef]
- Amin, P.; Akhavan-Ghalibaf, M. Assessment of Soils and Plants Loss as a Result of Darrezar Copper Mining in South-Eastern Iran. Eurasian J. Soil Sci. 2021, 54, 1608–1617. [Google Scholar] [CrossRef]
- Deng, G.; Li, M.; Hao, Z.; Shao, X. Responses to Climate Change of Maximum Latewood Density from Larix speciosa Cheng et Law and Abies delavayi Franch. in the Northwest of Yunnan Province, China. Forests 2022, 13, 720. [Google Scholar] [CrossRef]
- Pan, J.; Fan, X.; Luo, S.; Zhang, Y.; Qian, Z. Predicting the Potential Distribution of Two Varieties of Litsea coreana (Leopard-Skin Camphor) in China under Climate Change. Forests 2020, 11, 1159. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Wang, J.; Di, L.; Sikdar, A. The Effects of Leaf Extracts of Four Tree Species on Amygdalus pedunculata Seedlings Growth. Front Plant Sci. 2021, 11, 587579. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wu, T.; Wu, P.; Shi, S. Amygdalin isolated from Amygdalus mongolica protects against hepatic fibrosis in rats. Acta Pharm. 2021, 71, 459–471. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, R.; Bai, S.; Gao, X.; Liu, M.; Yan, W. Mongolian almond (Prunus mongolica Maxim): The morpho-physiological, biochemical and transcriptomic response to drought stress. PLoS ONE 2015, 10, e0124442. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, H.; Kong, D.; Yan, F.; Liu, D.; Zhang, Y. Effects of gradual soil drought stress on the growth, biomass partitioning, and chlorophyll fluorescence of Prunus mongolica seedlings. Turk. J. Biol. 2015, 39, 532–539. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, Z.S.; Lidon, F.J.; Kaimakanova, M. Plant physiological responses to UV-B radiation. Emir. J. Food Agric. 2012, 24, 481–501. [Google Scholar] [CrossRef]
- Zuk-Golaszewska, K.; Upadhyaya, M.; Golaszewski, J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003, 49, 135–140. [Google Scholar] [CrossRef]
- Mannucci, A.; Mariotti, L.; Castagna, A.; Santin, M.; Trivellini, A.; Reyes, T.H.; Mensuali-Sodi, A.; Ranieri, A.; Quartacci, M.F. Hormone profile changes occur in roots and leaves of Micro-Tom tomato plants when exposing the aerial part to low doses of UV-B radiation. Plant Physiol Bioch. 2020, 148, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021, 187, 1096–1103. [Google Scholar] [CrossRef]
- Garcia-Corral, L.S.; Holding, J.M.; Carrillo-De-Albornoz, P.; Steckbauer, A.; Pérez-Lorenzo, M.; Navarro, N.; Serret, P.; Duarte, C.M.; Agusti, S. Effects of UVB radiation on net community production in the upper global ocean. Glob. Ecol. Biogeogr. 2016, 26, 54–64. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Rozema, J.; Björn, L.O.; Bornman, J.; Gaberščik, A.; Häder, D.-P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R. The role of UV-B radiation in aquatic and terrestrial ecosystems—An experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Miao, G.; Zhao, Y.; Wang, Y.; Yu, C.; Xiong, F.; Sun, Y.; Cao, Y. Suitable Habitat Prediction and Analysis of Dendrolimus houi and Its Host Cupressus funebris in the Chinese Region. Forests 2024, 15, 162. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Tatzber, M.; Gerzabek, M.H. Soil organic-matter stocks and characteristics along an Alpine elevation gradient. J. Plant Nutr. Soil Sci. 2010, 173, 30–38. [Google Scholar] [CrossRef]
- Neina, D.J.A. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Grabherr, G. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; The Australian National University: Canberra, Australia, 2023; pp. 1–34. [Google Scholar]
- Hong, D.Y.; Zhou, S.; He, X.; Yuan, J.; Zhang, X. Current status of wild tree peony species with special reference to conservation. Biodivers. Sci. 2017, 25, 781–793. [Google Scholar] [CrossRef]
- Lu, K.; He, Y.-M.; Mao, W.; Zy, D.; Wang, L.-J.; Liu, G.-M.; Feng, W.-J.; Duan, Y.-Z. Potential geographical distribution and changes of Artemisia ordosica in China under future climate change. J. Appl. Ecol. 2020, 31, 3758–3766. [Google Scholar]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Zhang, J.M.; Song, M.; Li, Z.J.; Peng, X.; Su, S.; Li, B.; Xu, X.Q.; Wang, W. Effects of Climate Change on the Distribution of Akebia quinata. Front. Ecol. Evol. 2021, 9, 752682. [Google Scholar] [CrossRef]
- Temirbayeva, K.; Zhang, M.-L. Molecular phylogenetic and biogeographical analysis of Nitraria based on nuclear and chloroplast DNA sequences. Plant Syst. Evol. 2015, 301, 1897–1906. [Google Scholar] [CrossRef]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J. Arid Environ. 2021, 184, 104328. [Google Scholar] [CrossRef]
- Lioubimtseva, E.; Henebry, G.M. Climate and environmental change in arid Central Asia: Impacts, vulnerability, and adaptations. J. Arid Environ. 2009, 73, 963–977. [Google Scholar] [CrossRef]
- VanDerWal, J.; Murphy, H.T.; Kutt, A.S.; Perkins, G.C.; Bateman, B.L.; Perry, J.J.; Reside, A.E. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Chang. 2013, 3, 239–243. [Google Scholar] [CrossRef]
- Tian, L.; Benton, M.J. Predicting biotic responses to future climate warming with classic ecogeographic rules. Curr. Biol. 2020, 30, R744–R749. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tao, J. Predicting the areas of suitable distribution for Zelkova serrata in China under climate change. Sustainability 2021, 13, 1493. [Google Scholar] [CrossRef]
- Quamme, H.A.; Layne, R.E.C.; Ronald, W.G. Relationship of supercooling to cold hardiness and the northern distribution of several cultivated and native Prunus species and hybrids. Can. J. Plant Sci. 1982, 62, 137–148. [Google Scholar] [CrossRef]



| Environment Variable | Description | Unit |
|---|---|---|
| Bio1 | Annual mean temperature temp | °C |
| Bio2 | Mean diurnal temperature range | °C |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | - |
| Bio4 | Temperature seasonality (standard deviation×100) | - |
| Bio5 | Maximum temperature of the warmest month | |
| Bio6 | Min temperature of coldest month | °C |
| Bio7 | Range of annual temperature variation | °C |
| Bio8 | Mean temperature of the wettest quarter | °C |
| Bio9 | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm |
| Bio14 | Precipitation of driest month | mm |
| Bio15 | Precipitation seasonality (coefficient of variation) | mm |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of the driest quarter | mm |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
| SC | Soil organic carbon | g/kg |
| SpH | Soil pH | - |
| ST | Soil texture | - |
| UVB1 | Annual mean UV-B | J/m2/day |
| UVB2 | UV-B seasonality | J/m2/day |
| UVB3 | Mean UV-B of lightest month | J/m2/day |
| UVB4 | Mean UV-B of lowest month | J/m2/day |
| DEM | Digital Elevation Model | m |
| Aspect | Aspect | - |
| Slope | Slope | ° |
| Environment Variable | Unit | Contribution (%) | |||||
|---|---|---|---|---|---|---|---|
| P. amygdalus | P. tenella | P. mongolica | P. pedunculata | P. tangutica | P. triloba | ||
| Bio1 | °C | × | × | × | × | × | 59.9 |
| Bio2 | °C | 3.3 | 5.6 | × | × | × | 4.2 |
| Bio4 | - | × | × | 26.7 | × | × | × |
| Bio6 | °C | 20.2 | × | × | × | × | × |
| Bio8 | °C | 11.6 | × | × | × | × | × |
| Bio9 | °C | × | × | × | × | 20.3 | × |
| Bio11 | °C | × | 31.0 | × | × | × | × |
| Bio12 | mm | × | 5.5 | × | × | × | × |
| Bio13 | mm | × | × | × | 2.9 | × | × |
| Bio14 | mm | × | 38.4 | 3.8 | 0.3 | 3.8 | 1.8 |
| Bio15 | - | × | × | × | 19.6 | × | 1.1 |
| Bio16 | mm | 8.1 | × | × | × | × | × |
| Bio18 | mm | × | × | 6.8 | × | 24.0 | 15.9 |
| Bio19 | mm | 24.0 | × | 36.3 | 19.8 | 15.0 | 11.6 |
| SpH | - | × | 3.7 | × | 2.6 | × | × |
| UVB2 | J/m2/day | 15.5 | × | × | × | × | × |
| UVB4 | J/m2/day | 17.2 | 15.4 | × | 41.3 | × | × |
| DEM | m | × | × | 26.4 | 13.2 | 36.9 | 3.6 |
| Slope | ° | × | × | × | 0.3 | × | 1.9 |
| Species | Period | Poorly Suitable Area | Moderately Suitable Area | Highly Suitable Area | Total Suitable Area | |
|---|---|---|---|---|---|---|
| Area of each suitable area ×106 km2 (change in the area compared to current) | ||||||
| P. amygdalus | Current | - | 13.76 | 5.14 | 3.82 | 22.16 |
| ssp2.45 | 2050 | 21.49 (56.18%) | 7.06 (37.33%) | 3.75 (14.94%) | 32.30 (45.74%) | |
| 2070 | 12.98 (−5.70%) | 5.64 (9.69%) | 3.19 (−2.10%) | 21.81 (−1.60%) | ||
| ssp5.85 | 2050 | 12.71 (−7.62%) | 5.79 (12.48%) | 3.51 (7.55%) | 22.00 (−0.72%) | |
| 2070 | 12.36 (−10.18%) | 5.46 (6.24%) | 3.47 (6.31%) | 21.29 (−3.95%) | ||
| P. tenella | Current | - | 9.70 | 5.04 | 3.82 | 18.56 |
| ssp2.45 | 2050 | 9.45 (−2.58%) | 4.73 (−6.16%) | 4.03 (5.42%) | 18.21 (−1.91%) | |
| 2070 | 10.42 (7.41%) | 5.19 (3.06%) | 4.05 (6.11%) | 19.67 (5.96%) | ||
| ssp5.85 | 2050 | 9.93 (2.29%) | 4.80 (−4.66%) | 3.80 (−0.55%) | 18.53 (0.18%) | |
| 2070 | 10.91 (12.42%) | 4.51 (−10.56%) | 3.98 (4.29%) | 19.40 (4.51%) | ||
| P. mongolica | Current | - | 1.88 | 1.07 | 0.82 | 3.77 |
| ssp2.45 | 2050 | 2.38 (26.31%) | 1.47 (37.06%) | 1.16 (41.42%) | 5.00 (32.65%) | |
| 2070 | 2.32 (23.11%) | 1.51 (40.68%) | 1.12 (36.53%) | 4.94 (31.02%) | ||
| ssp5.85 | 2050 | 2.35 (24.89%) | 1.26 (17.80%) | 0.98 (19.24%) | 4.59 (21.65%) | |
| 2070 | 2.38 (26.60%) | 1.43 (33.22%) | 1.05 (28.49%) | 4.86 (28.89%) | ||
| P. tangutica | Current | - | 0.98 | 0.38 | 0.16 | 1.52 |
| ssp2.45 | 2050 | 0.96 (−1.92%) | 0.46 (19.70%) | 0.21 (33.13%) | 1.63 (7.17%) | |
| 2070 | 0.81 (−16.63%) | 0.37 (−3.58%) | 0.18 (13.53%) | 1.36 (−10.22%) | ||
| ssp5.85 | 2050 | 0.98 (0.54%) | 0.41 (7.58%) | 0.19 (20.37%) | 1.58 (4.37%) | |
| 2070 | 0.87 (−10.78%) | 0.40 (3.53%) | 0.18 (15.33%) | 1.45 (−4.47%) | ||
| P. pedunculata | Current | - | 3.06 | 1.23 | 1.33 | 5.62 |
| ssp2.45 | 2050 | 2.44 (−20.00%) | 1.08 (−12.71%) | 1.35 (1.92%) | 4.87 (−13.22%) | |
| 2070 | 2.78 (−9.01%) | 1.12(−8.93%) | 1.23 (−6.94%) | 5.14 (−8.50%) | ||
| ssp5.85 | 2050 | 2.80 (−8.20%) | 1.07 (−13.52%) | 1.39 (4.45%) | 5.26 (−6.38%) | |
| 2070 | 2.64 (−13.71%) | 0.99 (−20.11%) | 1.32 (−0.44%) | 4.94 (−11.98%) | ||
| P. triloba | Current | - | 15.56 | 7.34 | 2.05 | 24.95 |
| ssp2.45 | 2050 | 19.25 (23.69%) | 8.60 (17.24%) | 2.44 (18.90%) | 30.29 (21.40%) | |
| 2070 | 19.14 (23.00%) | 8.92 (21.54%) | 2.72 (32.60%) | 30.78 (23.36%) | ||
| ssp5.85 | 2050 | 20.58 (32.28%) | 8.69 (18.41%) | 2.51 (22.50%) | 31.79 (27.40%) | |
| 2070 | 17.70 (13.76%) | 8.13 (10.78%) | 2.47 (20.19%) | 28.30 (13.42%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Liu, M.; Hu, K.; Liu, Y.; He, Y.; Bai, H.; Du, Z.; Duan, Y. Potential Global Distribution and Habitat Shift of Prunus subg. Amygdalus Under Current and Future Climate Change. Forests 2024, 15, 1848. https://doi.org/10.3390/f15111848
Lu K, Liu M, Hu K, Liu Y, He Y, Bai H, Du Z, Duan Y. Potential Global Distribution and Habitat Shift of Prunus subg. Amygdalus Under Current and Future Climate Change. Forests. 2024; 15(11):1848. https://doi.org/10.3390/f15111848
Chicago/Turabian StyleLu, Ke, Mili Liu, Kui Hu, Yang Liu, Yiming He, Huihui Bai, Zhongyu Du, and Yizhong Duan. 2024. "Potential Global Distribution and Habitat Shift of Prunus subg. Amygdalus Under Current and Future Climate Change" Forests 15, no. 11: 1848. https://doi.org/10.3390/f15111848
APA StyleLu, K., Liu, M., Hu, K., Liu, Y., He, Y., Bai, H., Du, Z., & Duan, Y. (2024). Potential Global Distribution and Habitat Shift of Prunus subg. Amygdalus Under Current and Future Climate Change. Forests, 15(11), 1848. https://doi.org/10.3390/f15111848





