Conservation Genetics of the Only Honeysuckle Azalea (Rhododendron luteum) Population Present in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction
2.3. PCR Amplification Using Inter-Simple Sequence Repeat (ISSR)
2.4. Gel Electrophoresis
2.5. Data Analysis
3. Results
3.1. DNA Extraction and Genetic Diversity
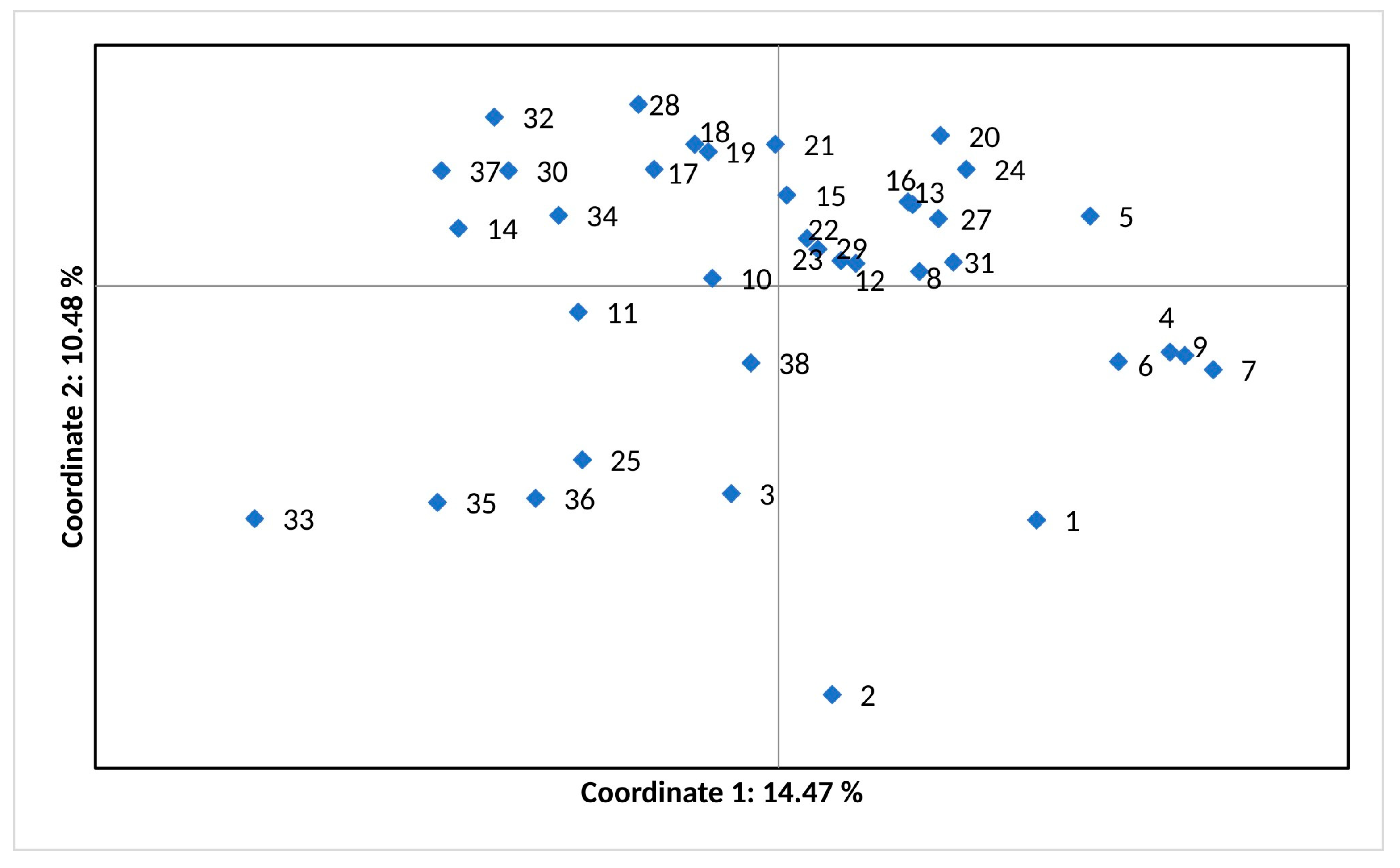
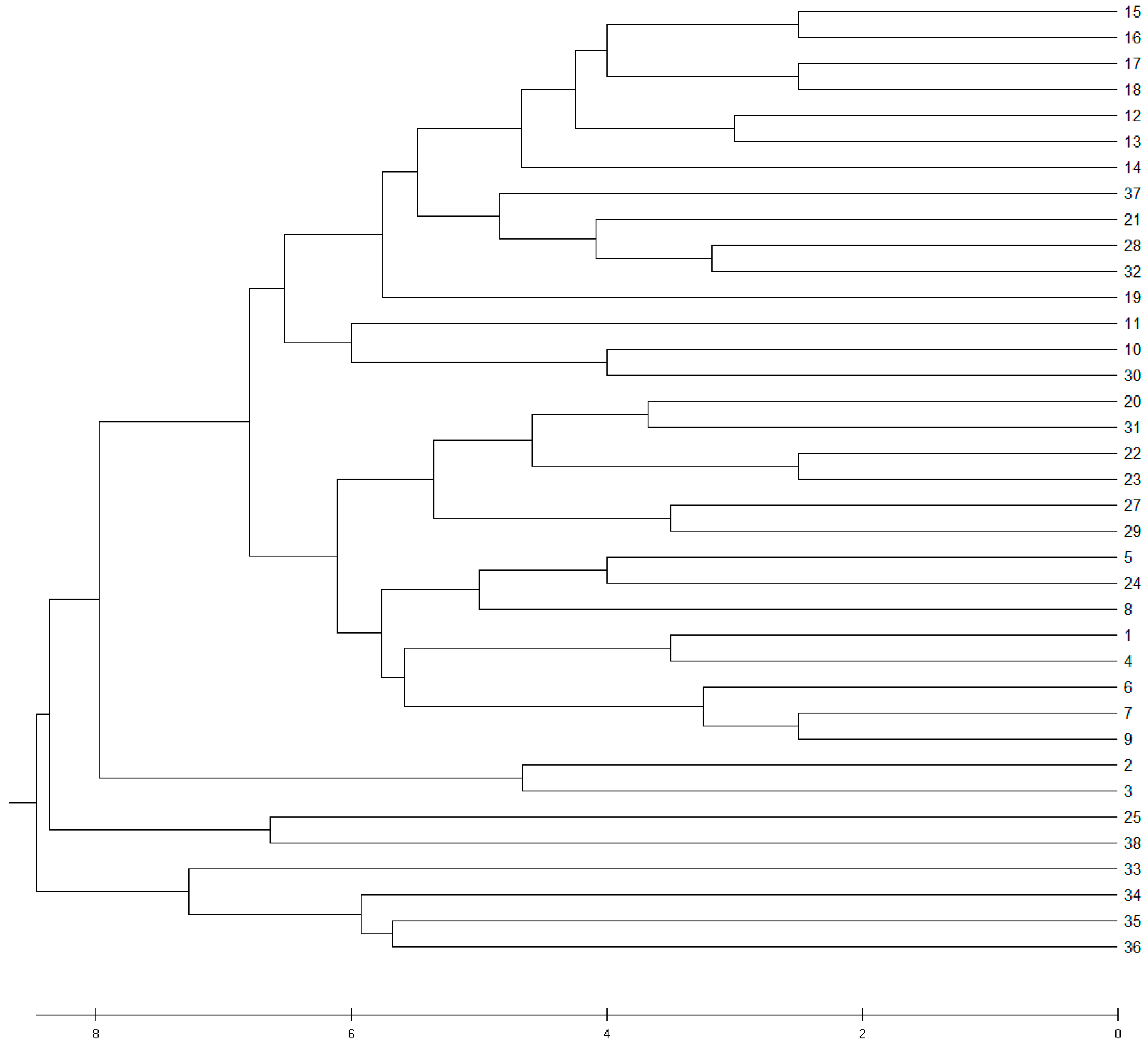
3.2. Genetic Differentiation
3.3. Spatial Autocorrelation Analysis
4. Discussion
5. Conclusions
- Expansion of the GR4110003 NATURA2000 protected area in order to include all of the R. luteum natural distribution in Lesvos Island.
- Establishment of an in situ gene conservation unit (GCU) within the European Forest Genetic Resources Programme (EUFORGEN; https://www.euforgen.org/) network; as all the respective minimum requirements [52] are met, the species is characterised in Greece as vulnerable (VU; IUCN) while this population is the only natural occurrence of the species in the country. This unit can also take the form of a conglomeration of small conservation micro-reserves [53], depending on the distribution of genetic variability within and among sub-populations, and given the association between altitude and genotype already found.
- Ex situ conservation, which can be enacted initially by the establishment of (a) a seed collection according to genetic conservation principles and (b) a plantation by seed outside the natural range.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zonneveld, B.J.M. New Record Holders for Maximum Genome Size in Eudicots and Monocots. J. Bot. 2010, 2010, 527357. [Google Scholar] [CrossRef]
- Kron, K.A. A Revision of Rhododendron Section Pentanthera. Edinb. J. Bot. 1993, 50, 249–364. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Zhou, Y.; Tu, M.; Wu, Z.; Gui, D.; Ma, Y.; Wang, J.; Zhang, C. The Rhododendron Plant Genome Database (RPGD): A Comprehensive Online Omics Database for Rhododendron. BMC Genom. 2021, 22, 376. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.S. Grayanotoxins of Rhododendron ponticum. Phytochemistry 1985, 24, 2225–2227. [Google Scholar]
- Qiang, Y.; Zhou, B.; Gao, K. Chemical Constituents of Plants from the Genus Rhododendron. Chem. Biodivers. 2011, 8, 792–815. [Google Scholar] [CrossRef] [PubMed]
- Caprar, M.; Cantor, M.; Sicora, O.; Copaci, C.; Sicora, C. Optimization of DNA Isolation from Four Species of Rhododendron from Europe. J. Hortic. Sci. For. Biotechnol. 2014, 18, 117–122. [Google Scholar]
- Demir, S.; Turan, I.; Aliyazicioglu, Y. Selective Cytotoxic Effect of Rhododendron luteum Extract on Human Colon and Liver Cancer Cells. J. BUON 2016, 21, 883–888. [Google Scholar]
- Popescu, R.; Kopp, B. The Genus Rhododendron: An Ethnopharmacological and Toxicological Review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef]
- Tasdemir, D.; Demirci, B.; Demirci, F.; Dönmez, A.A.; Baser, K.H.C.; Rüedia, P. Analysis of the Volatile Components of Five Turkish Rhododendron Species by Headspace Solid-Phase Microextraction and GC-MS (HS-SPME-GC-MS). Z. Für Naturforsch. C 2003, 58, 797–803. [Google Scholar] [CrossRef]
- Usta, A.; Yayli, B.; Kahrinman, N.; Karaoglu, S.A.; Yayli, N. Composition and Antimicrobial Activity of Essential Oil from the Flower of Rhododendron luteum Sweet. Asian J. Chem. 2012, 24, 4229. [Google Scholar]
- Erdemoglu, N.; Küpeli, E.; Yeşilada, E. Anti-Inflammatory and Antinociceptive Activity Assessment of Plants Used as Remedy in Turkish Folk Medicine. J. Ethnopharmacol. 2003, 89, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Katsikaros, G.; Matargas, D.; Migiros, G.; Triantafyllidis, E. Geological Survey of Lesvos; Institute of Geology and Mineral Exploration: Thessaloniki, Greece, 1982. [Google Scholar]
- Biel, B. Contributions to the Flora of the Aegean Islands of Lesvos and Limnos, Greece. Willdenowia 2002, 32, 209–219. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A.; Panetsos, K.P. Genetics of the Evolutionary Process of Trees and Shrubs in Lesvos Island. In Proceedings of the 1st Scientific Symposium: Petrified Forest of Lesvos: A Natural Monument, Mytilene, Greece, 26–28 April 1996; Museum of the Petrified Forest of Lesvos Publication: Mytilene, Greece, 1996; pp. 117–135. [Google Scholar]
- Aravanopoulos, F.A.; Panetsos, K.P. Genetics and Breeding of Brutia Pine (Pinus brutia Ten.) in Lesvos Island. Geotech. Sci. Issues 1998, 9, 10–19. [Google Scholar]
- Hecht, J. Zur Geologie von Südost-Lesbos (Griechenland). Z. Der Dtsch. Geol. Ges. 1972, 123, 423–432. [Google Scholar]
- Bazos, I. Study of the Flora and Vegetation of Lesvos (East Aegean Islands, Greece). Ph.D. Thesis, National and Capodistrian University of Athens, Athens, Greece, 2005. [Google Scholar]
- Bazos, I.; Yannitsaros, A. Floristic Reports from the Island of Lesvos (Greece) I. Dicotyledones: Aceraceae to Guttiferae. Edinb. J. Bot. 2004, 61, 49–86. [Google Scholar] [CrossRef]
- Sawidis, T.; Theodoridou, T.; Weryszko-Chmielewska, E.; Bosabalidis, A. Structural Features of Rhododendron luteum Flower. Biologia 2011, 66, 610–617. [Google Scholar] [CrossRef]
- Xystrakis, F.; Theodorou, K.; Maniati, A.; Eleftheriadou, E.; Bazos, I.; Dimopoulos, P. Plant Communities of Rhododendron luteum Sweet in Its Unique Appearance in Greece, in The Island of Lesvos. In Proceedings of the 18th Pan-Hellenic Forestry Conference, Edessa, Greece, 8–11 October 2017; pp. 401–409. [Google Scholar]
- Gribilakou, L.; Krallis, F.; Petanidou, T. Mapping of the Rhododendron luteum Sweet Distribution in the Forests of Western Lesvos. In Proceedings of the 1st Pan-Hellenic Ecology Conference, Thessaloniki, Greece, 5–7 May 2004; Hellenic Ecological Society Publication: Thessaloniki, Greece, 2004. [Google Scholar]
- Reisch, C.; Rosbakh, S. Patterns of Genetic Variation in European Plant Species Depend on Altitude. Divers. Distrib. 2021, 27, 157–163. [Google Scholar] [CrossRef]
- Ohsawa, T.; Ide, Y. Global Patterns of Genetic Variation in Plant Species along Vertical and Horizontal Gradients on Mountains. Glob. Ecol. Biogeogr. 2008, 17, 152–163. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Kobayashi, N. A Simple and Efficient DNA Extraction Method for Plants, Especially Woody Plants. Plant Tissue Cult. Biotech. 1998, 4, 76–80. [Google Scholar]
- Antoniadi, A. Investigation of Protocols for High Quality DNA Isolation from Rhododendron luteum Sweet Originating from Lesvos Island for Downstream Application in the Conservation of Genetic Resources and Molecular Breeding. Master’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2018. [Google Scholar]
- Xu, J.J.; Zhang, L.Y.; Zhao, B.; Shen, H.F. Assessment of Genetic Diversity among Six Populations of Rhododendron triflorum in Tibet Using ISSR and AFLP Markers. South Afr. J. Bot. 2017, 108, 175–183. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Xing, M.; Zhao, W.; Fan, R.-J.; Luo, S.; Chen, X. Genetic Diversity Analysis of Rhododendron aureum Georgi (Ericaceae) Located on Changbai Mountain Using ISSR and RAPD Markers. Plant Syst. Evol. 2012, 298, 921–930. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, H.; Zhang, M.; Qiao, G.; Shen, X. IRAPs in Combination with Highly Informative ISSRs Confer Effective Potentials for Genetic Diversity and Fidelity Assessment in Rhododendron. Int. J. Mol. Sci. 2023, 24, 6902. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhao, B.; Shen, H.F.; Huang, W.M.; Yuan, L.X. Assessment of Genetic Relationship among Rhododendron Cultivars Using Amplified Fragment Length Polymorphism and Inter-Simple Sequence Repeat Markers. Genet. Mol. Res. 2016, 15, gmr.15038467. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research--an Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Huff, D.R.; Peakall, R.; Smouse, P.E. RAPD Variation within and among Natural Populations of Outcrossing Buffalograss [Buchloë Dactyloides (Nutt.) Engelm. Theor. Appl. Genet. 1993, 86, 927–934. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R. Spatial Autocorrelation Analysis of Individual Multiallele and Multilocus Genetic Structure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R.; Gonzales, E. A Heterogeneity Test for Fine-Scale Genetic Structure. Mol. Ecol. 2008, 17, 3389–3400. [Google Scholar] [CrossRef]
- Jones, O.R.; Wang, J. COLONY: A Program for Parentage and Sibship Inference from Multilocus Genotype Data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. A New Method for Estimating Effective Population Sizes from a Single Sample of Multilocus Genotypes. Mol. Ecol. 2009, 18, 2148–2164. [Google Scholar] [CrossRef] [PubMed]
- Trung, L.T.; Duong, L.A.; Tien, T.V.; Trieu, L.N.; Hai, P.H. Genetic Diversity of Naturally Distributed Rhododendron moulmainense Hook. f. Populations in Lam Vien Plateau, Vietnam Revealed by ISSR and SCoT Markers. Malays. Appl. Biol. 2020, 49, 41–52. [Google Scholar] [CrossRef]
- Wu, F.Q.; Shen, S.K.; Zhang, X.J.; Wang, Y.H.; Sun, W.B. Genetic Diversity and Population Structure of an Extremely Endangered Species: The World’s Largest Rhododendron. AoB Plants 2015, 7, plu082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yin, Z.; Xu, M.; Wang, Q. AFLP Analysis of Genetic Variation in Wild Populations of Five Rhododendron Species in Qinling Mountain in China. Biochem. Syst. Ecol. 2012, 45, 198–205. [Google Scholar] [CrossRef]
- Zhou, L.; Wan, Y.; Zhang, L. Genetic Diversity and Relationship of 43 Rhododendron Sp. Based on RAPD Analysis. Bot. Res. J. 2009, 2, 1–6. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Hale, M.L.; Burg, T.M.; Steeves, T.E. Sampling for Microsatellite-Based Population Genetic Studies: 25 to 30 Individuals per Population Is Enough to Accurately Estimate Allele Frequencies. PLoS ONE 2012, 7, e45170. [Google Scholar] [CrossRef]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002; ISBN 0-521-63985-9. [Google Scholar]
- Yoichi, W.; Takahashi, M.; Nagano, A.J.; Uehara, K.; Abe, H. Evolutionary Effects of Geographic and Climatic Isolation between Rhododendron tsusiophyllum Populations on the Izu Islands and Mainland Honshu of Japan. Heredity 2021, 126, 859–868. [Google Scholar] [CrossRef]
- Kwon, J.A.; Morden, C.W. Population Genetic Structure of Two Rare Tree Species (Colubrina oppositifolia and Alphitonia ponderosa, Rhamnaceae) from Hawaiian Dry and Mesic Forests Using Random Amplified Polymorphic DNA Markers. Mol. Ecol. 2002, 11, 991–1001. [Google Scholar] [CrossRef]
- Funk, W.C.; Lovich, R.E.; Hohenlohe, P.A.; Hofman, C.A.; Morrison, S.A.; Sillett, T.S.; Ghalambor, C.K.; Maldonado, J.E.; Rick, T.C.; Day, M.D.; et al. Adaptive Divergence despite Strong Genetic Drift: Genomic Analysis of the Evolutionary Mechanisms Causing Genetic Differentiation in the Island Fox (Urocyon littoralis). Mol. Ecol. 2016, 25, 2176–2194. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, Y.; Isagi, Y.; Nakagoshi, N. Patterns and Levels of Gene Flow in Rhododendron metternichii var. Hondoense Revealed by Microsatellite Analysis. Mol. Ecol. 2001, 10, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Koskela, J.; Lefèvre, F.; Schueler, S.; Kraigher, H.; Olrik, D.C.; Hubert, J.; Longauer, R.; Bozzano, M.; Yrjänä, L.; Alizoti, P.; et al. Translating Conservation Genetics into Management: Pan-European Minimum Requirements for Dynamic Conservation Units of Forest Tree Genetic Diversity. Biol. Conserv. 2013, 157, 39–49. [Google Scholar] [CrossRef]
- Eliades, N.-G.H.; Papageorgiou, A.C.; Fady, B.; Gailing, O.; Leinemann, L.; Finkeldey, R. An Approach to Genetic Resources Conservation of Peripheral Isolated Plant Populations: The Case of an Island Narrow Endemic Species. Biol. Conserv. 2019, 28, 3005–3035. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Genetic Monitoring in Natural Perennial Plant Populations. Botany 2011, 89, 75–81. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Conservation and Monitoring of Tree Genetic Resources in Temperate Forests. Curr. For. Rep. 2016, 2, 119–129. [Google Scholar] [CrossRef]
- Bajc, M.; Aravanopoulos, F.A.; Westergren, M.; Fussi, B.; Kavaliauskas, D.; Alizoti, P.; Kiourtsis, F.; Kraigher, H. (Eds.) Manual for Forest Genetic Monitoring; Slovenian Forestry Institute, Silva Slovenica Publishing Centre: Ljubljana, Slovenia, 2020. [Google Scholar] [CrossRef]
| Species | Genetic Entry | Marker | P (%) | Ne | He | I | Reference |
|---|---|---|---|---|---|---|---|
| R. luteum | Natural population | ISSR | 90.20 | 1.446 | 0.275 | 0.410 | This study |
| R. triflorum | Natural populations | ISSR | 98.30 | 1.575 | 0.338 | 0.508 | [27] |
| R. aureum | Natural populations | ISSR | 87.43 | - | - | 0.459 | [28] |
| R. moulmainense | Natural populations | ISSR | 57.58 | - | 0.163 | 0.256 | [39] |
| Rhododendron sp. | Accessions | ISSR | 91.90 | 1.690 | 0.390 | 0.570 | [29] |
| Rhododendron sp. | Cultivars | ISSR | 96.99 | - | - | - | [30] |
| R. triflorum | Natural populations | AFLP | 95.86 | 1.959 | 0.306 | 0.464 | [27] |
| R. protistum var. giganteum | Natural populations | AFLP | 66.67 | - | 0.240 | 0.358 | [40] |
| Rhododendron, 5 species | Natural populations | AFLP | 92.84 | - | - | 0.556 | [41] |
| R. aureum | Natural populations | RAPD | 95.16 | - | - | 0.479 | [28] |
| Rhododendron, 29 species | Accessions | RAPD | 98.03 | - | - | - | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravanopoulos, F.A.; Tourvas, N.; Fotsinos, S.; Michailidou, C.; Antoniadi, A. Conservation Genetics of the Only Honeysuckle Azalea (Rhododendron luteum) Population Present in Greece. Forests 2024, 15, 5. https://doi.org/10.3390/f15010005
Aravanopoulos FA, Tourvas N, Fotsinos S, Michailidou C, Antoniadi A. Conservation Genetics of the Only Honeysuckle Azalea (Rhododendron luteum) Population Present in Greece. Forests. 2024; 15(1):5. https://doi.org/10.3390/f15010005
Chicago/Turabian StyleAravanopoulos, F. A., N. Tourvas, S. Fotsinos, C. Michailidou, and A. Antoniadi. 2024. "Conservation Genetics of the Only Honeysuckle Azalea (Rhododendron luteum) Population Present in Greece" Forests 15, no. 1: 5. https://doi.org/10.3390/f15010005
APA StyleAravanopoulos, F. A., Tourvas, N., Fotsinos, S., Michailidou, C., & Antoniadi, A. (2024). Conservation Genetics of the Only Honeysuckle Azalea (Rhododendron luteum) Population Present in Greece. Forests, 15(1), 5. https://doi.org/10.3390/f15010005







