Research History of Forest Gap as Small-Scale Disturbances in Forest Ecosystems
Abstract
1. Introduction
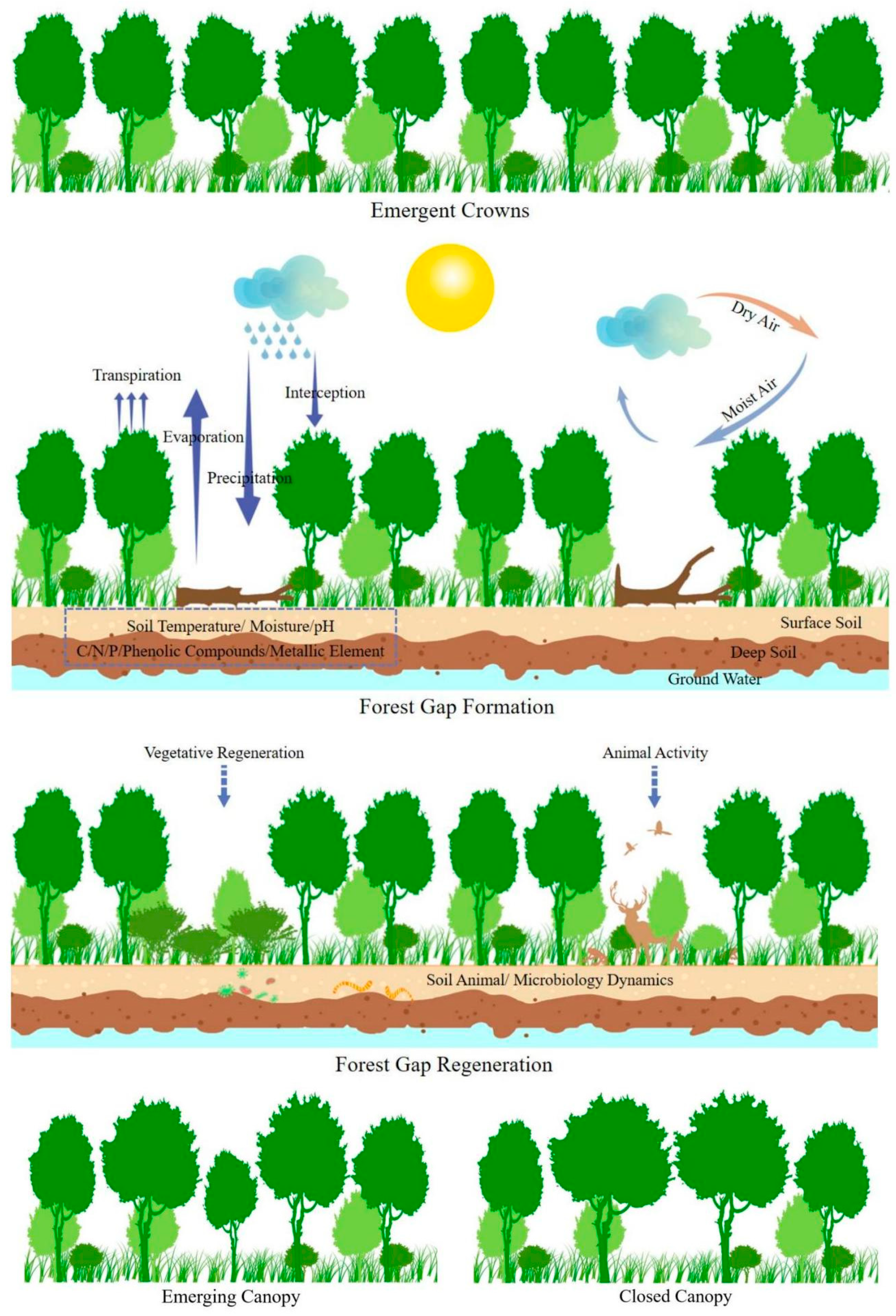
2. How/Why Forest Gaps Are Formed
3. Basic Characteristics and Measurement of Forest Gaps
4. Microenvironmental Changes within Forest Gaps
5. Effects of Forest Gap Disturbance on Plants
6. Effects of Forest Gap Disturbance on Animals
7. Effects of Forest Gap Disturbance on Soil Fauna and Microorganisms
8. Effects of Forest Gap Disturbance on Regeneration
9. Effects of Forest Gap Disturbance on Forest Succession
10. Conclusions and Recommendations
- The concept of forest gaps has yet to be standardized. Forest gaps occur for various and complicated reasons; researchers always define them using the occurrence reasons, which makes the research on forest gaps too fragmented. Whether and how to standardize the concept of forest gaps is important for global research on forest gaps.
- With the advances in science and technology, many new methods have been used in forest gap research, but there needs to be a uniform specification. The same or similar principles of research methods help the horizontal comparative analysis of the research. Forest gap models are diversified, with their focus and minor scope of application. Multi-dimensional and multi-parameter forest gap models must be established to improve the accuracy of model prediction so that the model can be applied more widely.
- Current research needs coherence among forest gaps, and the focus of research varies from one area to another, lacking comparison of different types of areas and long-term multi-directional monitoring of a single area, which makes it difficult to break out of the current situation. In China, forest gaps are mainly researched in the secondary forests of Northeast China and the alpine forests of Southwest China. While some progress has been made, there still needs to be more understanding of the role of forest gaps in the forest succession process and new forests at the end of the succession.
- The renewal succession process of forest gaps provides a reference for different types of forest ecosystem services. Forest managers can enhance ecosystem services by controlling the renewal of forest gaps, promoting the aesthetic services of forest landscapes, and strengthening the coordinated development of the economy, society, management, and ecology [217,218].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watt, A.S. On the Ecology of British Beechwoods with Special Reference to Their Regeneration: Part II, Sections II and III. The Development and Structure of Beech Communities on the Sussex Downs. J. Ecol. 1925, 13, 27–73. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, J.; Zhang, G.; Sun, Y.; Sun, Y.; Hu, L.; Wang, G.G. Disentangling regeneration by vertical stratification: A 17-year gap-filling process in a temperate secondary forest. For. Ecol. Manag. 2023, 539, 120994. [Google Scholar] [CrossRef]
- Horváth, C.V.; Kovács, B.; Tinya, F.; Schadeck Locatelli, J.; Németh, C.; Crecco, L.; Illés, G.; Csépányi, P.; Ódor, P. A matter of size and shape: Microclimatic changes induced by experimental gap openings in a sessile oak–hornbeam forest. Sci. Total Environ. 2023, 873, 162302. [Google Scholar] [CrossRef] [PubMed]
- Runkle, J.R. Patterns of Disturbance in Some Old-Growth Mesic Forests of Eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Patterson, C.P.; Hackworth, Z.J.; Lhotka, J.M.; Stringer, J.W. Light and regeneration patterns following silvicultural gap establishment in Quercus dominated stands of the northern Cumberland Plateau, USA. For. Ecol. Manag. 2022, 505, 119871. [Google Scholar] [CrossRef]
- Lima, J.; Guilherme, E. Birds associated with treefall gaps in a lowland forest in southwestern Brazilian Amazonia. Acta Amaz. 2021, 51, 42–51. [Google Scholar] [CrossRef]
- Izbicki, B.J.; Alexander, H.D.; Paulson, A.K.; Frey, B.R.; McEwan, R.W.; Berry, A.I. Prescribed fire and natural canopy gap disturbances: Impacts on upland oak regeneration. For. Ecol. Manag. 2020, 465, 18107. [Google Scholar] [CrossRef]
- Greenberg, C.H. Long-term recovery dynamics following hurricane-related wind disturbance in a southern Appalachian forest. For. Ecol. Manag. 2021, 502, 119704. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Hogan, J.A.; Rocha, O.J. Effects of canopy openness on seedling survival and growth after selective logging in a monodominant lowland swamp forest in Costa Rica. J. Trop. For. Sci. 2022, 34, 34–47. [Google Scholar] [CrossRef]
- Modrow, T.; Kuehne, C.; Saha, S.; Bauhus, J.; Pyttel, P.L. Photosynthetic performance, height growth, and dominance of naturally regenerated sessile oak (Quercus petraea [Mattuschka] Liebl.) seedlings in small-scale canopy openings of varying sizes. Eur. J. For. Res. 2020, 139, 41–52. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Harvey, B.D.; Reyes, G.P.; Caron, M.N.; Barlow, S. Spruce budworm, windthrow and partial cutting: Do different partial disturbances produce different forest structures? For. Ecol. Manag. 2011, 262, 482–490. [Google Scholar] [CrossRef]
- York, R.A.; Noble, H.; Quinn-Davidson, L.N.; Battles, J.J. Pyrosilviculture: Combining prescribed fire with gap-based silviculture in mixed-conifer forests of the Sierra Nevada. Can. J. For. Res. 2021, 51, 781–791. [Google Scholar] [CrossRef]
- Panferov, O.; Sogachev, A. Influence of gap size on wind damage variables in a forest. Agric. For. Meteorol. 2008, 148, 1869–1881. [Google Scholar] [CrossRef]
- McNab, W.H.; Greenberg, C.H.; Berg, E.C. Landscape distribution and characteristics of large hurricane-related canopy gaps in a southern Appalachian watershed. For. Ecol. Manag. 2004, 196, 435–447. [Google Scholar] [CrossRef]
- Gora, E.M.; Bitzer, P.M.; Burchfield, J.C.; Gutierrez, C.; Yanoviak, S.P. The contributions of lightning to biomass turnover, gap formation and plant mortality in a tropical forest. Ecology 2021, 102, e03541. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, B.; Nadeau, D.F.; Domine, F. Comparison of snowpack structure in gaps and under the canopy in a humid boreal forest. Hydrol. Process 2022, 36, e14681. [Google Scholar] [CrossRef]
- Keram, A.; Halik, U.; Keyimu, M.; Aishan, T.; Mamat, Z.; Rouzi, A. Gap dynamics of natural Populus euphratica floodplain forests affected by hydrological alteration along the Tarim River: Implications for restoration of the riparian forests. For. Ecol. Manag. 2019, 438, 103–113. [Google Scholar] [CrossRef]
- Amir, A.A. Canopy gaps and the natural regeneration of Matang mangroves. For. Ecol. Manag. 2012, 269, 60–67. [Google Scholar] [CrossRef]
- Trindade, A.D.; Ferraz, J.B.S.; DeArmond, D. Removal of Woody Debris from Logging Gaps Influences Soil Physical and Chemical Properties in the Short Term: A Study Case in Central Amazonia. For. Sci. 2021, 67, 711–720. [Google Scholar] [CrossRef]
- Villemaire-Cote, O.; Ruel, J.C.; Tremblay, J.P. Reduction in local white-tailed deer abundance allows the positive response of northern white cedar regeneration to gap dynamics. Can. J. For. Res. 2022, 52, 1383–1397. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Lindner, D.L.; Jusino, M.A.; Fraver, S.; Banik, M.T.; Mladenoff, D.J. Linking wood-decay fungal communities to decay rates: Using a long-term experimental manipulation of deadwood and canopy gaps. Fungal Ecol. 2023, 62, 101220. [Google Scholar] [CrossRef]
- Xi, W.M.; Peet, R.K.; Lee, M.T.; Urban, D.L. Hurricane disturbances, tree diversity, and succession in North Carolina Piedmont forests, USA. J. For. Res. 2019, 30, 219–231. [Google Scholar] [CrossRef]
- Yang, H.; Liu, S.R.; Cao, K.F.; Wang, J.X.; Li, Y.D.; Xu, H. Characteristics of typhoon disturbed gaps in an old-growth tropical montane rainforest in Hainan Island, China. J. For. Res. 2017, 28, 1231–1239. [Google Scholar] [CrossRef]
- Chao, K.J.; Lin, Y.C.; Song, G.Z.M.; Liao, C.H.; Kuo, Y.L.; Hsieh, C.F.; Schupp, E.W. Understorey light environment impacts on seedling establishment and growth in a typhoon-disturbed tropical forest. Plant Ecol. 2022, 223, 1007–1021. [Google Scholar] [CrossRef]
- Dietz, L.; Collet, C.; Dupouey, J.L.; Lacombe, E.; Laurent, L.; Gegout, J.C. Windstorm-induced canopy openings accelerate temperate forest adaptation to global warming. Glob. Ecol. Biogeogr. 2020, 29, 2067–2077. [Google Scholar] [CrossRef]
- Dietz, L.; Gegout, J.-C.; Dupouey, J.-L.; Lacombe, E.; Laurent, L.; Collet, C. Beech and hornbeam dominate oak 20 years after the creation of storm-induced gaps. For. Ecol. Manag. 2022, 503, 119758. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Sarker, T.C.; Stinca, A.; Motti, R.; Cesarano, G.; Teobaldelli, M.; Saulino, L.; Cona, F.; et al. Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest. iFor.-Biogeosci. For. 2018, 11, 64–71. [Google Scholar] [CrossRef]
- Arii, K.; Lechowicz, M.J. Changes in understory light regime in a beech-maple forest after a severe ice storm. Can. J. For. Res. 2007, 37, 1770–1776. [Google Scholar] [CrossRef]
- Lafon, C.W. Ice-storm disturbance and long-term forest dynamics in the Adirondack Mountains. J. Veg. Sci. 2004, 15, 267–276. [Google Scholar] [CrossRef]
- Ravnjak, B.; Bavcon, J.; Carni, A. Plant Species Turnover on Forest Gaps after Natural Disturbances in the Dinaric Fir Beech Forests (Omphalodo-Fagetum sylvaticae). Diversity 2022, 14, 209. [Google Scholar] [CrossRef]
- Jokela, J.; Siitonen, J.; Koivula, M. Short-term effects of selection, gap, patch and clear cutting on the beetle fauna in boreal spruce-dominated forests. For. Ecol. Manag. 2019, 446, 29–37. [Google Scholar] [CrossRef]
- Kramer, K.; Brang, P.; Bachofen, H.; Bugmann, H.; Wohlgemuth, T. Site factors are more important than salvage logging for tree regeneration after wind disturbance in Central European forests. For. Ecol. Manag. 2014, 331, 116–128. [Google Scholar] [CrossRef]
- Berg, E.C.; Zarnoch, S.J.; McNab, W.H. Twenty-year survivorship of tree seedlings in wind-created gaps in an upland hardwood forest in the eastern US. New For. 2019, 50, 323–344. [Google Scholar] [CrossRef]
- Molina, J.R.; Ortega, M.; Silva, F.R.Y. Scorch height and volume modeling in prescribed fires: Effects of canopy gaps in Pinus pinaster stands in Southern Europe. For. Ecol. Manag. 2022, 506, 119979. [Google Scholar] [CrossRef]
- de Almeida, B.N.; dos Santos, R.M.; Morel, J.D.; Salles Pereira, D.G. Variations in canopy gaps in different phytophysiognomies under fire effect. Cienc. Florest. 2020, 30, 632–643. [Google Scholar] [CrossRef]
- Mitamura, M.; Yamamura, Y.; Nakano, T. Large-scale canopy opening causes decreased photosynthesis in the saplings of shade-tolerant conifer, Abies veitchii. Tree Physiol. 2009, 29, 137–145. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, Y.; Dechant, B.; Lee, H.; Kim, H.S.; Kornfeld, A.; Berry, J.A. Solar-induced chlorophyll fluorescence is non-linearly related to canopy photosynthesis in a temperate evergreen needleleaf forest during the fall transition. Remote Sens. Environ. 2021, 258, 112362. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Zhu, J.J.; Zheng, X.; Lu, D.L.; Li, X.F. Comparison of gap formation and distribution pattern induced by wind/snowstorm and flood in a temperate secondary forest ecosystem, Northeast China. Silva Fenn. 2017, 51, 7693. [Google Scholar] [CrossRef][Green Version]
- Rader, A.M.; Cottrell, A.; Kudla, A.; Lum, T.; Henderson, D.; Karandikar, H.; Letcher, S.G. Tree functional traits as predictors of microburst-associated treefalls in tropical wet forests. Biotropica 2020, 52, 410–414. [Google Scholar] [CrossRef]
- Worrall, J.J.; Lee, T.D.; Harrington, T.C. Forest dynamics and agents that initiate and expand canopy gaps in Picea–Abies forests of Crawford Notch, New Hampshire, USA. J. Ecol. 2005, 93, 178–190. [Google Scholar] [CrossRef]
- Orman, O.; Dobrowolska, D.; Szwagrzyk, J. Gap regeneration patterns in Carpathian old-growth mixed beech forests—Interactive effects of spruce bark beetle canopy disturbance and deer herbivory. For. Ecol. Manag. 2018, 430, 451–459. [Google Scholar] [CrossRef]
- Nagel, T.A.; Diaci, J.; Jerina, K.; Kobal, M.; Rozenbergar, D. Simultaneous influence of canopy decline and deer herbivory on regeneration in a conifer-broadleaf forest. Can. J. For. Res. 2015, 45, 266–275. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Julio Camarero, J.; Manzanedo, R.D.; Sanchez-Cuesta, R.; Lopez Quintanilla, J.; Sanchez Salguero, R. Regeneration of Abies pinsapo within gaps created by Heterobasidion annosuminduced tree mortality in southern Spain. iFor.-Biogeosci. For. 2014, 7, 209–215. [Google Scholar] [CrossRef]
- Takahashi, K. Spatial distribution and size of small canopy gaps created by Japanese black bears: Estimating gap size using dropped branch measurements. BMC Ecol. 2013, 13, 23. [Google Scholar] [CrossRef]
- Mohammadi, L.; Mohadjer, M.R.M.; Etemad, V.; Sefidi, K.; Nasiri, N. Natural Regeneration within Natural and Man-Made Canopy Gaps in Caspian Natural Beech (Fagus orientalis Lipsky) Forest, Northern Iran. J. Sustain. For. 2020, 39, 61–75. [Google Scholar] [CrossRef]
- Goulamoussene, Y.; Bedeau, C.; Descroix, L.; Linguet, L.; Herault, B. Environmental control of natural gap size distribution in tropical forests. Biogeosciences 2017, 14, 353–364. [Google Scholar] [CrossRef]
- Rangel, C.D.; Reif, A.; Hernandez, L. Understanding small-scale disturbances in Guayana’s montane forest: Gap characterization in the Sierra de Lema, Venezuela. Interciencia 2011, 36, 272–280. [Google Scholar]
- Reis, C.R.; Jackson, T.D.; Gorgens, E.B.; Dalagnol, R.; Jucker, T.; Nunes, M.H.; Ometto, J.P.; Aragao, L.; Rodriguez, L.C.E.; Coomes, D.A. Forest disturbance and growth processes are reflected in the geographical distribution of large canopy gaps across the Brazilian Amazon. J. Ecol. 2022, 110, 2971–2983. [Google Scholar] [CrossRef]
- Parhizkar, P.; Sagheb-Talebi, K.; Zenner, E.K.; Hassani, M.; Hallaj, M.H.S. Gap and stand structural characteristics in a managed and an unmanaged old-growth oriental beech (Fagus orientalis Lipsky) forest. Forestry 2021, 94, 691–703. [Google Scholar] [CrossRef]
- Mazdi, R.A.; Mataji, A.; Fallah, A. Canopy gap dynamics, disturbances, and natural regeneration patterns in a beech-dominated Hyrcanian old-growth forest. Balt. For. 2021, 27, 535. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorny, R.; Okae-Anti, D.; Gyedu, A.; Obeng, I.O. The composition and diversity of natural regeneration of tree species in gaps under different intensities of forest disturbance. J. For. Res. 2021, 32, 1843–1853. [Google Scholar] [CrossRef]
- Lusk, C.H.; Sendall, K.; Kooyman, R. Latitude, solar elevation angles and gap-regenerating rain forest pioneers. J. Ecol. 2011, 99, 491–502. [Google Scholar] [CrossRef]
- Schmid, U.; Bigler, C.; Frehner, M.; Bugmann, H. Abiotic and biotic determinants of height growth of Picea abies regeneration in small forest gaps in the Swiss Alps. For. Ecol. Manag. 2021, 490, 119076. [Google Scholar] [CrossRef]
- Salvador-Van Eysenrode, D.; Bogaert, J.; Van Hecke, P.; Impens, I. Forest canopy perforation in time and space in Amazonian Ecuador. Acta Oecolog. 2000, 21, 285–291. [Google Scholar] [CrossRef]
- Richards, J.D.; Hart, J.L. Canopy gap dynamics and development patterns in secondary Quercus stands on the Cumberland Plateau, Alabama, USA. For. Ecol. Manag. 2011, 262, 2229–2239. [Google Scholar] [CrossRef]
- Sefidi, K.; Mohadjer, M.R.M.; Mosandl, R.; Copenheaver, C.A. Canopy gaps and regeneration in old-growth Oriental beech (Fagus orientalis Lipsky) stands, northern Iran. For. Ecol. Manag. 2011, 262, 1094–1099. [Google Scholar] [CrossRef]
- Fahey, R.T.; Puettmann, K.J. Patterns in spatial extent of gap influence on understory plant communities. For. Ecol. Manag. 2008, 255, 2801–2810. [Google Scholar] [CrossRef]
- Whyte, H.D.; Lusk, C.H. Woody debris in treefall gaps shelters palatable plant species from deer browsing, in an old-growth temperate forest. For. Ecol. Manag. 2019, 448, 198–207. [Google Scholar] [CrossRef]
- Coates, K.D. Conifer seedling response to northern temperate forest gaps. For. Ecol. Manag. 2000, 127, 249–269. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhu, C.Y.; Zhu, J.J.; Song, L.N.; Wang, G.G.; Lu, D.L. Estimating gap age using tree-ring width in combination with carbon isotope discrimination in a temperate forest, Northeast China. Ann. For. Sci. 2022, 79, 25. [Google Scholar] [CrossRef]
- Jansen, P.A.; Van Der Meer, P.J.; Bongers, F. Spatial Contagiousness of Canopy Disturbance in Tropical Rain Forest: An Individual-Tree-Based Test. Ecology 2008, 89, 3490–3502. [Google Scholar] [CrossRef]
- de Lima, R.A.F. Gap size measurement: The proposal of a new field method. For. Ecol. Manag. 2005, 214, 413–419. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, G.Q.; Wang, G.G.; Yan, Q.L.; Lu, D.L.; Li, X.F.; Zheng, X. On the size of forest gaps: Can their lower and upper limits be objectively defined? Agric. For. Meteorol. 2015, 213, 64–76. [Google Scholar] [CrossRef]
- McNab, W.H.; Kilgo, J.C.; Blake, J.I.; Zarnoch, S.J. Effect of gap size on composition and structure of regeneration 19 years after harvest in a southeastern bottomland forest, USA. Can. J. For. Res. 2021, 51, 380–392. [Google Scholar] [CrossRef]
- de Lima, R.A.F.; Prado, P.I.; Martini, A.M.Z.; Fonseca, L.J.; Gandolfi, S.; Rodrigues, R.R. Improving methods in gap ecology: Revisiting size and shape distributions using a model selection approach. J. Veg. Sci. 2013, 24, 484–495. [Google Scholar] [CrossRef]
- Hu, L.L.; Gong, Z.W.; Li, J.S.; Zhu, J.J. Estimation of canopy gap size and gap shape using a hemispherical photograph. Trees-Struct. Funct. 2009, 23, 1101–1108. [Google Scholar] [CrossRef]
- Menges, E.S.; Craddock, A.; Salo, J.; Zinthefer, R.; Weekley, C.W. Gap ecology in Florida scrub: Species occurrence, diversity and gap properties. J. Veg. Sci. 2008, 19, 503–514. [Google Scholar] [CrossRef]
- Lassalle, G.; de Souza, C.R. Tracking canopy gaps in mangroves remotely using deep learning. Remote Sens. Ecol. Conserv. 2022, 8, 890–903. [Google Scholar] [CrossRef]
- Vepakomma, U.; Kneeshaw, D.; Fortin, M.J. Spatial contiguity and continuity of canopy gaps in mixed wood boreal forests: Persistence, expansion, shrinkage and displacement. J. Ecol. 2012, 100, 1257–1268. [Google Scholar] [CrossRef]
- Feldmann, E.; Drossler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- Yamamoto, S.I.; Nishimura, N.; Torimaru, T.; Manabe, T.; Itaya, A.; Becek, K. A comparison of different survey methods for assessing gap parameters in old-growth forests. For. Ecol. Manag. 2011, 262, 886–893. [Google Scholar] [CrossRef]
- Frazer, G.W.; Fournier, R.A.; Trofymow, J.A.; Hall, R.J. A comparison of digital and film fisheye photography for analysis of forest canopy structure and gap light transmission. Agric. For. Meteorol. 2001, 109, 249–263. [Google Scholar] [CrossRef]
- Torimaru, T.; Itaya, A.; Yamamoto, S.I. Quantification of repeated gap formation events and their spatial patterns in three types of old-growth forests: Analysis of long-term canopy dynamics using aerial photographs and digital surface models. For. Ecol. Manag. 2012, 284, 1–11. [Google Scholar] [CrossRef]
- Getzin, S.; Lons, C.; Yizhaq, H.; Erickson, T.E.; Munoz-Rojas, M.; Huth, A.; Wiegand, K. High-resolution images and drone-based LiDAR reveal striking patterns of vegetation gaps in a wooded spinifex grassland of Western Australia. Landsc. Ecol. 2022, 37, 829–845. [Google Scholar] [CrossRef]
- Kellner, J.R.; Asner, G.P. Convergent structural responses of tropical forests to diverse disturbance regimes. Ecol. Lett. 2009, 12, 887–897. [Google Scholar] [CrossRef]
- Seidel, D.; Ammer, C.; Puettmann, K. Describing forest canopy gaps efficiently, accurately, and objectively: New prospects through the use of terrestrial laser scanning. Agric. For. Meteorol. 2015, 213, 23–32. [Google Scholar] [CrossRef]
- Komarov, A.V.; Ershov, D.V.; Tikhonova, E.V. Informativeness of the Spectral and Morphometric Characteristics of the Canopy-Gap Structure Based on Remote Sensing Data. Contemp. Probl. Ecol. 2021, 14, 733–742. [Google Scholar] [CrossRef]
- Beeles, K.L.; Tourville, J.C.; Dovciak, M. Characterizing Canopy Openness Across Large Forested Landscapes Using Spherical Densiometer and Smartphone Hemispherical Photography. J. For. 2022, 120, 37–50. [Google Scholar] [CrossRef]
- Botkin, D.B.; Janak, J.F.; Wallis, J.R. Some ecological consequences of a computer model of forest growth. J. Ecol. 1972, 60, 849–872. [Google Scholar] [CrossRef]
- Risch, A.C.; Heiri, C.; Bugmann, H. Simulating structural forest patterns with a forest gap model: A model evaluation. Ecol. Model. 2005, 181, 161–172. [Google Scholar] [CrossRef]
- Lutz, D.A.; Shugart, H.H.; Ershov, D.V.; Shuman, J.K.; Isaev, A.S. Boreal forest sensitivity to increased temperatures at multiple successional stages. Ann. For. Sci. 2013, 70, 299–308. [Google Scholar] [CrossRef][Green Version]
- Kathke, S.; Bruelheide, H. Gap dynamics in a near-natural spruce forest at Mt. Brocken, Germany. For. Ecol. Manag. 2010, 259, 624–632. [Google Scholar] [CrossRef]
- Brazhnik, K.; Shugart, H.H. SIBBORK: A new spatially-explicit gap model for boreal forest. Ecol. Model. 2016, 320, 182–196. [Google Scholar] [CrossRef]
- Larocque, G.R.; Archambault, L.; Delisle, C. Development of the gap model ZELIG-CFS to predict the dynamics of North American mixed forest types with complex structures. Ecol. Model. 2011, 222, 2570–2583. [Google Scholar] [CrossRef]
- Kazmierczak, M.; Wiegand, T.; Huth, A. A neutral vs. non-neutral parametrizations of a physiological forest gap model. Ecol. Model. 2014, 288, 94–102. [Google Scholar] [CrossRef]
- Zhou, D.H.; He, H.S.; Sun, G.C.; Li, X.Z. Gap model and its application in studies of global climate change. Chin. J. Ecol. 2007, 26, 1303–1310. [Google Scholar]
- Orman, O.; Wrzesinski, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2021, 482, 118866. [Google Scholar] [CrossRef]
- Kermavnar, J.; Ferlan, M.; Marinsek, A.; Eler, K.; Kobler, A.; Kutnar, L. Effects of various cutting treatments and topographic factors on microclimatic conditions in Dinaric fir-beech forests. Agric. For. Meteorol. 2020, 295, 108186. [Google Scholar] [CrossRef]
- Pritchard, J.M.; Comeau, P.G. Effects of opening size and stand characteristics on light transmittance and temperature under young trembling aspen stands. For. Ecol. Manag. 2004, 200, 119–128. [Google Scholar] [CrossRef]
- Ma, S.Y.; Concilio, A.; Oakley, B.; North, M.; Chen, J.Q. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For. Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Prevost, M.; Raymond, P. Effect of gap size, aspect and slope on available light and soil temperature after patch-selection cutting in yellow birch-conifer stands, Quebec, Canada. For. Ecol. Manag. 2012, 274, 210–221. [Google Scholar] [CrossRef]
- Li, X.F.; Wen, Y.J.; Zhang, J.X.; Liu, L.M.; Jin, L.; Yan, T.; Wang, Y. The effect of low-temperature event on the survival and growth of Juglans mandshurica seedlings within forest gaps. J. For. Res. 2018, 29, 943–951. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorny, R. Effects of gap size on natural regeneration and micro-environmental soil conditions in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst) dominated mixed forest. Plant Soil Environ. 2020, 66, 607–615. [Google Scholar] [CrossRef]
- Velazquez, E.; Wiegand, T. Competition for light and persistence of rare light-demanding species within tree-fall gaps in a moist tropical forest. Ecology 2020, 101, e03034. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Q.L.; Wang, J.; Zhu, J.J. Restoring temperate secondary forests by promoting sprout regeneration: Effects of gap size and within-gap position on the photosynthesis and growth of stump sprouts with contrasting shade tolerance. For. Ecol. Manag. 2018, 429, 267–277. [Google Scholar] [CrossRef]
- Annighofer, P. Stress relief through gap creation? Growth response of a shade tolerant species (Fagus sylvatica L.) to a changed light environment. For. Ecol. Manag. 2018, 415, 139–147. [Google Scholar] [CrossRef]
- Pardonnet, S.; Beck, H.; Milberg, P.; Bergman, K.O. Effect of Tree-Fall Gaps on Fruit-Feeding Nymphalid Butterfly Assemblages in a Peruvian Rain Forest. Biotropica 2013, 45, 612–619. [Google Scholar] [CrossRef]
- Habashi, H. Spatial correlation of pit and mound topography with canopy gaps in a virgin mixed beech forest, northern Iran. J. For. Res. 2019, 30, 295–303. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Chen, Y.J.; Bongers, F. Seasonal changes in photosynthesis and growth of Zizyphus attopensis seedlings in three contrasting microhabitats in a tropical seasonal rain forest. Tree Physiol. 2007, 27, 827–836. [Google Scholar] [CrossRef]
- Mollinari, M.M.; Peres, C.A.; Edwards, D.P. Rapid recovery of thermal environment after selective logging in the Amazon. Agric. For. Meteorol. 2019, 278, 107637. [Google Scholar] [CrossRef]
- Van Couwenberghe, R.; Collet, C.; Lacombe, E.; Pierrat, J.C.; Gegout, J.C. Gap partitioning among temperate tree species across a regional soil gradient in windstorm-disturbed forests. For. Ecol. Manag. 2010, 260, 146–154. [Google Scholar] [CrossRef]
- Miller, S.D.; Goulden, M.L.; da Rocha, H.R. The effect of canopy gaps on subcanopy ventilation and scalar fluxes in a tropical forest. Agric. For. Meteorol. 2007, 142, 25–34. [Google Scholar] [CrossRef]
- Forrester, J.A.; Mladenoff, D.J.; Gower, S.T.; Stoffel, J.L. Interactions of temperature and moisture with respiration from coarse woody debris in experimental forest canopy gaps. For. Ecol. Manag. 2012, 265, 124–132. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Mladenoff, D.J.; Lewandowski, T.E. Deadwood Reduces the Variation in Soil Microbial Communities Caused by Experimental Forest Gaps. Ecosystems 2021, 24, 1928–1943. [Google Scholar] [CrossRef]
- Han, M.G.; Tang, M.; Shi, B.K.; Jin, G.Z. Effect of canopy gap size on soil respiration in a mixed broadleaved-Korean pine forest: Evidence from biotic and abiotic factors. Eur. J. Soil. Biol. 2020, 99, 103194. [Google Scholar] [CrossRef]
- Ficano, N.; Porder, S.; McCulloch, L.A. Tripartite legume-rhizobia-mycorrhizae relationship is influenced by light and soil nitrogen in Neotropical canopy gaps. Ecology 2021, 102, e03489. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Bockheim, J.G. Impacts of forest gaps on soil properties and processes in old growth northern hardwood-hemlock forests. Plant Soil. 2007, 294, 219–233. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J.G. Influence of gap size on carbon and nitrogen biogeochemical cycling in Northern hardwood forests of the Upper Peninsula, Michigan. Plant Soil 2014, 377, 323–335. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, J.; Yang, W.Q.; Yin, R.; Xu, Z.F.; Liu, Y.; Zhang, L.; Li, H.; You, C.M. Forest gaps retard carbon and nutrient release from twig litter in alpine forest ecosystems. Eur. J. For. Res. 2020, 139, 53–65. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.Z.; Yang, W.Q.; Xu, L.Y.; Ni, X.Y.; He, J.; Tan, B.; Hu, Y.; Justin, M.F. The losses of condensed tannins in six foliar litters vary with gap position and season in an alpine forest. iFor. Biogeosci. For. 2016, 9, 910–918. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Bagnato, S.; Mallamaci, C.; Mercurio, R. Gap size effects on above- and below-ground processes in a silver fir stand. Eur. J. For. Res. 2010, 129, 355–365. [Google Scholar] [CrossRef]
- Ozcan, M.; Gokbulak, F. Effect of size and surrounding forest vegetation on chemical properties of soil in forest gaps. iFor. Biogeosci. For. 2015, 8, 67–72. [Google Scholar] [CrossRef]
- He, J.; Yang, W.Q.; Xu, L.Y.; Ni, X.Y.; Li, H.; Wu, F.Z. Copper and zinc dynamics in foliar litter during decomposition from gap center to closed canopy in an alpine forest. Scand. J. For. Res. 2016, 31, 355–367. [Google Scholar] [CrossRef]
- Tan, S.Y.; Dong, Q.; Ni, X.Y.; Yue, K.; Liao, S.; Wu, F.Z. Gap edge canopy buffering of throughfall deposition in a subalpine natural forest. For. Ecosyst. 2022, 9, 100047. [Google Scholar] [CrossRef]
- Lin, N.; Bartsch, N.; Heinrichs, S.; Vor, T. Long-term effects of canopy opening and liming on leaf litter production, and on leaf litter and fine-root decomposition in a European beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2015, 338, 183–190. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, B.; Yang, W.Q.; Wang, Q.; Chang, C.H.; Wang, L.F.; Li, H.; You, C.M.; Cao, R.; Jiang, Y.R.; et al. Forest gaps accelerate the degradation of cellulose and lignin in decaying logs in a subalpine forest. Eur. J. For. Res. 2023, 142, 27–36. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Eirthorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Lu, D.L.; Wang, G.G.; Yu, L.Z.; Zhang, T.; Zhu, J.J. Seedling survival within forest gaps: The effects of gap size, within-gap position and forest type on species of contrasting shade-tolerance in Northeast China. Forestry 2018, 91, 470–479. [Google Scholar] [CrossRef]
- Sariyildiz, T. Effects of gap-size classes on long-term litter decomposition rates of beech, oak and chestnut species at high elevations in northeast Turkey. Ecosystems 2008, 11, 841–853. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.J.; Zhang, Y.; Zhang, J.; Song, S.M.; Zhou, Y. Forest gap size can efficiently promote litter decomposition and nutrient release in south-western China. South For. A J. For. Sci. 2019, 81, 185–194. [Google Scholar] [CrossRef]
- Nygaard, P.H.; Strand, L.T.; Stuanes, A.O. Gap formation and dynamics after long-term steady state in an old-growth Picea abies stand in Norway: Above- and belowground interactions. Ecol. Evol. 2018, 8, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.D.; Forrester, J.A.; Mladenoff, D.J. Spatial Patterns of Soil Surface C Flux in Experimental Canopy Gaps. Ecosystems 2012, 15, 616–623. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.K.; Zhou, Z.H. Thinning promotes the nitrogen and phosphorous cycling in forest soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Maas, G.C.B.; Sanquetta, C.R.; Marques, R.; Machado, S.D.; Sanquetta, M.N.I.; Corte, A.P.D.; Barberena, I.M. Carbon production from seasonal litterfall in the Brazilian Atlantic Forest. South For. A J. For. Sci. 2021, 83, 128–134. [Google Scholar] [CrossRef]
- Martin, M.; Woodbury, D.; Glogower, Y.; Duguid, M.; Frey, B.; Ashton, M. Within-gap position shapes fifty years of forest dynamics in a temperate hardwood forest in Connecticut, USA. For. Ecol. Manag. 2021, 494, 119311. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Q.L.; Yan, T.; Song, Y.; Sun, Y.R.; Zhu, J.J. Rodent-mediated seed dispersal of Juglans mandshurica regulated by gap size and within-gap position in larch plantations: Implication for converting pure larch plantations into larch-walnut mixed forests. For. Ecol. Manag. 2017, 404, 205–213. [Google Scholar] [CrossRef]
- Wang, Z.B.; Jiang, L.A.; Gao, J.F.; Qing, S.Q.; Pan, C.; Wu, Y.; Yang, H.J.; Wang, D.H. The influence of microhabitat factors on the regeneration and species composition of understory woody plants in Pinus tabuliformis plantations on the Loess Plateau. For. Ecol. Manag. 2022, 509, 120080. [Google Scholar] [CrossRef]
- Newsom, L.A.; Yan, Q.-L.; Zhu, J.-J.; Yu, L.-Z. Seed Regeneration Potential of Canopy Gaps at Early Formation Stage in Temperate Secondary Forests, Northeast China. PLoS ONE 2012, 7, e39502. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhu, J.J.; Zhang, J.P.; Yu, L.Z.; Hu, Z.B. Spatial distribution pattern of soil seed bank in canopy gaps of various sizes in temperate secondary forests, Northeast China. Plant Soil 2010, 329, 469–480. [Google Scholar] [CrossRef]
- Daws, M.I.; Ballard, C.; Mullins, C.E.; Garwood, N.C.; Murray, B.; Pearson, T.R.H.; Burslem, D. Allometric relationships between seed mass and seedling characteristics reveal trade-offs for neotropical gap-dependent species. Oecologia 2007, 154, 445–454. [Google Scholar] [CrossRef]
- Puerta-Pinero, C.; Muller-Landau, H.C.; Calderon, O.; Wright, S.J. Seed arrival in tropical forest tree fall gaps. Ecology 2013, 94, 1552–1562. [Google Scholar] [CrossRef]
- Xia, Q.Q.; Ando, M.; Seiwa, K. Interaction of seed size with light quality and temperature regimes as germination cues in 10 temperate pioneer tree species. Funct. Ecol. 2016, 30, 866–874. [Google Scholar] [CrossRef]
- da Silva, P.H.M.; Bouillet, J.-P.; de Paula, R.C. Assessing the invasive potential of commercial Eucalyptus species in Brazil: Germination and early establishment. For. Ecol. Manag. 2016, 374, 129–135. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Zhu, D.H.; Xing, C.; Jin, M.R.; Liu, J.F.; He, Z.S. Forest gaps regulate seed germination rate and radicle growth of an endangered plant species in a subtropical natural forest. Plant Divers. 2022, 44, 445–454. [Google Scholar] [CrossRef]
- Vandenberghe, C.; Frelechoux, F.; Gadallah, F.; Buttler, A. Competitive effects of herbaceous vegetation on tree seedling emergence, growth and survival: Does gap size matter? J. Veg. Sci. 2006, 17, 481–488. [Google Scholar] [CrossRef]
- Sharma, L.N.; Shrestha, K.B.; Maren, I.E. Tree regeneration in gap-understory mosaics in a subtropical Shorea robusta (Sal) forest. J. For. Res. 2019, 30, 2061–2068. [Google Scholar] [CrossRef]
- Zhu, J.J.; Matsuzaki, T.; Lee, F.Q.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Totland, O.; Nyeko, P.; Bjerknes, A.L.; Hegland, S.J.; Nielsen, A. Does forest gap size affects population size, plant size, reproductive success and pollinator visitation in Lantana camara, a tropical invasive shrub? For. Ecol. Manag. 2005, 215, 329–338. [Google Scholar] [CrossRef]
- Keram, A.; Halik, U.; Aishan, T.; Keyimu, M.; Jiapaer, K.; Li, G.L. Tree mortality and regeneration of Euphrates poplar riparian forests along the Tarim River, Northwest China. For. Ecosyst. 2021, 8, 49. [Google Scholar] [CrossRef]
- Dumais, D.; Raymond, P.; Prevost, M. Eight-year ecophysiology and growth dynamics of Picea rubens seedlings planted in harvest gaps of partially cut stands. For. Ecol. Manag. 2020, 478, 118514. [Google Scholar] [CrossRef]
- Mountford, E.P.; Savill, P.S.; Bebber, D.P. Patterns of regeneration and ground vegetation associated with canopy gaps in a managed beechwood in southern England. Forestry 2006, 79, 389–408. [Google Scholar] [CrossRef]
- Wang, Z.B.; Yang, H.J.; Wang, D.H.; Zhao, Z. Spatial distribution and growth association of regeneration in gaps of Chinese pine (Pinus tabuliformis Carr.) plantation in northern China. For. Ecol. Manag. 2019, 432, 387–399. [Google Scholar] [CrossRef]
- Lu, D.L.; Zhu, J.J.; Wu, D.N.; Chen, Q.D.; Yu, Y.; Wang, J.; Zhu, C.Y.; Liu, H.Q.; Gao, T.; Wang, G.G. Detecting dynamics and variations of crown asymmetry induced by natural gaps in a temperate secondary forest using terrestrial laser scanning. For. Ecol. Manag. 2020, 473, 118289. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Pang, X.Y.; Bao, W.K.; He, Q.H. Effects of gap-model thinning intensity on the radial growth of gap-edge trees with distinct crown classes in a spruce plantation. Trees-Struct. Funct. 2015, 29, 1861–1870. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; van der Heijden, G.; Mascaro, J.; Carson, W.P. Lianas in gaps reduce carbon accumulation in a tropical forest. Ecology 2014, 95, 3008–3017. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; DeFilippis, D.M.; Visser, M.; Estrada-Villegas, S.; Rivera-Camana, R.; Bernal, B.; Perez, S.; Valdez, A.; Valdez, S.; Aguilar, A.; et al. Local canopy disturbance as an explanation for long-term increases in liana abundance. Ecol. Lett. 2021, 24, 2635–2647. [Google Scholar] [CrossRef]
- Koorem, K.; Moora, M. Positive association between understory species richness and a dominant shrub species (Corylus avellana) in a boreonemoral spruce forest. For. Ecol. Manag. 2010, 260, 1407–1413. [Google Scholar] [CrossRef]
- Yu, J.T.; Zhang, X.N.; Xu, C.Y.; Hao, M.H.; Choe, C.; He, H.J. Thinning can increase shrub diversity and decrease herb diversity by regulating light and soil environments. Front. Plant Sci. 2022, 13, 948648. [Google Scholar] [CrossRef]
- Lu, D.L.; Zhu, J.J.; Wang, X.Y.; Hao, G.Y.; Wang, G.G. A systematic evaluation of gap size and within-gap position effects on seedling regeneration in a temperate secondary forest, Northeast China. For. Ecol. Manag. 2021, 490, 119140. [Google Scholar] [CrossRef]
- Diaci, J.; Adamic, T.; Rozman, A. Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: Influences of light regime, herb competition and browsing. For. Ecol. Manag. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Takano, T.; Kominami, Y.; Mizunaga, H. Do Coarser Gap Mosaics in Conifer Plantations Induce More Seed Dispersal by Birds? Temporal Changes during 12 Years after Gap Creation. Forests 2019, 10, 918. [Google Scholar] [CrossRef]
- Lewandowski, P.; Przepiora, F.; Ciach, M. Single dead trees matter: Small-scale canopy gaps increase the species richness, diversity and abundance of birds breeding in a temperate deciduous forest. For. Ecol. Manag. 2021, 481, 118693. [Google Scholar] [CrossRef]
- Champlin, T.B.; Kilgo, J.C.; Gumpertz, M.L.; Moorman, C.E. Avian Response to Microclimate in Canopy Gaps in a Bottomland Hardwood Forest. Southeast. Nat. 2009, 8, 107–120. [Google Scholar] [CrossRef]
- Shea, E.L.; Schulte, L.A.; Palik, B.J. Decade-long bird community response to the spatial pattern of variable retention harvesting in red pine (Pines resinosa) forests. For. Ecol. Manag. 2017, 402, 272–284. [Google Scholar] [CrossRef]
- Nerlekar, A.N.; Kamath, V.; Saravanan, A.; Ganesan, R. Successional dynamics of a regenerated forest in a plantation landscape in Southern India. J. Trop. Ecol. 2019, 35, 57–67. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, Z.Y.; Liu, X.L.; Yi, X.F. Seedling predation of Quercus mongolica by small rodents in response to forest gaps. New For. 2017, 48, 83–94. [Google Scholar] [CrossRef]
- Piiroinen, T.; Valtonen, A.; Roininen, H. Vertebrate herbivores are the main cause of seedling mortality in a logged African rainforest—Implications for forest restoration. Restor. Ecol. 2017, 25, 442–452. [Google Scholar] [CrossRef]
- Kuijper, D.P.J.; Cromsigt, J.; Churski, M.; Adam, B.; Jedrzejewska, B.; Jedrzejewski, W. Do ungulates preferentially feed in forest gaps in European temperate forest? For. Ecol. Manag. 2009, 258, 1528–1535. [Google Scholar] [CrossRef]
- Burton, J.I.; Mladenoff, D.J.; Forrester, J.A.; Clayton, M.K. Effects of forest canopy gaps on the ground-layer plant community depend on deer: Evidence from a controlled experiment. J. Veg. Sci. 2021, 32, e12969. [Google Scholar] [CrossRef]
- VanderMolen, M.S.; Webster, C.R. Influence of deer herbivory on regeneration dynamics and gap capture in experimental gaps, 18 years post-harvest. For. Ecol. Manag. 2021, 501, 119675. [Google Scholar] [CrossRef]
- Cory, B.J.; Russell, F.L. Deer browsing and light availability limit post oak (Quercus stellata) sapling growth and post-fire recovery in a xeric woodland. For. Ecol. Manag. 2022, 519, 120346. [Google Scholar] [CrossRef]
- Agha, M.; Todd, B.D.; Augustine, B.; Lhotka, J.M.; Fleckenstein, L.J.; Lewis, M.; Patterson, C.; Stringer, J.W.; Price, S.J. Effects of gap-based silviculture on thermal biology of a terrestrial reptile. Wildl. Res. 2018, 45, 72–81. [Google Scholar] [CrossRef]
- Pringle, R.M.; Webb, J.K.; Shine, R. Canopy structure, microclimate, and habitat selection by a nocturnal snake, Hoplocephalus bungaroides. Ecology 2003, 84, 2668–2679. [Google Scholar] [CrossRef]
- Horn, S.; Hanula, J.L.; Ulyshen, M.D. Abundance of green tree frogs and insects in artificial canopy gaps in a bottomland hardwood forest. Am. Midl. Nat. 2005, 153, 321–326. [Google Scholar] [CrossRef]
- Smith, M.J.; Forbes, G.J.; Betts, M.G. Landscape configuration influences gap-crossing decisions of northern flying squirrel (Glaucomys sabrinus). Biol. Conserv. 2013, 168, 176–183. [Google Scholar] [CrossRef]
- Fukui, D.; Hirao, T.; Murakami, M.; Hirakawa, H. Effects of treefall gaps created by windthrow on bat assemblages in a temperate forest. For. Ecol. Manag. 2011, 261, 1546–1552. [Google Scholar] [CrossRef]
- Erasmy, M.; Leuschner, C.; Balkenhol, N.; Dietz, M. Shed light in the dark—How do natural canopy gaps influence temperate bat diversity and activity? For. Ecol. Manag. 2021, 497, 119509. [Google Scholar] [CrossRef]
- Hagglund, R.; Dynesius, M.; Lofroth, T.; Olsson, J.; Roberge, J.M.; Hjalten, J. Restoration measures emulating natural disturbances alter beetle assemblages in boreal forest. For. Ecol. Manag. 2020, 462, 117934. [Google Scholar] [CrossRef]
- Mullally, H.L.; Buckley, D.S.; Fordyce, J.A.; Collins, B.; Kwit, C. Bee Communities across Gap, Edge, and Closed-Canopy Microsites in Forest Stands with Group Selection Openings. For. Sci. 2019, 65, 751–757. [Google Scholar] [CrossRef]
- Piper, F.I.; Altmann, S.H.; Lusk, C.H. Global patterns of insect herbivory in gap and understorey environments, and their implications for woody plant carbon storage. Oikos 2018, 127, 483–496. [Google Scholar] [CrossRef]
- Felton, A.M.; Engstrom, L.M.; Felton, A.; Knott, C.D. Orangutan population density, forest structure and fruit availability in hand-logged and unlogged peat swamp forests in West Kalimantan, Indonesia. Biol. Conserv. 2003, 114, 91–101. [Google Scholar] [CrossRef]
- Jourgholami, M.; Feghhi, J.; Tavankar, F.; Latterini, F.; Venanzi, R.; Picchio, R. Short-term effects in canopy gap area on the recovery of compacted soil caused by forest harvesting in old-growth Oriental beech (Fagus orientalis Lipsky) stands. iFor. Biogeosci. For. 2021, 14, 370–377. [Google Scholar] [CrossRef]
- Matveinen-Huju, K.; Koivula, M. Effects of alternative harvesting methods on boreal forest spider assemblages. Can. J. For. Res. 2008, 38, 782–794. [Google Scholar] [CrossRef]
- Patrick, M.; Fowler, D.; Dunn, R.R.; Sanders, N.J. Effects of Treefall Gap Disturbances on Ant Assemblages in a Tropical Montane Cloud Forest. Biotropica 2012, 44, 472–478. [Google Scholar] [CrossRef]
- Xu, J.X.; Lie, G.W.; Xue, L. Effects of gap size on diversity of soil fauna in a Cunninghamia lanceolata stand damaged by an ice storm in southern China. J. For. Res. 2016, 27, 1427–1434. [Google Scholar] [CrossRef]
- Richards, L.A.; Windsor, D.M. Seasonal variation of arthropod abundance in gaps and the understorey of a lowland moist forest in Panama. J. Trop. Ecol. 2007, 23, 169–176. [Google Scholar] [CrossRef]
- Zurita, G.A.; Zuleta, G.A. Bird use of logging gaps in a subtropical mountain forest: The influence of habitat structure and resource abundance in the Yungas of Argentina. For. Ecol. Manag. 2009, 257, 271–279. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.Y.; Bao, W.K.; Zhou, K.X. Thinning-induced canopy opening exerted a specific effect on soil nematode community. Ecol. Evol. 2018, 8, 3851–3861. [Google Scholar] [CrossRef]
- Gilarte, P.; Pendall, E.; Carrillo, Y.; Nielsen, U.N. Plant functional identity has predictable effects on nematode communities across successional stages. Soil Biol. Biochem. 2021, 162, 108406. [Google Scholar] [CrossRef]
- Kim, S.; Li, G.L.; Han, S.H.; Kim, C.; Lee, S.T.; Son, Y. Microbial biomass and enzymatic responses to temperate oak and larch forest thinning: Influential factors for the site-specific changes. Sci. Total Environ. 2019, 651, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, C.K.; Zhou, Z.H. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- Lyu, Q.; Luo, Y.; Liu, S.Z.; Zhang, Y.; Li, X.J.; Hou, G.R.; Chen, G.; Zhao, K.J.; Fan, C.; Li, X.W. Forest gaps alter the soil bacterial community of weeping cypress plantations by modulating the understory plant diversity. Front. Plant Sci. 2022, 13, 920905. [Google Scholar] [CrossRef] [PubMed]
- Brazee, N.J.; Lindner, D.L.; D’Amato, A.W.; Fraver, S.; Forrester, J.A.; Mladenoff, D.J. Disturbance and diversity of wood-inhabiting fungi: Effects of canopy gaps and downed woody debris. Biodivers. Conserv. 2014, 23, 2155–2172. [Google Scholar] [CrossRef]
- Yang, Y.G.; Geng, Y.Q.; Zhou, H.J.; Zhao, G.L.; Wang, L. Effects of gaps in the forest canopy on soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Curzon, M.T.; D’Amato, A.W.; Fraver, S.; Palik, B.J.; Bottero, A.; Foster, J.R.; Gleason, K.E. Harvesting influences functional identity and diversity over time in forests of the northeastern USA. For. Ecol. Manag. 2017, 400, 93–99. [Google Scholar] [CrossRef]
- Manabe, T.; Shimatani, K.; Kawarasaki, S.; Aikawa, S.I.; Yamamoto, S.I. The patch mosaic of an old-growth warm-temperate forest: Patch-level descriptions of 40-year gap-forming processes and community structures. Ecol. Res. 2009, 24, 575–586. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef]
- Kasper, J.; Weigel, R.; Walentowski, H.; Gröning, A.; Petritan, A.M.; Leuschner, C. Climate warming-induced replacement of mesic beech by thermophilic oak forests will reduce the carbon storage potential in aboveground biomass and soil. Ann. For. Sci. 2021, 78, 89. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Huang, Z.J.; Wang, Z.N.; Chen, Y.F.; Wen, Z.M.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef]
- Schmitt, S.; Tysklind, N.; Heuertz, M.; Herault, B. Selection in space and time: Individual tree growth is adapted to tropical forest gap dynamics. Mol. Ecol. 2022; preprint. [Google Scholar] [CrossRef] [PubMed]
- Wulantuya; Masaka, K.; Bayandala; Fukasawa, Y.; Matsukura, K.; Seiwa, K. Gap creation alters the mode of conspecific distance-dependent seedling establishment via changes in the relative influence of pathogens and mycorrhizae. Oecologia 2020, 192, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Karki, L.; Hallgren, S.W. Tree-Fall Gaps and Regeneration in Old-Growth Cross Timbers Forests. Nat. Areas J. 2015, 35, 533–541. [Google Scholar] [CrossRef]
- Hart, J.L.; Bhuta, A.A.R.; Schneider, R.M. Canopy Disturbance Patterns in Secondary Hardwood Stands on the Highland Rim of Alabama. Castanea 2011, 76, 55–63. [Google Scholar] [CrossRef]
- de Lima, R.A.F.; de Moura, L.C. Gap disturbance regime and composition in the Atlantic Montane Rain Forest: The influence of topography. Plant Ecol. 2008, 197, 239–253. [Google Scholar] [CrossRef]
- de Oliveira, C.D.C.; de Oliveira, I.R.C.; Suganuma, M.S.; Durigan, G. Overstory trees in excess: A threat to restoration success in Brazilian Atlantic forest. For. Ecol. Manag. 2019, 449, 117453. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Ren, S.Y.; Ali, A.; Liu, H.M.; Yuan, Z.Q.; Yang, Q.S.; Shen, G.C.; Zhou, S.S.; Wang, X.H. Response of community diversity and productivity to canopy gap disturbance in subtropical forests. For. Ecol. Manag. 2021, 502, 119740. [Google Scholar] [CrossRef]
- Nuttle, T.; Royo, A.A.; Adams, M.B.; Carson, W.P. Historic disturbance regimes promote tree diversity only under low browsing regimes in eastern deciduous forest. Ecol. Monogr. 2013, 83, 3–17. [Google Scholar] [CrossRef]
- Liu, F.; Tan, C.; Yang, Z.G.; Li, J.J.; Xiao, H.S.; Tong, Y. Regeneration and growth of tree seedlings and saplings in created gaps of different sizes in a subtropical secondary forest in southern China. For. Ecol. Manag. 2022, 511, 120143. [Google Scholar] [CrossRef]
- Terborgh, J.; Nunez, N.H.; Feeley, K.; Beck, H. Gaps present a trade-off between dispersal and establishment that nourishes species diversity. Ecology 2020, 101, e02996. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; dos Santos, F.A.M. Variation in the population structure between a natural and a human-modified forest for a pioneer tropical tree species not restricted to large gaps. Ecol. Evol. 2015, 5, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Lydersen, J.M.; North, M.P.; Knapp, E.E.; Collins, B.M. Quantifying spatial patterns of tree groups and gaps in mixed-conifer forests: Reference conditions and long-term changes following fire suppression and logging. For. Ecol. Manag. 2013, 304, 370–382. [Google Scholar] [CrossRef]
- Diaci, J.; Rozman, J.; Rozman, A. Regeneration gap and microsite niche partitioning in a high alpine forest: Are Norway spruce seedlings more drought-tolerant than beech seedlings? For. Ecol. Manag. 2020, 455, 117688. [Google Scholar] [CrossRef]
- Moreau, G.; Chagnon, C.; Achim, A.; Caspersen, J.; D’Orangeville, L.; Sanchez-Pinillos, M.; Thiffault, N. Opportunities and limitations of thinning to increase resistance and resilience of trees and forests to global change. Forestry 2022, 95, 595–615. [Google Scholar] [CrossRef]
- Staab, M.; Achury, R.; Ammer, C.; Ehbrecht, M.; Irmscher, V.; Mohr, H.; Schall, P.; Weisser, W.W.; Bluthgen, N. Negative effects of forest gaps on dung removal in a full-factorial experiment. J. Anim. Ecol. 2022, 91, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Royo, A.A.; Carson, W.P. Stasis in forest regeneration following deer exclusion and understory gap creation: A 10-year experiment. Ecol. Appl. 2022, 32, e2569. [Google Scholar] [CrossRef]
- Wang, G.L.; Liu, F. The influence of gap creation on the regeneration of Pinus tabuliformis planted forest and its role in the near-natural cultivation strategy for planted forest management. For. Ecol. Manag. 2011, 262, 413–423. [Google Scholar] [CrossRef]
- Dupuy, J.M.; Chazdon, R.L. Effects of vegetation cover on seedling and sapling dynamics in secondary tropical wet forests in Costa Rica. J. Trop. Ecol. 2006, 22, 65–76. [Google Scholar] [CrossRef]
- De Grandpre, L.; Boucher, D.; Bergeron, Y.; Gagnon, D. Effects of small canopy gaps on boreal mixedwood understory vegetation dynamics. Community Ecol. 2011, 12, 67–77. [Google Scholar] [CrossRef]
- Manrique-Ascencio, A.; Williams-Linera, G.; Badano, E.I. Experimental drought on seedlings in a canopy gap and understory of a tropical cloud forest, Veracruz, Mexico. Acta Bot. Mex. 2022, 129, e2009. [Google Scholar] [CrossRef]
- Tourville, J.C.; Wason, J.W.; Dovciak, M. Canopy gaps facilitate upslope shifts in montane conifers but not in temperate deciduous trees in the Northeastern United States. J. Ecol. 2022, 110, 2870–2882. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhu, C.Y.; Lu, D.L.; Wang, G.G.; Zheng, X.; Cao, J.S.; Zhang, J.X. Regeneration and succession: A 50-year gap dynamic in temperate secondary forests, Northeast China. For. Ecol. Manag. 2021, 484, 118943. [Google Scholar] [CrossRef]
- Li, X.N.; Wang, Y.H.; Yang, Z.H.; Liu, T.; Mu, C.C. Photosynthesis adaption in Korean pine to gap size and position within Populus davidiana forests in Xiaoxing’anling, China. J. For. Res. 2022, 33, 1517–1527. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Zhu, J.J.; Wang, G.G.; Zheng, X.; Lu, D.L.; Gao, T. Dynamics of gaps and large openings in a secondary forest of Northeast China over 50 years. Ann. For. Sci. 2019, 76, 72. [Google Scholar] [CrossRef]
- Flinn, K.M.; Dolnicek, M.N.; Cox, A.L. Gap dynamics and disease-causing invasive species drive the development of an old-growth forest over 250 years. For. Ecol. Manag. 2022, 508, 120045. [Google Scholar] [CrossRef]
- Li, Q.Y.; Du, Y.; Liu, Y.; Chen, J.; Zhang, X.J.; Liu, J.C.; Tao, J.P. Canopy Gaps Improve Landscape Aesthetic Service by Promoting Autumn Color-Leaved Tree Species Diversity and Color-Leaved Patch Properties in Subalpine Forests of Southwestern China. Forests 2021, 12, 199. [Google Scholar] [CrossRef]
- Massad, T.J.; Williams, G.L.; Wilson, M.; Hulsey, C.E.; Deery, E.; Bridges, L.E. Regeneration dynamics in old-growth urban forest gaps. Urban For. Urban Gree 2019, 43, 126364. [Google Scholar] [CrossRef]
| Formation of Forest Gaps | Disturbance Forms | Reference |
|---|---|---|
| Artificial disturbance | Selective logging | Valverde-Barrantes, O.J. (2022) [10] Trindade, A.D. (2021) [20] VanderMolen, M.S. (2021) [21] Moreau, G. (2022) [22] |
| Natural disturbance | Hurricane | Greenberg, C.H. (2021) [9] Xi, W.M. (2019) [23] |
| Typhoon | Yang, H. (2017) [24] Chao, K.J. (2022) [25] | |
| Windstorms | Dietz, L. (2020, 2022) [26,27] Bonanomi, G. (2018) [28] | |
| Ice-storm | Arii, K (2007) [29] Aarssen, L.W. (2004) Lafon, C.W. (2004) [30] | |
| Sleet windthrow | Ravnjak, B. (2022) [31] Fukui, D. (2011) [32] | |
| Wind | Kramer, K. (2014) [33] Berg, E.C. (2019) [34] | |
| Burning | Izbicki, B.J. (2020) [8] Molina, J.R. (2022) [35] de Almeida, BN (2020) [36] | |
| Lightning | Gora, E.M. (2021) [16] Amir, A.A. (2012) [19] | |
| Snow-covered | Bouchard, B. (2022) [17] Mitamura, M. (2009) [37] | |
| Intermittent flooding | Keram, A. (2019, 2021) [18,38] Zhu, C.Y. (2017) [39] | |
| Microburst | Rader, A.M. (2020) [40] | |
| Bark beetle | Worrall, J.J. (2005) [41] Orman, O. (2018) [42] | |
| Epidemics | Nagel, T.A. (2015) [43] Navarro-Cerrillo, R.M. (2014) [44] | |
| Animals (black bears) | Takahashi, K. (2013) [45] |
| Name of the Model | Year of the Model | Author of the Model | Main Views |
|---|---|---|---|
| JABOWA | 1972 | Botkin et al. | The first forest gap model |
| FORET | 1977 | Shugart and West | First time considering sprout tillers update |
| FORTHITE | 1982 | Aber and Melillo | First time considering nutrient cycling and litter decomposition |
| LINKAGES | 1986 | Pastor and Post | The first comprehensive model that includes the whole soil process |
| FORSKA | 1987 | Leemans and Prentice | First time designing the 3D geometry of a forest canopy |
| ZELIG | 1988 | Smith and Urban | The first forest gap model considering the spatial relationships of the samples |
| SORTIE | 1993 | Pacala et al. | The first spatial model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, A.; Halik, Ü.; Fu, W.; Sawirdin, S.; Cheng, S.; Lei, J. Research History of Forest Gap as Small-Scale Disturbances in Forest Ecosystems. Forests 2024, 15, 21. https://doi.org/10.3390/f15010021
Tian A, Halik Ü, Fu W, Sawirdin S, Cheng S, Lei J. Research History of Forest Gap as Small-Scale Disturbances in Forest Ecosystems. Forests. 2024; 15(1):21. https://doi.org/10.3390/f15010021
Chicago/Turabian StyleTian, Aolei, Ümüt Halik, Wentao Fu, Subinur Sawirdin, Shengyuan Cheng, and Jiaqiang Lei. 2024. "Research History of Forest Gap as Small-Scale Disturbances in Forest Ecosystems" Forests 15, no. 1: 21. https://doi.org/10.3390/f15010021
APA StyleTian, A., Halik, Ü., Fu, W., Sawirdin, S., Cheng, S., & Lei, J. (2024). Research History of Forest Gap as Small-Scale Disturbances in Forest Ecosystems. Forests, 15(1), 21. https://doi.org/10.3390/f15010021








