Abstract
The natural distribution of Cycas micronesica includes three island groups. Damage to the widespread tree from the armored scale Aulacaspis yasumatsui was initiated with the 2003 invasion of Guam and the 2007 invasion of Rota. This herbivore has threatened the unique gymnosperm species with extinction. The number and identity of co-occurring consumers are dissimilar among disjunct insular subpopulations, and six of these habitats were used to assess tree mortality trends to confirm that A. yasumatsui stands alone as the greatest threat to species persistence. Following the initial infestation outbreak of this pest into each new subpopulation, the standing seedlings and saplings were the first to be culled, the juvenile plants were the next to be culled, and then the adult trees were killed more slowly thereafter. The timing of this plant population behavior did not differ among habitats with five other consumers, three other consumers, one other consumer, or no other consumers. We have shown that A. yasumatsui acting as the sole biotic threat in an isolated subpopulation can generate a decline in survival that is as rapid as when it is acting in conjunction with up to five other consequential consumers. This armored scale is the most acute threat to C. micronesica, and adding other specialist herbivores to the scale herbivory does not alter the speed and extent of initial plant mortality.
1. Introduction
Invasive alien species comprise one of the consequential drivers of anthropogenic global change [1], and the damage that newly added invasive species cause to resident native species may differ between island habitats and continental habitats [2]. The isolated, insular features that define the evolution of island flora may lead to a lack of resistance to invasive herbivores. Additionally, specialist herbivores that invade an island may proliferate in the absence of natural enemies. For example, the cycad species from Micronesia is described as Cycas micronesica K.D. Hill [3], and there are no known native herbivores that consume leaves. The native Anatrachyntis sp. Meyrick larvae consume microstrobilus tissues after the adults provide entomophilous pollination services [4,5]. The rapid removal of the strobilus tissue is beneficial for hastening the timing of subsequent reproductive events [6]. The native flying fox, Pteropus mariannus Desmarest, consumes the seed integuments [7], which may improve seed germination and may provide a zoochory service. The native stem borer, Acalolepta marianarum Aurivillius, exploits C. micronesica stem cortex tissue as larval food [5,8]. This stem borer exhibits typical stem and bark borer behaviors by avoiding ovipositioning on healthy host trees and selectively targeting unhealthy individuals. In the absence of novel non-native stressors, these three native herbivores do not pose threats to the host tree with which they coevolved.
Non-native species that invade these Micronesian islands with a specialized affinity for consuming cycad leaf tissue may be at an advantage in the insular habitats because no pre-existing natural enemies exist and the host tree possesses no evolved resistance. The beginning of the 21st century was not a good time in this regard for C. micronesica, as the armored scale Aulacaspis yasumatsui Takagi, the specialist butterfly Luthrodes pandava Horsfield, and the microlepidoptera leaf miner Erechthias Meyrick sp. were first identified on Guam between 2003 and 2005 [5,8,9]. These three herbivores exhibited rapid movement throughout the island because the host tree was the most abundant tree in Guam’s forests at the time [10]. The newly developed residence of A. yasumatsui in Micronesia led to secondary invasions of Rota in 2007 and the urban areas of Palau in 2008 [11]. The islands of Yap contain C. micronesica populations that remain free of the threats of these invasive insect herbivores.
This case study has been extensively studied. For example, interactions among the invasive insect herbivores [5,12,13] and long-term changes to Guam’s C. micronesica populations [14,15,16] have been reported as consequences of the A. yasumatsui invasion. The number of herbivores and omnivores that feed on C. micronesica is considerable, but A. yasumatsui is the only one that is known to be lethal when acting alone [5]. One factor that has not been adequately reported involves how the consequential co-occurring herbivores in a subpopulation influence the plant’s initial mortality response to the A. yasumatsui invasion. Therefore, the aims of this study were to determine how the C. micronesica population within each of six disparate subpopulations with differing abiotic conditions and numbers of competing consumers responded immediately following the onset of A. yasumatsui infestations. Would the addition of other consumers exert any consequential influence on the features of early host tree mortality caused by A. yasumatsui? The answer to this question may aid conservationists by improving predictions of the timing and extent of host plant mortality as the A. yasumatsui invasive range continues to expand to other islands.
2. Materials and Methods
Six sites on the islands of Guam and Rota were selected for the establishment of plots designed to determine changes to C. micronesica plant density and demography over a period of three years following the initial outbreak of A. yasumatsui within each site. The sites were selected to ensure disparity in abiotic factors and the number of co-occurring herbivore threats to the host tree. In Guam, site selection was further defined by geographic distance and high plant density. In Rota, site selection was further defined by the inclusion of the three disjunct areas of occupancy.
2.1. Site Descriptions
The initial A. yasumatsui infestations ranged from May 2005 in the northern Guam site until April 2010 in the western Rota site (Table 1). The invasive Rota population required three years to enter a western Rota habitat because the small forest fragment was located on an isolated peninsula that was separated from the remaining forested habitats by an urban area. These sites exhibited great disparity in aspect and ranged in elevation from 6 to 334 masl. Although α-diversity was similar in all of the habitats, β-diversity was dissimilar. The dominant species were not the same within the footprint of the designated plots (Table 1).

Table 1.
Characteristics of six habitats containing high-density Cycas micronesica plants on Guam and Rota. The armored scale Aulacaspis yasumatsui invaded each of the habitats, leading to rapid host plant mortality.
The six sites were highly contrasting in number and identity of co-occurring consequential consumer species (Table 2). In addition to the two invasive lepidopteran leaf herbivores and the native stem borer, some C. micronesica habitats in Guam were impacted by non-native ungulates. These included the omnivore feral pig (Sus scrofa L.) and the herbivore naturalized deer (Rusa marianna Desmarest). The level of damage to plant health that each of these consumers causes has been discussed elsewhere [5]. The disparity in ungulate damage among the Guam sites was a result of differential access by local hunters. Site 1 in Guam was located within a military installation where local hunting is highly regulated and restricted [17], and this was the site with abundant pig and deer damage. In contrast, local hunters are allowed unrestricted access to Sites 2 and 3, and these sites never revealed ungulate damage to C. micronesica trees. One of the six sites exhibited signs of direct plant herbivory from all five consumers, and one of the sites was devoid of all five consumers (Table 2). The other four sites contained one to three of the co-occurring consumers.

Table 2.
The occurrence of consequential herbivores and omnivores among six subpopulations containing high-density Cycas micronesica plants in Guam and Rota. The armored scale Aulacaspis yasumatsui invaded each of the habitats, adding to the threats to the host tree species. A check mark indicates the presence of an herbivore or omnivore.
Sites 1 (centered at 13.5541 N, 144.9376 E) and 2 (13.3376 N, 144.6683 E) were located in calcareous soils with abundant karst outcrops (Clayey-skeletal, gibbsitic, nonacid, isohyperthermic Lithic Ustorthents). Site 3 (13.2651 N, 144.7119 E) was supported with a volcanic clay soil that was poorly drained and subject to erosion (Clayey, montmorillonitic, isohyperthermic, shallow Udic Haplustolls). The littoral soils in Site 4 (14.1825 N, 145.2094 E) were sands (Loamy-skeletal, carbonatic, isohyperthermic Lithic Haplustolls). The alkaline soils in Site 5 (14.1376 N, 145.2247 E) contained fewer rock outcrops than sites 1 and 2 (Clayey-skeletal, kaolinitic, isohyperthermic Lithic Haplustolls). The soils in Site 6 (14.1272 N, 145.1262 E) exhibited chemical and physical properties that were similar to those in Sites 1, 2, and 5, although the soil series differed (Loamy, oxidic, nonacid, isohyperthermic Lithic Ustorthents). The latitudinal range of this study was approximately 100 km, and the longitudinal range was 60 km. The two closest sites were separated by 6 km. Although the sites varied in edaphic and topographic characteristics, there were no climate differences within the footprint of this study. The most influential weather factor that may have caused heterogeneous plant damage among the sites was the occurrence of tropical cyclones. There were no consequential tropical cyclones that influenced the plant population between 2005, when data collection was initiated at Site 1, and 2013, when data collection was terminated at Site 6.
2.2. Data Collection
Within each of the sites, four 20 m × 20 m plots were established when A yasumatsui had entered the habitat but before there were any signs of infestation within each plot’s boundaries. From experience, this meant A. yasumatsui would enter the plots within weeks of the initial plant survey. The distance among the four plots within each site was not fixed, and the maximum distance ranged from 830 m in the western Rota site to 470 m in the northeast Guam site. The stem count and height of every C. micronesica stem were recorded initially, and then these same measurements were repeated over a three-year period. The sequential population counts were conducted more often on Guam, but the Rota counts were never separated by more than 12 months. These methods enabled an annual assessment of plant mortality using the date of initial A. yasumatsui herbivory as the starting date for each site.
Plants with stems less than 20 cm in height were designated as newly emerged seedlings if the leaf number was four or less and the stem height was difficult to measure, and designated as saplings if the leaf number was greater than four and the stem height could be easily measured. Plants with stems between 20 and 100 cm in height were designated as juveniles, as reproductive structures are rarely observed on in situ individuals less than 100 cm in height. The adult plants were separated into 100–200 cm, 200–300 cm, and 300+ cm demographic categories. As a result, there were six plant size categories. There were 548 individuals in the initial census of Site 1, 513 individuals in Site 2, and 563 individuals in Site 3. Rota habitats contained more initial plants within the areas of occupancy than did Guam habitats. There were 1992 individuals in the initial census of Site 4, 1772 individuals in Site 5, and 1816 individuals in Site 6.
2.3. Data Analysis
Our primary objective was to determine how annual changes in alive C. micronesica plants caused by A. yasumatsui herbivory differed among the sites in order to tease apart the influence of co-occurring consumers. Long-term survival of C. micronesica has been studied in many habitats on Guam [14]. In order to determine if our six sites behaved similarly to the sites that were previously reported, the survival of the entire population within each plot was tabulated over the four measurement periods to compare overall survival among the years. The data did not conform to parametric prerequisites, so the non-parametric Kruskal–Wallis H test was used to compare the four observation years. The means were separated using a post-hoc Dunn-Bonferroni test. Second, the data from each of the years were tabulated separately within size categories to visualize the changes in population demography. Frequency distributions of plant size within each site and year were created, then kurtosis (κ) and skewness (Skp) were calculated [18]. Third, models were created based on coefficients that described the proportion of surviving plants within each size category at the end of each year. Models were developed from the Guam data, and these models were used with pre-invasion Rota data to generate predicted survival after one, two, or three years. Models were developed from the Rota data, and these models were used with pre-invasion Guam data to generate predicted survival after one, two, or three years. Fourth, the results from each of the individual plots were subjected to linear regression to determine significance and fit for the actual versus predicted population of surviving plants.
3. Results
The number of initial C. micronesica plants within the 24 plots in this study exemplified why this tree was considered a foundation species prior to the A. yasumatsui invasion. A total of 7204 individual plants were monitored in this study. The first year of A. yasumatsui herbivory caused a 70% reduction in stem count among these sites (Figure 1). By the end of year two, the population had been reduced by 81%. The surviving plants after three years of herbivory represented 6% of the original population.
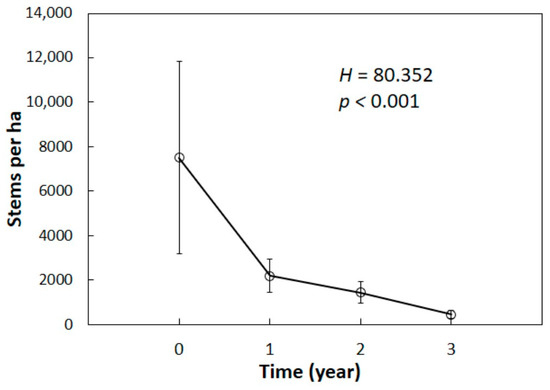
Figure 1.
The number of Cycas micronesica stems per hectare increased four years after being invaded by Aulacaspis yasumatsui. It means 24 plots throughout Guam and Rota. Each year was different from the other years, according to the Dunn-Bonferroni test. Mean ± SD, n = 24.
3.1. Initial Population Characteristics
The three Guam sites were dominated by plants in the smallest size categories, with newly emerged C. micronesica seedlings and saplings combining to represent most of the population (Figure 2). Site 1 exhibited platykurtic kurtosis (negative κ), indicating similar frequencies among the size categories, but the other two sites exhibited moderate to high leptokurtic kurtosis with many outliers (positive κ). The substantial number of juvenile plants in Site 1 compared to the other five sites caused the frequency relationships to be less peaked. All three Guam sites exhibited positive skewness. The total population consisted of 3422 ± 138 plants per ha in Site 1, 3208 ± 221 plants per ha in Site 2, and 3519 ± 415 plants per ha in Site 3. Site 1 was the only site with fewer newly emerged seedlings than juveniles, although the sum of the seedlings and saplings exceeded the stem count for juveniles.
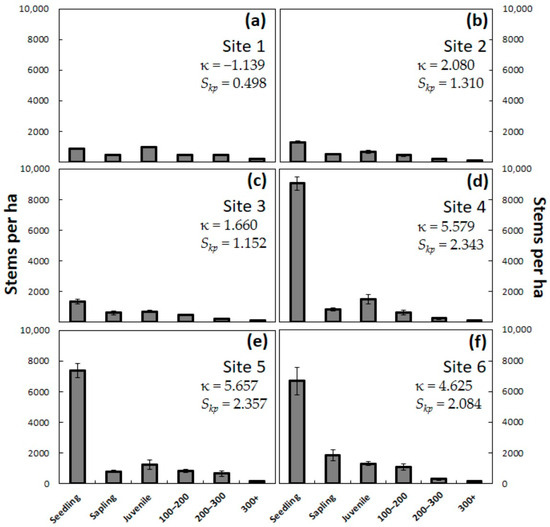
Figure 2.
The number of Cycas micronesica stems per hectare for six plant size categories before the invasions of Aulacaspis yasumatsui into the habitats. (a) Northeast Guam; (b) Southwest Guam; (c) South Guam; (d) Northeast Rota; (e) Southeast Rota; (f) West Rota. Mean ± SE, n = 4.
The three Rota sites exhibited an even greater proportional representation of the smallest plant size categories and plant densities that greatly exceeded those of the Guam sites (Figure 2). In contrast to the Guam sites, the Rota populations universally exhibited high leptokurtic kurtosis (positive κ), indicating uneven frequency distributions among the size categories. The positive skewness at the Rota sites greatly exceeded the skewness at the Guam sites. The total population consisted of 12,450 ± 628 plants per ha in Site 4, 11,075 ± 638 plants per ha in Site 5, and 11,350 ± 821 plants per ha in Site 6. The isolated forest fragment in Site 6 was the only site with more saplings than juveniles, indicating greater potential for recruiting from the seedling to the sapling stage than for the other sites.
This population structure containing numerous seedlings, saplings, and juveniles indicated healthy C. micronesica populations with abundant regeneration and recruitment potential for all sites prior to the invasions of A. yasumatsui. The extremely high seedling counts in Rota indicated greater regeneration potential than in Guam. In contrast, the juvenile plant counts were more similar among the six sites, indicating recruitment potential was similar for the two islands.
3.2. First Year Population Response
One year of A. yasumatsui damage eliminated all of the pre-existing C. micronesica seedlings and saplings from all six Guam and Rota habitats (Figure 3). From 3% to 5% of the juvenile plants also died during the first year of herbivory. In contrast, no trees greater than 100 cm died as a result of one year of A. yasumatsui herbivory. This population-level response caused a drastic change in kurtosis, with all sites exhibiting values closer to zero, which indicated less peaked frequency distributions. These changes were more dramatic at the Rota sites because the excessive frequencies of the smallest size categories were removed from the analysis. Skewness also became more similar among the six sites, with most sites exhibiting moderately positive Skp values. The complete absence of seedlings and saplings from these habitats after experiencing herbivory by this invasive insect indicated an unhealthy C. micronesica population with no evidence of regeneration. The total population of Guam sites consisted of 2052 ± 81 plants per ha in Site 1, 1383 ± 190 plants per ha in Site 2, and 1540 ± 183 plants per ha in Site 3. The total population of Rota sites consisted of 2481 ± 387 plants per ha in Site 4, 2861 ± 393 plants per ha in Site 5, and 2779 ± 132 plants per ha in Site 6. The total population was reduced by 40%–61% on Guam and by 74%–80% on Rota by the end of 12 months of herbivory, the difference reflecting the much greater proportion of Rota’s pre-invasion population consisting of seedlings and saplings.
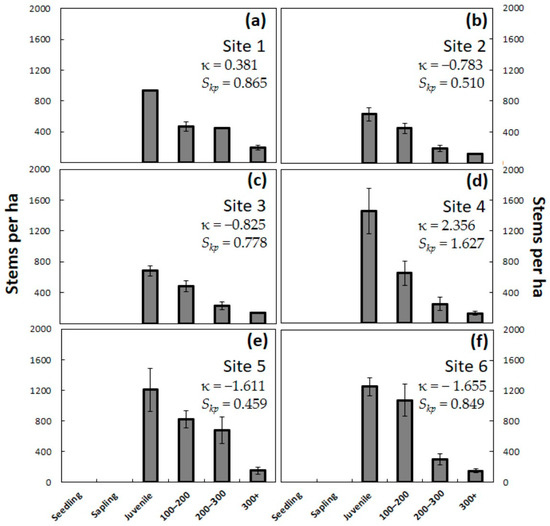
Figure 3.
The number of Cycas micronesica stems per hectare for six plant size categories after one year of Aulacaspis yasumatsui herbivory. (a) Northeast Guam; (b) Southwest Guam; (c) South Guam; (d) Northeast Rota; (e) Southeast Rota; (f) West Rota. Mean ± SE, n = 4.
3.3. Second Year Population Response
The second year of chronic A. yasumatsui herbivory began to take a heavy toll on the juvenile plants that were 20–100 cm in stem height (Figure 4). From 42% to 52% of these juvenile plants were dead at the end of two years of herbivory. After exhibiting no mortality during the first year, mature trees greater than 100 cm in stem height also began to die during the second year. From 23% to 26% of the mature trees died by the end of two years of herbivory. These ongoing changes in population-level survival caused increases in the platykurtic kurtosis compared to year one, indicating fewer outliers among the plant size categories as the smallest plants continued to be culled. Skewness became more dissimilar among the sites after two years of herbivory. For the first time, three of the sites exhibited weak Skp values, indicating approximate symmetry. The total population of Guam sites consisted of 1330 ± 56 plants per ha in Site 1, 954 ± 131 plants per ha in Site 2, and 998 ± 117 plants per ha in Site 3. The total population of Rota sites consisted of 1581 ± 272 plants per ha in Site 4, 1913 ± 232 plants per ha in Site 5, and 1823 ± 98 plants per ha in Site 6. The total population was reduced by 61%–72% on Guam and by 83%–87% on Rota by the end of 24 months of herbivory. This first year of the widespread deaths of juveniles included the smallest juveniles. As a result, there were no C. micronesica plants less than 50 cm in stem height by the end of two years of herbivory.
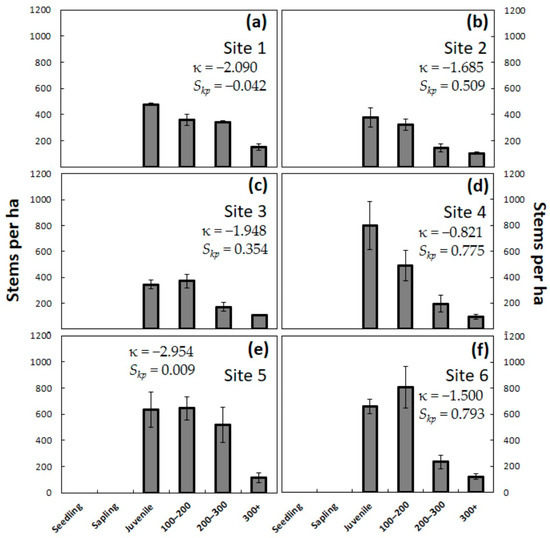
Figure 4.
The number of Cycas micronesica stems per hectare for six plant size categories after two years of Aulacaspis yasumatsui herbivory. (a) Northeast Guam; (b) Southwest Guam; (c) South Guam; (d) Northeast Rota; (e) Southeast Rota; (f) West Rota. Mean ± SE, n = 4.
3.4. Third Year Population Response
The third year of chronic A. yasumatsui herbivory eliminated almost all of the juvenile plants that were 20–100 cm in stem height (Figure 5). The speed of attrition of adult plants greater than 100 cm in height that began in the second year did not slow down, as an even greater number of adults died during year three. From 61% to 63% of the mature trees died during the second and third years of herbivory. These ongoing changes led to an increase in the peakedness of κ among the sites, with four sites exhibiting leptokurtic kurtosis for the first time. All six sites increased in skewness compared to year two, with Sites 2–6 exhibiting moderate to high positive Skp values. The total population of Guam sites consisted of 443 ± 30 plants per ha in Site 1, 301 ± 41 plants per ha in Site 2, and 349 ± 45 plants per ha in Site 3. The total population of Rota sites consisted of 399 ± 79 plants per ha in Site 4, 632 ± 79 plants per ha in Site 5, and 601 ± 71 plants per ha in Site 6. The total population was reduced by 87%–91% on Guam and by 94%–96% on Rota by the end of 36 months of herbivory. The difference again reflected the fact that seedlings and saplings comprised such a high percentage of the pre-invasion Rota population. There were no C. micronesica plants less than 90 cm in stem height by the end of three years of herbivory.
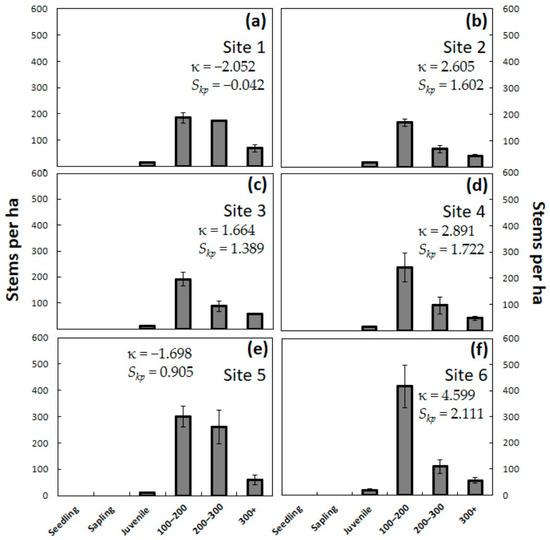
Figure 5.
The number of Cycas micronesica stems per hectare for six plant size categories after three years of Aulacaspis yasumatsui herbivory. (a) Northeast Guam; (b) Southwest Guam; (c) South Guam; (d) Northeast Rota; (e) Southeast Rota; (f) West Rota. Mean ± SE, n = 4.
3.5. Predictive Equations
The relative consistency of how the different plant size categories responded to initial A. yasumatsui herbivory enabled the creation of predictive population survival equations. One model was created based on survival from the 12 Guam plots for use with the pre-invasion Rota data, as this assured no autocorrelation in the regression methods. Using the population data from Guam, by the end of year one, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.957) + (adults × 1.000).
By the end of year two, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.517) + (adults × 0.757).
By the end of year three, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.023) + (adults × 0.380).
The initial population assessment that was conducted prior to any signs of A. yasumatsui herbivory was then used to calculate the predicted population within each of the 12 Rota plots for years 1, 2, and 3 using coefficients from the three Guam equations. This predicted population metric was regressed onto the actual Rota data from each plot after each of the years of herbivory to determine how well the actual data conformed to the predictive data for each plot (Figure 6). Every plot exhibited a tight fit between actual survival and predicted survival.
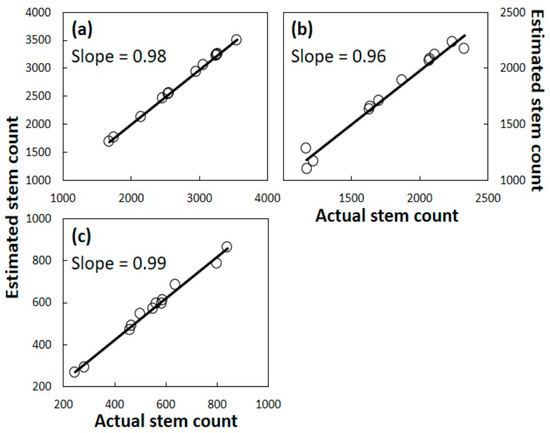
Figure 6.
Cycas micronesica stems per ha on Rota after mortality caused by Aulacaspis yasumatsui herbivory. Linear regression relationship between estimated stems based on models derived from Guam data and actual Rota data. (a) Year 1; (b) Year 2; (c) Year 3.
A second model was created from the 12 Rota plots for use with the pre-invasion Guam data. Using the population data from Rota, by the end of year one, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.967) + (adults × 1.000).
By the end of year two, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.517) + (adults × 0.763).
By the end of year three, the population will be comprised of:
Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.010) + (adults × 0.377).
The initial population assessment that was conducted prior to any signs of A. yasumatsui herbivory was then used to calculate the predicted population within each of the 12 Guam plots for years 1, 2, and 3 using coefficients from the three Rota equations. This predicted population metric was regressed onto the actual Guam data from each plot after each of the years of herbivory to determine how well the actual data conformed to the predictive data for each plot (Figure 7). As with the Rota plots, these Guam plots revealed a conformity between actual survival counts and predicted survival.
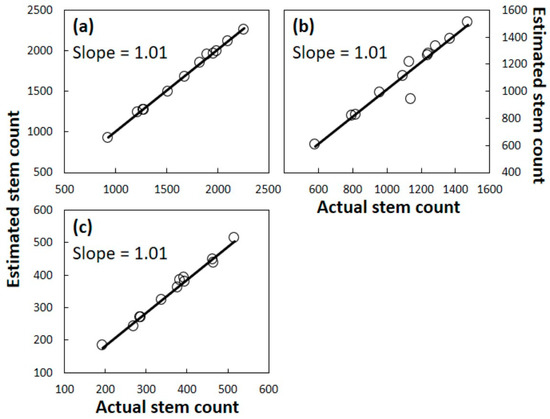
Figure 7.
Cycas micronesica stems per ha on Guam after mortality caused by Aulacaspis yasumatsui herbivory. Linear regression relationship between estimated stems based on models derived from Rota data and actual Guam data. (a) Year 1; (b) Year 2; (c) Year 3.
4. Discussion
Cycas micronesica experienced no major threats prior to 2003, when it was the most abundant tree species on Guam [10] and was thriving as a foundation species throughout its native range. Today, this unique tree is listed as Endangered under the IUCN Red List [19] and Threatened under the United States Endangered Species Act (ESA) [20]. The IUCN listing was initiated in 2006, revealing the speed with which invasive specialist herbivore species can alter insular ecosystems if an abundant native plant serves as a host for the herbivore. Although long-term trends in mortality of the tree have been reported [14,21], this is the first study to show that the presence of other consequential consumers [5] found on Guam and Rota did not influence the speed and demographic traits of the initial years of mortality after herbivory by A. yasumatsui was initiated within a new subpopulation. One of our sites contained five consequential co-occurring herbivores and omnivores, and one of our sites contained no other observable consumers, yet the influence of plant size on the mortality response was homogeneous among all 24 of our plots. Indeed, the Guam model coefficients were able to predict the Rota outcomes with accuracy, and the Rota model coefficients were able to predict the Guam outcomes with accuracy.
The pre-invasion C. micronesica populations contained seedlings and saplings as the most abundant demographic category, and this entire category was eliminated immediately during the first year. By the end of year one, the combination of data from all 24 plots in this study indicated a new subpopulation responding to A. yasumatsui herbivory, which can be predicted by subjecting the pre-invasions data to the equation: Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.962) + (adults × 1.000). The second year of scale damage eliminated all individuals less than 50 cm in height and began to cull some of the adult trees. By the end of year two, the combination of data from all 24 plots in this study indicated a new subpopulation responding to A. yasumatsui herbivory, which can be predicted by the equation: Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.517) + (adults × 0.760). By the end of the third year, there were no living individuals less than 90 cm in height, and more than half of the plants greater than 90 cm had been killed. By the end of year three, the combination of data from all 24 plots in this study indicated a new subpopulation responding to A. yasumatsui herbivory, which can be predicted by the equation: Stems per ha = (seedlings × 0.000) + (saplings × 0.000) + (juveniles × 0.016) + (adults × 0.378).
Our previous long-term studies on Guam indicated that no C. micronesica trees were less than 100 cm in height after 5 years of herbivory in one location [21] and after 9 years in all 12 locations [14]. Moreover, by 2020, the smallest individuals measured on Guam were 175 cm in height, indicating the elimination of every small individual by this hemipteran pest deleted at least 70 years of population-level recruitment [22]. Even if conservation efforts are successful in beginning to mitigate the biotic threats and the tree species begins to recover in the future, this extensive recruitment gap will leave a permanent mark on the demographic traits of the tree populations into the future. This permanent mark will be at least 2 m in vertical stem length and will persist for centuries into the future. Heeding the early advice to construct a coalition of biological control organisms [23,24] would not have allowed these permanent changes to demography to occur.
One profound confirmation for the assertion that A. yasumatsui is the most influential threat to C. micronesica is illuminated by looking more closely at the comparison of Site 1 and Site 6. Site 1 contained five co-occurring herbivores or omnivores, exhibited the least healthy pre-invasion population, and became infested in May 2005. In contrast, Site 6 was herbivore-free when A. yasumatsui initially infested the forest fragment in April 2010. While both sites experienced the elimination of seedlings, saplings, and most juveniles, 34% of the adults in Site 1 but only 31% of the adults in Site 6 were persisting after three years of damage by the armored scale herbivore. Clearly, the absence of co-occurring consumers in Site 6 did not offer any benefits to the host tree population when it was forced to respond to the first three years of the A. yasumatsui invasion.
4.1. Practical Applications
The utility of this new knowledge may be profound for predicting how a newly invaded C. micronesica subpopulation will respond for three years. Indeed, the predicted number of plant deaths based on pre-invasion subpopulation data in each of the 24 plots was remarkably consistent with the actual plant deaths over the timeline of this study. Therefore, conservationists may obtain pre-invasion density and demography data that can be used to accurately predict the dynamics of population changes during the first three years of A. yasumatsui herbivory. By the end of three years of A. yasumatsui herbivory, a once-thriving C. micronesica population is predicted to consist of about 2% of the initial juvenile population and about 38% of the initial adult population.
Over the course of the first two years, new seedlings are predicted to germinate from the pre-existing seed bank and from the dispersal of seeds from megastrobili that were close to maturity when A. yasumatsui herbivory was initiated. These seedlings are predicted to be killed shortly after germination, with no recruitment to the sapling stage. No newly germinated seedlings will be found after year two. Megastrobili that develop after the A. yasumatsui invasion are predicted to contain undeveloped ovules at the end of three years, but no developing seeds will reach maturity in the C. micronesica habitats at this stage.
4.2. The Other Consumers
Site 1 contained the least number and proportion of seedlings and saplings among the three Guam sites, even though the observed number of fecund female trees and seeds in the standing trees was no less at this site than at the other sites. Signs of soil disturbance due to pig rooting were widespread. These observations indicated that the relatively low seedling and sapling counts were likely due to the rooting and seed herbivory of this feral omnivore.
The pre-invasion data indicated that Site 6 exhibited the greatest evidence of recruitment from seedling to sapling stage among the Rota sites. This was the only site that did not contain L. pandava herbivory. These observations indicated that the decreased recruitment of seedlings to saplings in Rota Sites 4 and 5 was likely due to the long-term pressures of butterfly herbivory.
When a foundation tree species is selectively removed from an island’s forests, the conservation community is expected to understand the possible influences on other native organisms that rely on the tree species in order to consider the potential for coextinction. The microlepidoptera mutualist pollinators are the most important native animals to discuss in this regard. Our observations indicate that the Mariana, Western Caroline, and Palau islands contain three different taxa of pollinators. Moreover, we do not know of an alternate larval food that augments C. micronesica microstrobilus tissue herbivory. Therefore, the pollinator taxon from the Mariana Islands of Guam and Rota appears to be a two-island endemic species, and these native animals have been and remain threatened by the loss of C. micronesica trees.
The native flying fox, P. mariannus, is a frugivore and may rely on C. micronesica seed availability during seasons when other preferred fruits are difficult to find [7]. The invasion of A. yasumatsui has threatened this source of food for the flying fox communities in Guam and Rota [25].
The specialist butterfly, L. pandava, appears to be in direct competition with A. yasumatsui. In Guam, ephemeral irruptions of A. yasumatsui were accompanied by decreases in L. pandava herbivory, and periods of decreased A. yasumatsui infestations were accompanied by increases in L. pandava herbivory [12]. The incidence of A. yasumatsui on Guam has declined in recent years for unknown reasons [26], and this development may generate an increase in butterfly herbivory in the near future.
The native stem borer A. marianarum poses a secondary threat to C. micronesica because stem borer damage increases after any abiotic or biotic stress that reduces the health of the host tree, and therefore irruptions of the non-native A. yasumatsui are followed by irruptions of the native A. marianarum [12]. Subsequently, the incidence of windsnap during tropical cyclones is increased by the A. marianarum stem herbivory [26,27], illuminating how the invasion of A. yasumatusi has led to cascading negative interactive outcomes. This case study reveals a conservation conundrum because the native beetle is no less deserving of conservation than the host tree, C. micronesica.
Within the context of population dynamics and recruitment behaviors of Anatrachyntis sp., P. mariannus, and A. marianarum, we view C. micronesica as a keystone species [28]. The immense population loss and the less frequent production of strobili among the living trees have undoubtedly decimated the services that this native tree species provides for these native animals. In addition to fostering the recovery of regeneration and recruitment of C. micronesica, conservationists may also need to study and foster re-entanglement of the food webs that have been disrupted. We believe the Anatrachyntis populations may be at greater risk than any other involved taxon, including C. micronesica, because the Guam and Rota pollinator species likely occur nowhere else worldwide.
4.3. Biological Control in the Study Sites
Ongoing work within the native range of the armored scale in Thailand has revealed that C. micronesica trees are not threatened by chronic infestations of A. yasumatsui, and the ex situ plant populations are healthy and thriving as a result of widespread native biological control organisms that maintain the A. yasumatsui density below damaging levels [28]. These observations support the contention that the establishment of a biological control program on Guam and Rota would adequately mitigate the threats to C. micronesica that are imposed by A. yasumatsui [26,27].
The armored scale predator Rhyzobius lophanthae Blaisdell was intentionally released in Guam in 2005 and in Rota in 2007 [5]. The predator entered each of our six research sites shortly after A. yasumatsui entered each subpopulation, either naturally or by anthropogenic distribution and release. Therefore, the initial plant mortality in each plot occurred in the presence of this scale predator. Ongoing plant mortality has not ceased, and the predator is clearly not sufficient to fully mitigate the lethal threat caused by the armored scale. The limitations of R. lophanthae have been discussed elsewhere [5,11,28].
Attempts to introduce the parasitoids Aphytis lingnanensis Compere and Coccobius fulvus Compere & Annecke to Guam were unsuccessful [5]. However, the fortuitous presence of the parasitoid Arrhenophagus chionaspidis Aurivillius was first noticed on Guam in 2013 [5]. The Guam portion of the present study was terminated before this date, so there was no parasitoid biocontrol of A. yasumatsui within any of our six study sites.
4.4. Species Conservation
When a cornucopia of anthropogenic, abiotic, and biotic threats coalesce to endanger a tree species, developing a conservation action plan to slow down plant mortality may require a triage approach where the single greatest threat is identified and then countered with the greatest level of conservation efforts. For this approach to be successful, knowledge about each of the threats needs to be generated through observation and experimentation, and then those facts need to be communicated to the empowered decision-makers who control funding and programming of conservation efforts. In order to enable the most effective conservation actions, this communication best occurs through peer-reviewed journal publications that are filtered through the expert vetting process. Therefore, developing a conservation action plan to mitigate the threats and a recovery plan to rebuild the plant populations depends on bringing knowledgeable research biologists into the agenda, as these are the contributors who possess the wherewithal to compile and interpret the copious information from myriad directions.
What is the primary threat to C. micronesica? All evidence to date indicates that land conversion is not a consequential threat. The loss of C. micronesica trees due to construction activities has not occurred to a great extent on Rota, Yap, or Palau and is restricted to military lands on Guam. The forests that have experienced the loss of C. micronesica trees within federal construction sites contain numerous trees in the habitats adjacent to the construction sites, and in situ conservation of these trees constitutes the most effective mitigation endeavor to counteract the consequence of land conversion [26]. The other native and non-native consumers that have been discussed are also not primary threats to the tree species; they are secondary threats. The consequential historical C. micronesica plant mortality since 2003 has been a result of A. yasumatsui herbivory. The threat is manifested as direct threats caused by carbohydrate depletion that leads to individual plant mortality following A. yasumatsui damage [29], but there are also cascading threats that only emerge because of antecedent A. yasumatsui herbivory. These include lethal damage during and after tropical cyclones [26,27,30] and herbivory from other animals that exploit the increased vulnerability of the unhealthy trees [5,12].
The results herein provide more evidence to support the contention that A. yasumatsui alone persists as the greatest single threat to the host tree. A predictive equation based on Guam data calculated with accuracy the mortality from the three Rota subpopulations. Similarly, the predictive equations based on Rota data calculated with accuracy the mortality from the three Guam subpopulations. These outcomes occurred even though the number of co-occurring consumers and abiotic conditions varied greatly among the sites.
This new information is valuable for two reasons. First, the development of an adequate biological control program that manages A. yasumatsui below lethal densities would enable the recovery of the C. micronesica population [26]. Second, the focus of emergency conservation funding and planning to ensure the host tree species can persist into the future can justifiably ignore the other co-occurring threats, such as salvage from construction sites, to fully focus all resources on biological control of the armored scale. These secondary threats would inadvertently decline without any management if the primary threat were adequately addressed. Therefore, contemporary conservation projects designed to translocate trees from construction sites unjustifiably consume available financial resources and do not contribute to the actionable conservation of C. micronesica. These large-scale tree transplanting and propagation projects have consumed millions of dollars in attempts to rescue trees from federal construction sites on the island of Guam. Our results indicate that these expensive projects are ill-informed and will not generate any benefit to species conservation because the primary threat is not being addressed in any capacity.
Establishing a coalition of specialist biological control organisms to maintain the A. yasumatsui herbivory below lethal levels was the greatest conservation need in 2003 on Guam and in 2007 on Rota. This conservation action was the most important need when the species was Red-listed in 2006 [19] and ESA-listed in 2015 [20]. In order to address the persistence of C. micronesica into the future, this endeavor, which was recommended by the scientific community in 2005 [23,24] remains the greatest conservation need today. Invasive species impose an enormous cost on society [31], and group apathy toward the control of invasive species cannot be good for society. This is essentially what describes the past two decades in Guam. These types of difficult conservation needs define many biodiversity threats that are global in nature and are sometimes described as wicked problems [32,33,34,35]. While the management of biological control programs has been implemented for more than a century [36], many case studies are characterized by difficult hurdles [37]. Turning the corner toward success within these case studies cannot occur without collaborations among all stakeholders, including the scientists who possess the knowledge to inform each decision within information-deficit conditions [36,38]. Being successful in solving the wicked problems of conservation requires a change in the manner in which decision-makers interact with the problems. The Guam and Rota invasive species control needs demand a multi-year, multi-discipline approach [28].
4.5. Island Comparisons
There are three island groups that comprise the indigenous range of C. micronesica. The Mariana Island of Guam stands alone in containing vast areas of occupancy that stretch for tens of kilometers and traverse numerous soil and habitat types. The Mariana island of Rota and the Caroline island of Yap are similar in that three disjunct areas of occupancy make up three subpopulations. The plant density within these subpopulations is immense in Yap and was immense in Rota prior to the 2007 A. yasumatsui invasion. The independent state of Palau contains numerous island subpopulations, but all are limited in plant number and geographic range. Land conversion within any of the conscribed subpopulations in Rota, Yap, and Palau would be devastating to the global genetic diversity of the tree species. But land conversion in Guam is inconsequential to species survival because so many trees persist in the same habitats adjacent to the construction sites that are causing the land conversion. In situ conservation of these populations adjacent to the construction sites would benefit species survival without the expense and added health threat that is required to excavate, propagate, and translocate from the construction sites [39]. The greatest conservation needs for Guam and Rota are to obey the 2005 recommendations by scientists [23,24] and ensure biocontrol becomes a reality. The greatest conservation needs for Yap and the isolated islands of Palau are to implement appropriate hygiene protocols for human travelers to ensure A. yasumatsui is not vectored into the uninfested islands.
The data herein are the first C. micronesica density data to be reported from the island of Rota. There were three known areas of occupancy at the time of the A. yasumatsui invasion. The plant density within these Rota habitats was extensive, with more than 1000 adults per ha. These same densities were reported from the Yap subpopulations [14]. If the conservation deciders allow local extirpation of any of the six Rota or Yap subpopulations, the result will be extensive genetic erosion that cannot be recovered.
The unusually high absolute and proportional numbers of pre-invasion juvenile plants in northeast Guam (Site 1) were also reported from a northwest Guam location [21]. These two northern coastal habitats were positioned in the terrain that received massive environmental destruction from the bombing raids imposed by the United States military when Guam was recaptured following the Japanese occupation of WWII. This bombardment began during the first week of May 1944 and reached its greatest intensity in July 1944 [40]. Therefore, aircraft and battleship firepower destroyed Guam’s terrain, demolished civilian dwellings, and killed civilian residents for more than two months in order to lessen military casualties when United States troops began to land on 21 July 1944. This environmental destruction from months of conventional wartime firepower occurred because the United States enabled the Japanese occupation by abandoning the legal responsibility to defend Guam in 1941 [41]. In this study, Site 1 exhibited a number of juvenile plants, which was much greater in proportion to the adult tree categories than at any other site. These extreme numbers of juvenile plants may illuminate a lingering demographic consequence of the destruction of the forest resources 60 years prior and the permanent mark on C. micronesica subpopulations. Sites 2 and 3 in Guam were located in isolated forests in remote areas that were not likely targeted by the bombing of 1944. Similarly, the islands of Rota, Yap, and Palau did not receive military bombardment during WWII.
4.6. Lessons Learned
Our results directly inform the conservation decisions that are needed in Guam and Rota, with several applications previously discussed. But the cumulative actionable research in this case study highlights lessons that may have regional or global application.
Models applicable throughout the genus range. The genus Cycas is the most speciose and widespread genus of cycad [42], with numerous insular subpopulations throughout this range. This entire range remains at risk from A. yasumatsui. This armored scale has invaded the Ryukyu Archipelago in recent months [43], and these islands comprise the endemic range of Cycas revoluta Thunb. Therefore, the Yap and Palau subpopulations of C. micronesica and the uninvaded subpopulations of C. revoluta in the Ryukyu islands exhibit the greatest risk of future A. yasumatsui invasion. Improving our ability to predict future outcomes in conservation science is an emerging challenge for the global conservation community [44]. We believe our predictive equations from Guam and Rota will accurately define the plant response in all of these uninvaded subpopulations if an invasion occurs. Local scientists will be required to collect plant density and demography data prior to any plant mortality in order to capitalize on this predictive power.
Benchmarking. Our data illuminate the importance of securing population-level descriptors prior to the onset of damage by an acute threat in order to provide future conservationists with a benchmark or baseline foundation. When apathy and procrastination allow nascent threats to impose large-scale population changes before the conservation community decides to fund and conduct actionable research, subsequent species recovery goals are devoid of the requisite benchmark data that informs those goals. In the case of a Cycas subpopulation threatened by A. yasumatsui, a delay of only a few months will cause the collection of accurate benchmark data to be impossible. For example, four of our sites exhibited leptokurtic kurtosis before A. yasumatsui damage, then exhibited platykurtic kurtosis only 12 months after the damage ensued. Our results show that a delay of only 3 years will result in the failure to document 90% of the pre-existing population. How can one restore a plant population if one does not know the restoration goal as defined by pre-threat descriptors? This issue is at the core of the unsuccessful funded projects in Guam. The funding agency has employed a revolving door policy for the biologists empowered with managing the sequential projects, as none of them possess direct cycad biology education and all are replaced before they have enough time to develop appropriate knowledge. Newly hired practitioners who possess no verifiable cycad biology knowledge are allowed to spend millions of dollars. Parachute scientists [45] from United States universities are flown into Guam to guide decisions about tropical island invasive species despite never living and working within the native range of C. micronesica. The incomprehension leads to an inability to draw on the awareness of the physiognomy and gestalt appearance of the forests in which a pre-scale cycad plant thrived. These contemporary contributors, who are collectively spending more public funds on cycad conservation than any other humans worldwide, have no first-hand knowledge about how the habitats looked prior to the onset of the threats. This is a large part of why the funded activities have been unsuccessful.
Balancing current knowledge with uncertainties. Almost 40% of known vascular plants are currently threatened with extinction [46]. One of the challenges in conservation decision-making is how to manage the interactions between what is known from the literature and what is not known [47]. This C. micronesica case study has emerged as an unsuccessful example in this regard, as members of the International Union for Conservation of Nature’s Species Survival Council with international cycad conservation knowledge have been actively marginalized by empowered administrators, and actionable knowledge from the peer-reviewed literature has been actively ignored by funded practitioners [26]. The consequence has been ongoing plant mortality despite the millions of dollars invested in ill-conceived conservation actions, resulting in a federal lawsuit in the District Court of Guam designed to stop the ongoing ecocide [48]. The funded conservation actions to conserve Lodoicea maldivica Gmelin in the Seychelles provide a successful example that is in stark contrast to the approach that has been implemented in Guam. The empowered conservation deciders in this developing nation initiated conservation actions by first funding scientists to determine the bottlenecks and second by developing action plans based on the new information that was published in peer-reviewed journals [49,50]. These unsuccessful examples in Guam and successful examples in the Seychelles highlight the importance of establishing a management team that values independent review and fosters the synthesis of ongoing monitoring and research to achieve tangible conservation outcomes. The development of effective species conservation plans will require embracing the best available information and combining it with collaborative development among all stakeholders [51].
Long-term monitoring. Finally, our study provides an explicit example of the value of long-term monitoring of permanent plots. Relying on permanent plots to monitor long-term changes in tropical forests is a key theme in global change forest science [52]. Permanent plots have been exploited to learn many C. micronesica lessons from Guam, Rota, and Yap [14,21]. However, the value of using repeated measurements can be diminished if the observations are not sustained for an appropriate length of time. Indeed, rates of mortality at 5 years after perennial plant translocation were not similar to rates of mortality in the same sites at 15 years after translocation [53]. Monitoring to document the success of plant translocations should extend beyond documenting translocated plant survival and should continue until evidence of regeneration and recruitment is verified at the recipient site [54,55]. One of the reasons for information deficits in the translocation ecology literature is that unsuccessful outcomes are purposefully not reported [53]. Short-term observations of only 1 year have been used in Guam to declare the success of translocation projects [48]. These methods are indefensible, and taxpayers who fund conservation actions in every country deserve to know the success rate of plant translocations at 5, 10, and 15 years. Based on our decades of Guam-based research, we believe the use of an appropriate duration of monitoring will reveal that these recent translocation projects that were deemed successful have already reached 100% mortality.
5. Conclusions
Conservation of endangered tree species has become a global agenda as more tree species become threatened. When threats are numerous, research is needed to determine their relative importance and rank them as primary or ancillary threats in order to facilitate the efficient allocation of conservation efforts. We have added new mortality data herein to verify that A. yasumatsui herbivory has been and continues to be the single greatest threat to C. micronesica survival. Adding up to five ancillary biotic threats did not change the population-level mortality trends following A. yasumatsui invasions of new insular habitats. The collective knowledge that has been generated from Guam and Rota since 2003 indicates that any delay in establishing biological control within every newly invaded Cycas subpopulation will likely cause irreversible damage.
Author Contributions
Conceptualization, T.E.M. and G.N.C.; methodology, T.E.M. and G.N.C.; formal analysis, T.E.M.; resources, T.E.M. and G.N.C.; data curation, T.E.M.; writing—original draft preparation, T.E.M.; writing—review and editing, G.N.C.; project administration, T.E.M.; funding acquisition, T.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA CSREES grant number 2003-05495 and the United States Forest Service grant numbers 06-DG-11052021-206, 09-DG-11052021-173, and 10-DG-11059702-095.
Data Availability Statement
Data are available upon request.
Acknowledgments
We thank Maren Roe for the field survey work that accurately identified the dates of the initial Aulacaspis yasumatsui infestation among the Guam habitats and Irene Terry for skillfully finding the initial infestation on Rota before considerable spread had occurred.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analysis, or interpretation of data, in the writing of this manuscript, or in the decision to publish the results.
References
- Feng, Y.-L.; Du, D.; van Kleunen, M. Global change and biological invasions. J. Plant Ecol. 2022, 15, 425–428. [Google Scholar] [CrossRef]
- Chong, K.Y.; Corlett, R.T.; Nuñez, M.A.; Chiu, J.H.; Courchamp, F.; Dawson, W.; Kuebbing, S.; Liebhold, A.M.; Padmanaba, M.; Souza, L.; et al. Are terrestrial biological invasions different in the tropics? Annu. Rev. Ecol. Evol. Syst. 2021, 52, 291–314. [Google Scholar] [CrossRef]
- Hill, K.D. The Cycas rumphii complex (Cycadaceae) in New Guinea and the Western Pacific. Aust. Syst. Bot. 1994, 7, 543–567. [Google Scholar] [CrossRef]
- Terry, I.; Roe, M.; Tang, W.; Marler, T.E. Cone insects and putative pollen vectors of the endangered cycad, Cycas micronesica. Micronesica 2009, 41, 83–99. [Google Scholar]
- Deloso, B.E.; Terry, L.I.; Yudin, L.S.; Marler, T.E. Biotic threats to Cycas micronesica continue to expand to complicate conservation decisions. Insects 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Cycad mutualist offers more than pollen transport. Am. J. Bot. 2010, 97, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Wiles, G.J. Current research and future management of Marianas fruit bats (Chiroptera: Pteropodidae) on Guam. Aust. Mammal. 1987, 10, 93–95. [Google Scholar] [CrossRef]
- Marler, T.E.; Muniappan, R. Pests of Cycas micronesica leaf, stem, and male reproductive tissues with notes on current threat status. Micronesica 2006, 39, 1–9. [Google Scholar]
- Marler, T.E. Cycad Aulacaspis Scale Invades the Mariana Islands; New York Botanical Garden Press: New York, NY, USA, 2012; Volume 106, pp. 20–35. [Google Scholar]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources, 2002; Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004.
- Marler, T.E.; Lindström, A.J.; Watson, G.W. Aulacaspis yasumatsui delivers a blow to international cycad horticulture. Horticulturae 2021, 7, 147. [Google Scholar] [CrossRef]
- Marler, T.E. Temporal variations in leaf miner, butterfly, and stem borer infestations of Cycas micronesica in relation to Aulacaspis yasumatsui incidence. HortScience 2013, 48, 1334–1338. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N. Three invasive insects alter Cycas micronesica leaf chemistry and predict changes in biogeochemical cycling. Commun. Integr. Biol. 2016, 9, e1208324. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Marler, T.E.; Terry, L.I. Aulacaspis yasumatsui invasion reduced Cycas micronesica microstrobilus size and pollinator brood site competence. Insects 2021, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Terry, L.I. Cycas micronesica megastrobilus traits respond to chronic herbivory by Aulacaspis yasumatsui. Ecologies 2023, 4, 371–384. [Google Scholar] [CrossRef]
- Wiles, G.J. Rusa marianna (Philippine deer). In CABI Compendium; CABI: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Joanes, D.N.; Gill, C.A. Comparing measures of sample skewness and kurtosis. Stat. 1998, 47, 183–189. [Google Scholar] [CrossRef]
- Bösenberg, J.D. Cycas micronesica. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2022; p. e.T61316A68906033. Available online: https://apiv3.iucnredlist.org/api/v3/taxonredirect/61316 (accessed on 25 November 2023).
- United States Fish & Wildlife Service. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Fed. Regist. 2015, 80, 59424–59497. [Google Scholar]
- Marler, T.E.; Lawrence, J.H. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J. Trop. Ecol. 2012, 28, 233–242. [Google Scholar] [CrossRef]
- Marler, T.E.; Griffith, M.P.; Krishnapillai, M.V. Height increment of Cycas micronesica informs conservation decisions. Plant Signal. Behav. 2020, 15, e1830237. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Marler, T.; Miller, R.H.; Muniappan, R. Biological control of cycad aulacaspis scale on Guam. Cycad Newsl. 2005, 28, 6–8. [Google Scholar]
- Tang, W.; Donaldson, J.; Haynes, J.; Terry, I. International Union for Conservation of Nature Cycad Specialist Group Report and Recommendations on Cycad Aulacaspis Scale, Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae); IUCN: Gland, Switzerland, 2005. [Google Scholar]
- Wiles, G.J.; Brooke, A.P.; Fleming, T.H.; Racey, P.A. Conservation threats to bats in the tropical Pacific islands and insular Southeast Asia. In Island Bats: Evolution, Ecology, and Conservation; Fleming, T.H., Racey, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 405–459. [Google Scholar]
- Lindström, A.; Terry, I.; Deloso, B.; Tang, W.; Donaldson, J.; Marler, T. Typhoon Mawar enables an assessment of Cycas micronesica conservation plans. J. Geogr. Nat. Disasters 2023, 13, 280. [Google Scholar]
- Marler, T.E.; Lawrence, J.H. Phytophagous insects reduce cycad resistance to tropical cyclone winds and impair storm recovery. HortScience 2013, 48, 1224–1226. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. First, do no harm. Commun. Integr. Biol. 2017, 10, e1393593. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Cascasan, A.N.J. Carbohydrate depletion during lethal infestation of Aulacaspis yasumatsui on Cycas revoluta. Int. J. Plant Sci. 2018, 179, 497–504. [Google Scholar] [CrossRef]
- Marler, T.E.; Lawrence, J.H.; Cruz, G.N. Topographic relief, wind direction, and conservation management decisions influence Cycas micronesica K.D. Hill population damage during tropical cyclone. J. Geogr. Nat. Disasters 2016, 6, 3. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Woodford, D.J.; Richardson, D.M.; MacIsaac, H.J.; Mandrak, N.E.; van Wilgen, B.W.; Wilson, J.R.U.; Weyl, O.L.F. Confronting the wicked problem of managing biological invasions. NeoBiota 2016, 31, 63–86. [Google Scholar] [CrossRef]
- DeFries, R.; Nagendra, H. Ecosystem management as a wicked problem. Science 2017, 356, 265–270. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A. Grand challenges in global biodiversity threats. Front. Conserv. Sci. 2020, 1, 609007. [Google Scholar] [CrossRef]
- Ardoin, N.M.; Bowers, A.W.; Wheaton, M. Leveraging collective action and environmental literacy to address complex sustainability challenges. Ambio 2023, 52, 30–44. [Google Scholar] [CrossRef]
- Roy, H.E.; Pauchard, A.; Stoett, P.; Renard Truong, T.; Bacher, S.; Galil, B.S.; Hulme, P.E.; Ikeda, T.; Sankaran, K.V.; McGeoch, M.A.; et al. Intergovernmental Science Policy Platform on Biodiversity and Ecosystem Services Alien Species Assessment: Summary for Policymakers; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Poland, T.M.; Patel-Weynand, T.; Finch, D.C.; Miniat, C.F.; Hayes, D.C.; Lopez, V.M. (Eds.) Invasive Species in Forests and Rangelands of the United States; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Marler, T.E. Infestations of Aulacaspis yasumatsui reduce asexual propagation and transplantation success of Cycas revoluta plants. Horticulturae 2023, 9, 1108. [Google Scholar] [CrossRef]
- Rogers, R.F. Destiny’s Landfall; University of Hawaii Press: Honolulu, HI, USA, 1995. [Google Scholar]
- Bates, R.S. An American Shame—The Abandonment of an Entire American Population; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2016. [Google Scholar]
- Calonje, M.; Stevenson, D.W.; Osborne, R. The World List of Cycads, 2013–2023. Available online: http://www.cycadlist.org (accessed on 25 November 2023).
- Takagi, S. Outbreak of Aulacaspis yasumatsui in Japan (Sternorrhyncha:Coccoidea: Diaspididae). Insecta Matsumurana 2023, 79, 81–84. [Google Scholar]
- Yannelli, F.A.; Bazzichetto, M.; Conradi, T.; Pattison, Z.; Andrade, B.O.; Anibaba, Q.A.; Bonari, G.; Chelli, S.; Ćuk, M.; Damasceno, G.; et al. Fifteen emerging challenges and opportunities for vegetation science: A horizon scan by early career researchers. J. Veg. Sci. 2022, 33, e13119. [Google Scholar] [CrossRef]
- de Vos, A.; Schwartz, M.W. Confronting parachute science in conservation. Conserv. Sci. Pract. 2022, 4, e12681. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Michaels, S.; Auld, G.; Cooke, S.J.; Young, N.; Bennett, J.R.; Vermaire, J.C. Conservation, uncertainty and intellectual humility. Environ. Conserv. 2023, 50, 196–201. [Google Scholar] [CrossRef]
- Center for Biological Diversity and Prutehi Litekyan versus United States Department of Defense; Carlos del Toro; United States Fish and Wildlife Service; Haaland, D. CIV 23-00019. Complaint for Declaratory and Injunctive Relief under the Endangered Species Act, Administrative Procedure Act, and Freedom of Information Act. United States District Court of Guam. 2023. Available online: https://www.biologicaldiversity.org/programs/biodiversity/pdfs/Camp-Blaz-Complaint.pdf (accessed on 25 November 2023).
- Dogley, D.A. Government’s perspective on safeguarding biodiversity: The Seychelles experience. Biotropica 2010, 42, 572–575. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Fleischer-Dogley, F.; Dogley, D.; Bunbury, N. Scientists’ responsibilities towards evidence-based conservation in a small island developing state. J. Appl. Ecol. 2015, 52, 7–11. [Google Scholar] [CrossRef]
- Byers, O.; Copsey, J.; Lees, C.; Miller, P.; Traylor-Holzer, K. Reversing the decline in threatened species through effective conservation planning. Diversity 2022, 14, 754. [Google Scholar] [CrossRef]
- Phillips, O.L. Sensing forests directly: The power of permanent plots. Plants 2023, 12, 3710. [Google Scholar] [CrossRef]
- Drayton, B.; Primack, R.B. Success rates for reintroductions of eight perennial plant species after 15 Years. Restor. Ecol. 2012, 20, 299–303. [Google Scholar] [CrossRef]
- Maschinski, J.; Coates, D.; Monks, L.; Dillon, R.; Barrett, S.; Possley, J.; Lange, J.; Duquesnel, J.; Goodman, J.; Hermanutz, L.; et al. Rare and threatened plant conservation translocations: Lessons learned and future directions. In Ecological Restoration; Florentine, S., Gibson-Roy, P., Dixon, K.W., Broadhurst, L., Eds.; Springer: Cham, Switzerland, 2023; pp. 287–322. [Google Scholar] [CrossRef]
- Corli, A.; Rocchetti, G.A.; Orsenigo, S.; Possley, J.; Abeli, T. The role of aftercare in plant translocation. Biodivers. Conserv. 2023, 32, 4181–4197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).