Tracheids vs. Tree Rings as Proxies for Dendroclimatic Reconstruction at High Altitude: The Case of Pinus sibirica Du Tour
Abstract
1. Introduction
2. Materials and Methods
2.1. Climate Data
2.2. Tree Data Collection and Processing
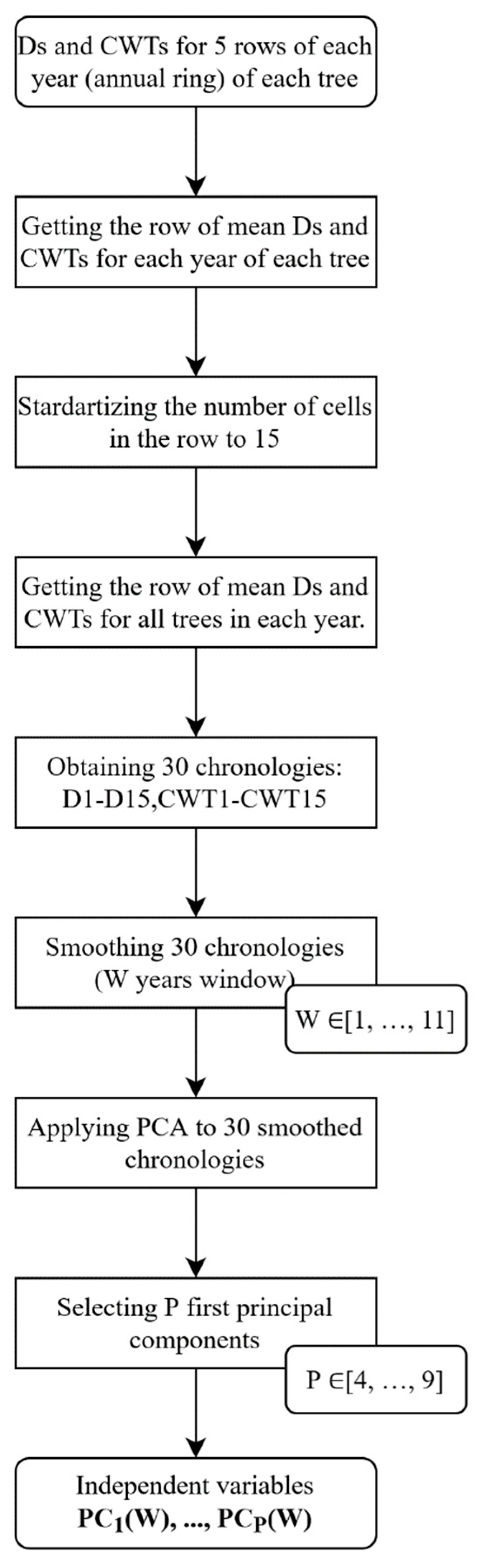
2.3. Reconstruction Models Development
- 1.
- Each th element (year) of is considered as a verification set.
- 2.
- The elements from are omitted ( is the floored division).This is done to prevent the data from the th element from getting into the calibration set due to smoothing with the inter-annual sliding window and affecting the elements from .All indices from or are ignored.
- 3.
- All other elements are considered as a calibration set.
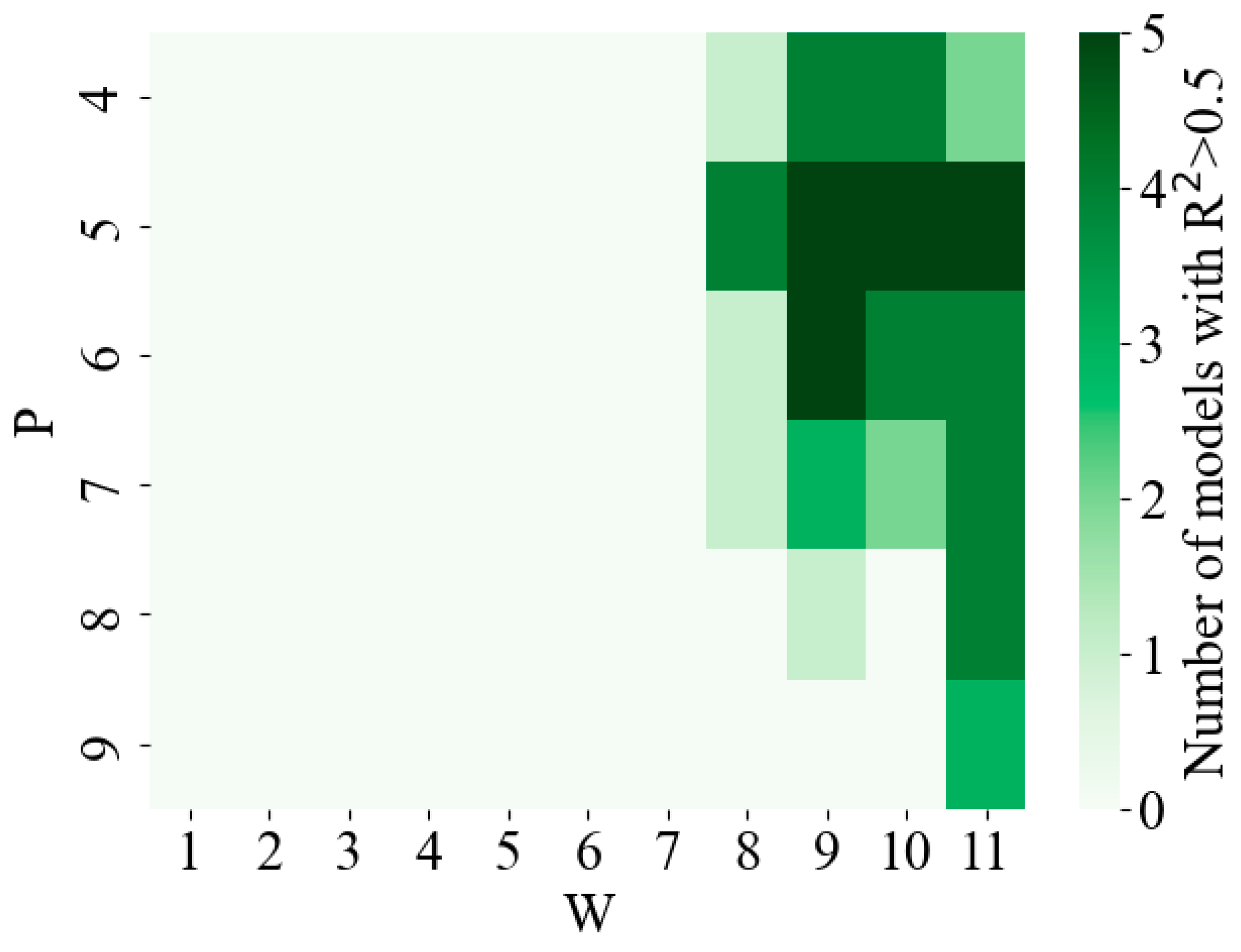
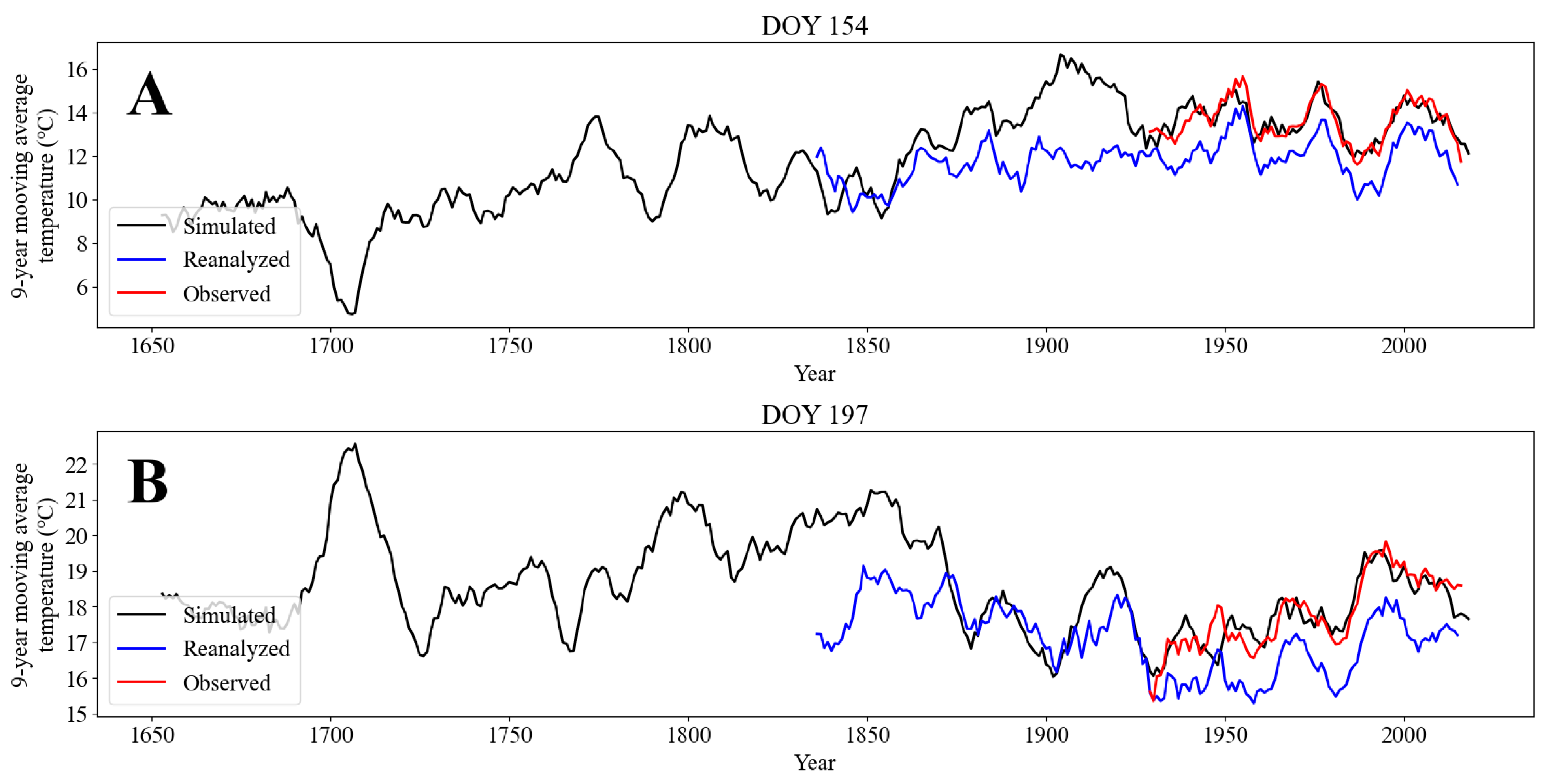
3. Results
3.1. Reconstruction of Temperature Dynamics
3.2. Reconstruction Model Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danchenko, A.M.; Beh, I.A. Cedar Forests of Western Siberia; Tomsk State University: Tomsk, Russia, 2010. [Google Scholar]
- White, A.; Cannell, M.G.R.; Friend, A.D. CO2 Stabilization, Climate Change and the Terrestrial Carbon Sink. Glob. Chang. Biol. 2000, 6, 817–833. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aaasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European Phenological Response to Climate Change Matches the Warming Pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Huang, J.G.; Zhang, Y.; Wang, M.; Yu, X.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. A Critical Thermal Transition Driving Spring Phenology of Northern Hemisphere Conifers. Glob. Chang. Biol. 2023, 29, 1606–1617. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Holtmeier, F.-K. Mountain Timberlines; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are Treelines Advancing? A Global Meta-Analysis of Treeline Response to Climate Warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Jochner, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Tree Growth Responses to Changing Temperatures across Space and Time: A Fine-Scale Analysis at the Treeline in the Swiss Alps. Trees-Struct. Funct. 2018, 32, 645–660. [Google Scholar] [CrossRef]
- Lett, S.; Dorrepaal, E. Global Drivers of Tree Seedling Establishment at Alpine Treelines in a Changing Climate. Funct. Ecol. 2018, 32, 1666–1680. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Parks, C.G.; Bernier, P. Adaptation of Forests and Forest Management to Changing Climate with Emphasis on Forest Health: A Review of Science, Policies and Practices. For. Ecol. Manag. 2010, 259, 657–659. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; García-Valdés, R.; Bugmann, H. Long-Term Response of Forest Productivity to Climate Change Is Mostly Driven by Change in Tree Species Composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of Forest Regrowth in Global Carbon Sink Dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Fonti, P.; Von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying Global Change through Investigation of the Plastic Responses of Xylem Anatomy in Tree Rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shao, X.; Fang, X.; Yin, Z.; Chen, L.; Zhao, D.; Wu, S. Responses of Pinus Koraiensis Tree Ring Cell Scale Parameters to Climate Elements in Changbai Mountains. Ying Yong Sheng Tai Xue Bao 2011, 22, 2643–2652. [Google Scholar]
- Wang, H.; Shao, X.; Fang, X.; Jiang, Y.; Liu, C.; Qiao, Q. Relationships between Tree-Ring Cell Features of Pinus Koraiensis and Climate Factors in the Changbai Mountains, Northeastern China. J. For. Res. 2017, 28, 105–114. [Google Scholar] [CrossRef]
- Zhirnova, D.F.; Belokopytova, L.V.; Upadhyay, K.K.; Tripathi, S.K.; Babushkina, E.A.; Vaganov, E.A. 495-Year Wood Anatomical Record of Siberian Stone Pine (Pinus Sibirica Du Tour) as Climatic Proxy on the Timberline. Forests 2022, 13, 247. [Google Scholar] [CrossRef]
- Anderegg, W.R.L. Spatial and Temporal Variation in Plant Hydraulic Traits and Their Relevance for Climate Change Impacts on Vegetation. New Phytol. 2015, 205, 1008–1014. [Google Scholar] [CrossRef]
- Sperry, J.S.; Love, D.M. What Plant Hydraulics Can Tell Us about Responses to Climate-Change Droughts. New Phytol. 2015, 207, 14–27. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Von Arx, G.; Archer, S.R.; Hughes, M.K. Long-Term Functional Plasticity in Plant Hydraulic Architecture in Response to Supplemental Moisture. Ann. Bot. 2012, 109, 1091–1100. [Google Scholar] [CrossRef]
- Castagneri, D.; Fonti, P.; Von Arx, G.; Carrer, M. How Does Climate Influence Xylem Morphogenesis over the Growing Season? Insights from Long-Term Intra-Ring Anatomy in Picea Abies. Ann. Bot. 2017, 119, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Tumajer, J.; Shishov, V.V.; Ilyin, V.A.; Camarero, J.J. Intra-Annual Growth Dynamics of Mediterranean Pines and Junipers Determines Their Climatic Adaptability. Agric. For. Meteorol. 2021, 311, 108685. [Google Scholar] [CrossRef]
- Tumajer, J.; Kašpar, J.; Kuželová, H.; Shishov, V.V.; Tychkov, I.I.; Popkova, M.I.; Vaganov, E.A.; Treml, V. Forward Modeling Reveals Multidecadal Trends in Cambial Kinetics and Phenology at Treeline. Front. Plant Sci. 2021, 12, 613643. [Google Scholar] [CrossRef] [PubMed]
- Pritzkow, C.; Wazny, T.; Heußner, K.U.; Słowiński, M.; Bieber, A.; Liñán, I.D.; Helle, G.; Heinrich, I. Minimum Winter Temperature Reconstruction from Average Earlywood Vessel Area of European Oak (Quercus Robur) in N-Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 449, 520–530. [Google Scholar] [CrossRef]
- Ziaco, E.; Biondi, F.; Heinrich, I. Wood Cellular Dendroclimatology: Testing New Proxies in Great Basin Bristlecone Pine. Front. Plant Sci. 2016, 7, 223658. [Google Scholar] [CrossRef]
- Valeriano, C.; Gutiérrez, E.; Colangelo, M.; Gazol, A.; Sánchez-Salguero, R.; Tumajer, J.; Shishov, V.; Bonet, J.A.; Martínez de Aragón, J.; Ibáñez, R.; et al. Seasonal Precipitation and Continentality Drive Bimodal Growth in Mediterranean Forests. Dendrochronologia 2023, 78, 126057. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Jacoby, G.; Frank, D.; Pederson, N.; Cook, E.; Buckley, B.; Nachin, B.; Mijiddorj, R.; Dugarjav, C. 1738 Years of Mongolian Temperature Variability Inferred from a Tree-Ring Width Chronology of Siberian Pine. Geophys. Res. Lett. 2001, 28, 543–546. [Google Scholar] [CrossRef]
- Cerrato, R.; Salvatore, M.C.; Gunnarson, B.E.; Linderholm, H.W.; Carturan, L.; Brunetti, M.; De Blasi, F.; Baroni, C. A Pinus cembra L. Tree-Ring Record for Late Spring to Late Summer Temperature in the Rhaetian Alps, Italy. Dendrochronologia 2019, 53, 22–31. [Google Scholar] [CrossRef]
- Zharkov, M.S.; Huang, J.G.; Yang, B.; Babushkina, E.A.; Belokopytova, L.V.; Vaganov, E.A.; Zhirnova, D.F.; Ilyin, V.A.; Popkova, M.I.; Shishov, V.V. Tracheidogram’s Classification as a New Potential Proxy in High-Resolution Dendroclimatic Reconstructions. Forests 2022, 13, 970. [Google Scholar] [CrossRef]
- Silkin, P.P. Methods of Multiparameter Analysis of Conifers Tree-Ring Structure; Siberian Federal University: Krasnoyarsk, Russia, 2010. [Google Scholar]
- Dyachuk, P.; Arzac, A.; Peresunko, P.; Videnin, S.; Ilyin, V.; Assaulianov, R.; Babushkina, E.A.; Zhirnova, D.; Belokopytova, L.; Vaganov, E.A.; et al. AutoCellRow (ACR)–A New Tool for the Automatic Quantification of Cell Radial Files in Conifer Images. Dendrochronologia 2020, 60, 125687. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cook, E.; Peters, K. The Smoothing Spline: A New Approach to Standardizing Forest Interior Tree-Ring Width Series for Dendroclimatic Studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Perez, L.V. Principal Component Analysis to Address Multicollinearity; Whitman College: Whitman, MA, USA, 2017. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Chen, B.X.; Sun, Y.F.; Zhang, H.B.; Han, Z.H.; Wang, J.S.; Li, Y.K.; Yang, X.L. Temperature Change along Elevation and Its Effect on the Alpine Timberline Tree Growth in the Southeast of the Tibetan Plateau. Adv. Clim. Chang. Res. 2018, 9, 185–191. [Google Scholar] [CrossRef]
- Baas, P.; Wheeler, E.A. Wood Anatomy and Climate Change. Clim. Chang. Ecol. Syst. 2011, 78, 141–155. [Google Scholar] [CrossRef]
- Arzac, A.; Fonti, M.V.; Vaganov, E.A. An Overview on Dendrochronology and Quantitative Wood Anatomy Studies of Conifers in Southern Siberia (Russia). Prog. Bot. 2021, 83, 161–181. [Google Scholar] [CrossRef]
- Pandey, S. Climatic Influence on Tree Wood Anatomy: A Review. J. Wood Sci. 2021, 67, 24. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Herrmann, V.; Rollinson, C.R.; Gonzalez, B.; Gonzalez-Akre, E.B.; Pederson, N.; Alexander, M.R.; Allen, C.D.; Alfaro-Sánchez, R.; Awada, T.; et al. Joint Effects of Climate, Tree Size, and Year on Annual Tree Growth Derived from Tree-Ring Records of Ten Globally Distributed Forests. Glob. Chang. Biol. 2022, 28, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.N.; Myglan, V.S. Application of Siberian Cedar for Reconstruction of Climate and Geomorphological Events in Altai. Izv. Ross. Akad. Nauk. Seriya Geogr. 2015, 2, 43–51. [Google Scholar] [CrossRef]
- Shah, S.; Liu, Q.; Ahmad, A.; Mannan, A. Climate Growth Response of Pinus Sibirica (SIBERIAN PINE) in the Altai Mountains, Northwestern China. Pak. J. Bot. 2020, 52, 593–600. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Ning, Q.R.; Gong, X.W.; Li, M.Y.; Hao, G.Y. Differences in Growth Pattern and Response to Climate Warming between Larix Olgensis and Pinus Koraiensis in Northeast China Are Related to Their Distinctions in Xylem Hydraulics. Agric. For. Meteorol. 2022, 312, 108724. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, Y.; Wang, M.; Kang, X.; Zhang, W.; Zhang, L.; Zhao, S. Responses to Climate Change in Radial Growth of Picea Schrenkiana along Elevations of the Eastern Tianshan Mountains, Northwest China. Dendrochronologia 2016, 40, 117–127. [Google Scholar] [CrossRef]
- Lei, J.; Feng, X.; Shi, Z.; Bai, D.; Xiao, W. Climate–Growth Relationship Stability of Picea Crassifolia on an Elevation Gradient, Qilian Mountain, Northwest China. J. Mt. Sci. 2016, 13, 734–743. [Google Scholar] [CrossRef]
- Ignatenko, M.M. Siberian Cedar: (Biology, Introduction, Culture); Nauka: Moscow, Russia, 1988. [Google Scholar]
- MacGillivray, C.W.; Grime, J.P. Genome Size Predicts Frost Resistance in British Herbaceous Plants: Implications for Rates of Vegetation Response to Global Warming. Funct. Ecol. 1995, 9, 320. [Google Scholar] [CrossRef]
- Pellicer, J.; Leitch, I.J. The Plant DNA C-Values Database (Release 7.1): An Updated Online Repository of Plant Genome Size Data for Comparative Studies. New Phytol. 2020, 226, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.N.; Myglan, V.S. The Possibility of Construction of the 6000-Year Chronology for Siberian Pine in the Central Altai. J. Sib. Fed. Univ. Biol. 2012, 5, 70–88. [Google Scholar]
- Kharuk, V.I.; Ranson, K.J.; Im, S.T.; Dvinskaya, M.L. Response of Pinus Sibirica and Larix Sibirica to Climate Change in Southern Siberian Alpine Forest–Tundra Ecotone. Scand. J. For. Res. 2009, 24, 130–139. [Google Scholar] [CrossRef]
- González-Cásares, M.; Camarero, J.J.; Colangelo, M.; Rita, A.; Pompa-García, M. High Responsiveness of Wood Anatomy to Water Availability and Drought near the Equatorial Rear Edge of Douglas-Fir. Can. J. For. Res. 2019, 49, 1114–1123. [Google Scholar] [CrossRef]
- Seftigen, K.; Fuentes, M.; Ljungqvist, F.C.; Björklund, J. Using Blue Intensity from Drought-Sensitive Pinus Sylvestris in Fennoscandia to Improve Reconstruction of Past Hydroclimate Variability. Clim. Dyn. 2020, 55, 579–594. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Tjoelker, M.G. Evidence That Longer Needle Retention of Spruce and Pine Populations at High Elevations and High Latitudes Is Largely a Phenotypic Response. Tree Physiol. 1996, 16, 643–647. [Google Scholar] [CrossRef]
- Sobchak, R.O.; Zotikova, A.P. Influence of High Altitude Conditions on Anatomo-Physiological Indices of Siberian Pine Needles. Bull. Tomsk. State Univ. 2009, 326, 200–202. [Google Scholar]
- Bender, O.G.; Zotikova, A.P.; Bender, A.G. The State of Photosynthetic Apparatus of Different-Aged Needles of Siberian Cedar at the Southern Limit of Growth in the Altai Mountains. In Proceedings of the Forest Biogeocenoses of the Boreal Zone: Geography, Structure, Functions, Dynamics, Krasnoyarsk, Russia, 16 September 2014; Sukachev Institute of Forest SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”: Krasnoyarsk, Russia, 2014; pp. 380–383. [Google Scholar]
- Günthardt, M.S.; Wanner, H. Die Menge des Cuticulären Wachses auf Nadeln von Pinus cembra L. und Picea abies (L.) Karsten in Abhängigkeit von Nadelalter und Standort. Flora 1982, 172, 125–137. [Google Scholar] [CrossRef]
- Nebel, B.; Matile, P. Longevity and Senescence of Needles in Pinus cembra L. Trees 1992, 6, 156–161. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The Dynamic Nature of Bud Dormancy in Trees: Environmental Control and Molecular Mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Drolet, G.; Sylvain, J.D.; Paré, M.C.; Rossi, S. Assessment of Spatio-Temporal Patterns of Black Spruce Bud Phenology across Quebec Based on MODIS-NDVI Time Series and Field Observations. Remote Sens. 2019, 11, 2745. [Google Scholar] [CrossRef]
- Silvestro, R.; Brasseur, S.; Klisz, M.; Mencuccini, M.; Rossi, S. Bioclimatic Distance and Performance of Apical Shoot Extension: Disentangling the Role of Growth Rate and Duration in Ecotypic Differentiation. For. Ecol. Manag. 2020, 477, 118483. [Google Scholar] [CrossRef]
- Lopez-Saez, J.; Corona, C.; von Arx, G.; Fonti, P.; Slamova, L.; Stoffel, M. Tree-Ring Anatomy of Pinus Cembra Trees Opens New Avenues for Climate Reconstructions in the European Alps. Sci. Total Environ. 2023, 855, 158605. [Google Scholar] [CrossRef]
- Meko, D.M.; Baisan, C.H. Pilot Study of Latewood-Width of Conifers as an Indicator of Variability of Summer Rainfall in the North American Monsoon Region. Int. J. Climatol. 2001, 21, 697–708. [Google Scholar] [CrossRef]


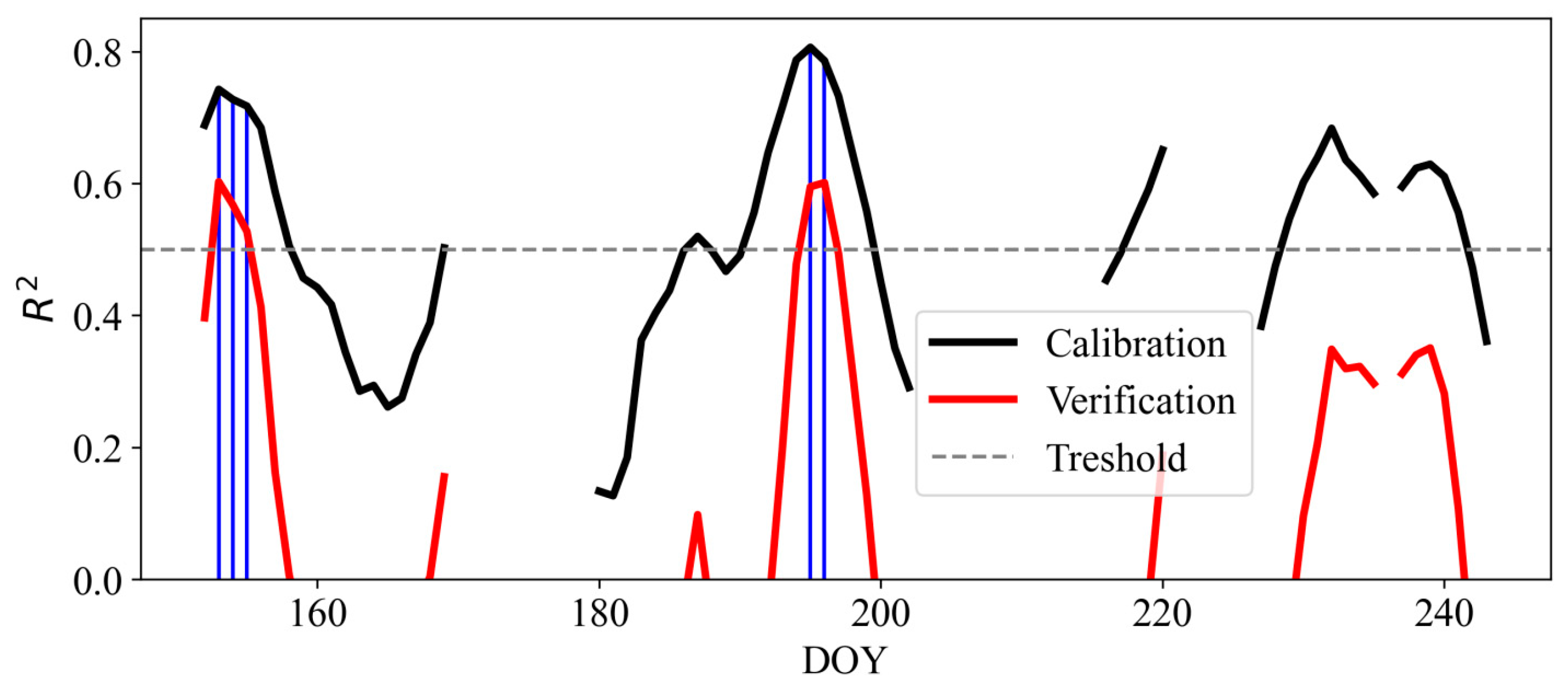
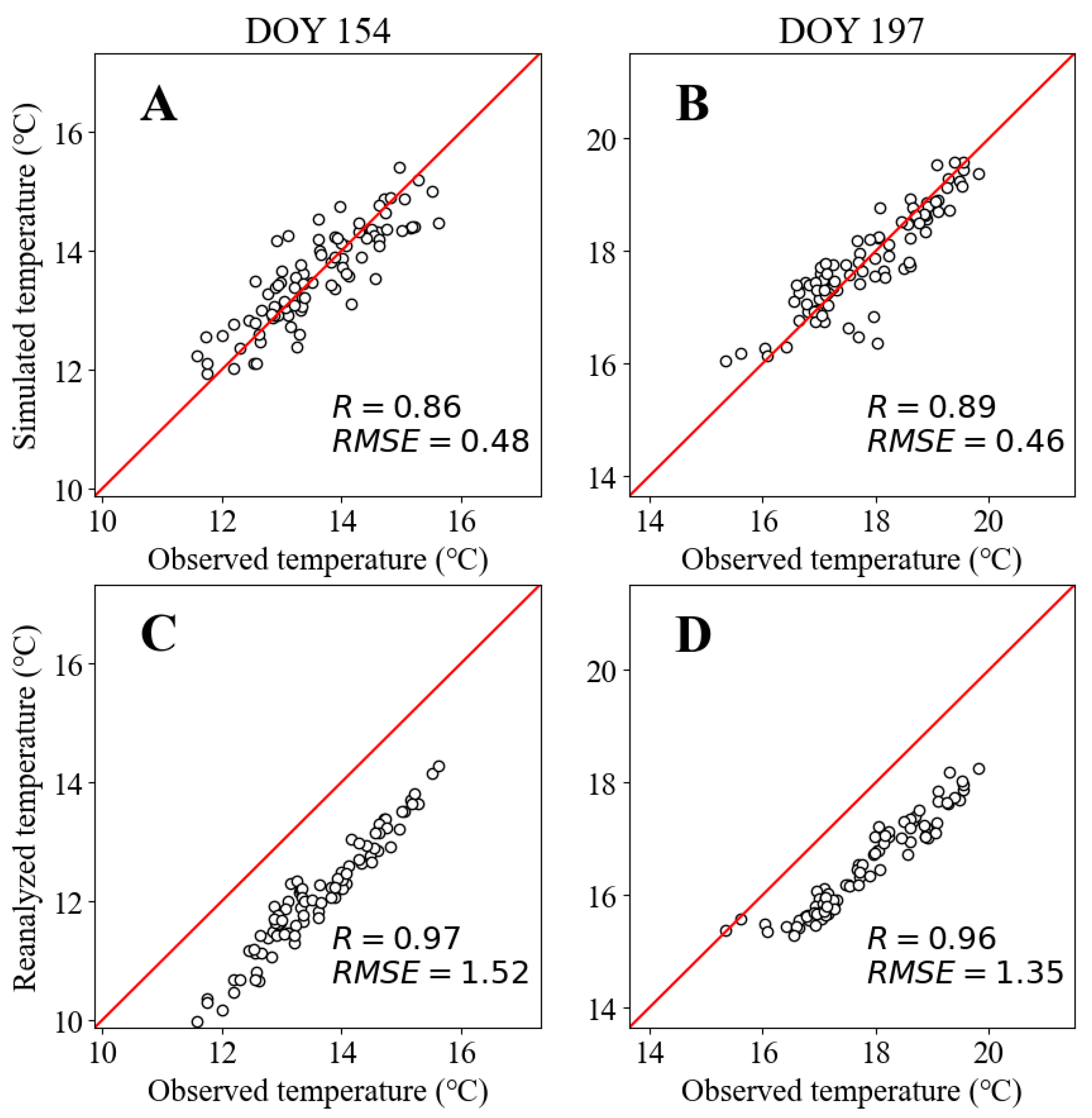
| Period | DOY | R2 Calibration | R2 Verification | R2 Simulated | RMSE Calibration | RMSE Verification | RMSE Simulated |
|---|---|---|---|---|---|---|---|
| A | 154 | 0.74 ± 0.03 | 0.60 | 0.74 | 0.48 ± 0.15 | 0.60 | 0.48 |
| B | 197 | 0.79 ± 0.03 | 0.60 | 0.78 | 0.46 ± 0.15 | 0.63 | 0.46 |
| Years | Period | Observed\Reanalyzed | Observed\Simulated | Simulated\Reanalyzed | |||
|---|---|---|---|---|---|---|---|
| Pearson R | p-Value | Pearson R | p-Value | Pearson R | p-Value | ||
| 1836–1928 | A | - | - | - | - | 0.67 | <10−12 |
| B | - | - | - | - | 0.57 | <10−8 | |
| 1929–2016 | A | 0.97 | <10−51 | 0.86 | <10−26 | 0.79 | <10−18 |
| B | 0.96 | <10−45 | 0.89 | <10−29 | 0.81 | <10−20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharkov, M.S.; Yang, B.; Babushkina, E.A.; Zhirnova, D.F.; Vaganov, E.A.; Shishov, V.V. Tracheids vs. Tree Rings as Proxies for Dendroclimatic Reconstruction at High Altitude: The Case of Pinus sibirica Du Tour. Forests 2024, 15, 167. https://doi.org/10.3390/f15010167
Zharkov MS, Yang B, Babushkina EA, Zhirnova DF, Vaganov EA, Shishov VV. Tracheids vs. Tree Rings as Proxies for Dendroclimatic Reconstruction at High Altitude: The Case of Pinus sibirica Du Tour. Forests. 2024; 15(1):167. https://doi.org/10.3390/f15010167
Chicago/Turabian StyleZharkov, Mikhail S., Bao Yang, Elena A. Babushkina, Dina F. Zhirnova, Eugene A. Vaganov, and Vladimir V. Shishov. 2024. "Tracheids vs. Tree Rings as Proxies for Dendroclimatic Reconstruction at High Altitude: The Case of Pinus sibirica Du Tour" Forests 15, no. 1: 167. https://doi.org/10.3390/f15010167
APA StyleZharkov, M. S., Yang, B., Babushkina, E. A., Zhirnova, D. F., Vaganov, E. A., & Shishov, V. V. (2024). Tracheids vs. Tree Rings as Proxies for Dendroclimatic Reconstruction at High Altitude: The Case of Pinus sibirica Du Tour. Forests, 15(1), 167. https://doi.org/10.3390/f15010167








