Elevation-Dependent Natural Regeneration of Abies georgei var. smithii Forest in Southeastern Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
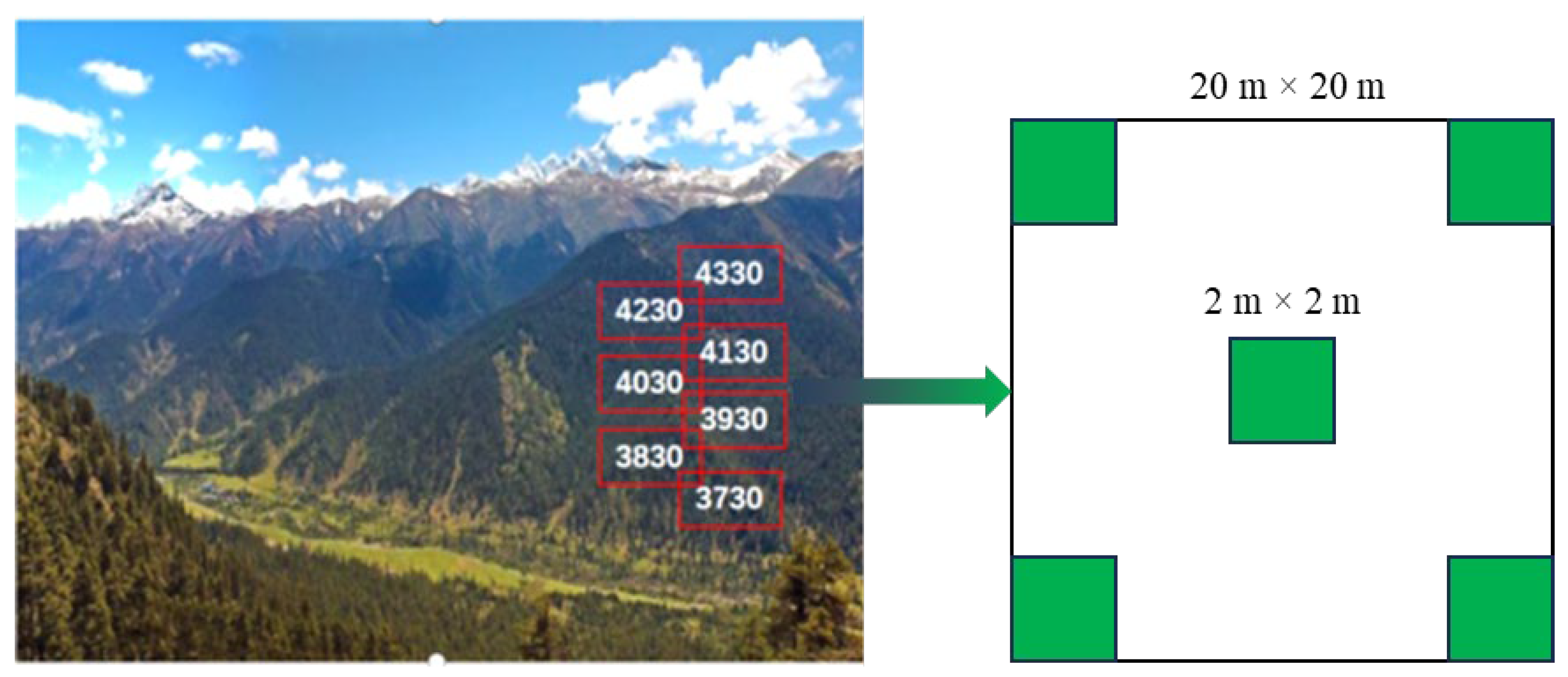
2.2. Sampling and Data Collection
2.3. Statistical Analysis
3. Results
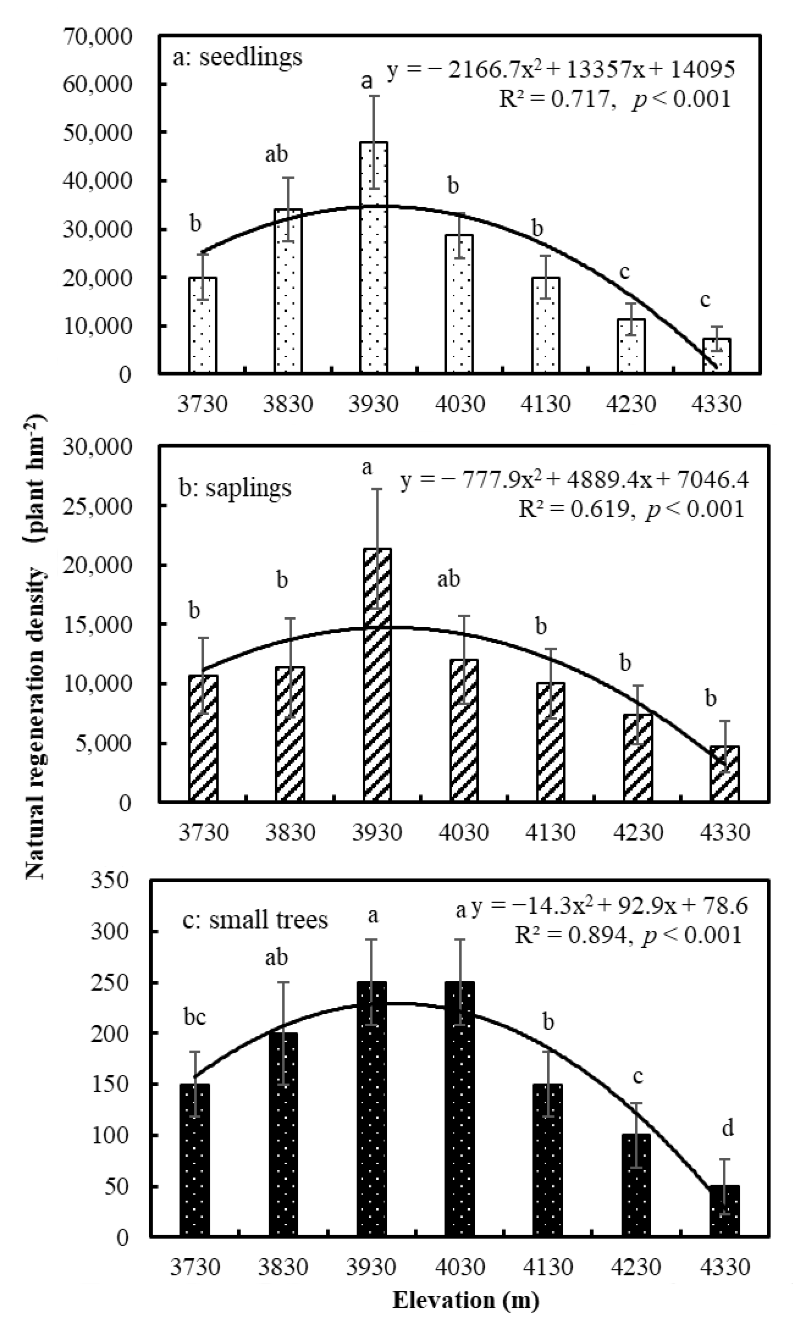
3.1. Natural Regeneration Characteristics of Abies Forest in Relation to Elevation
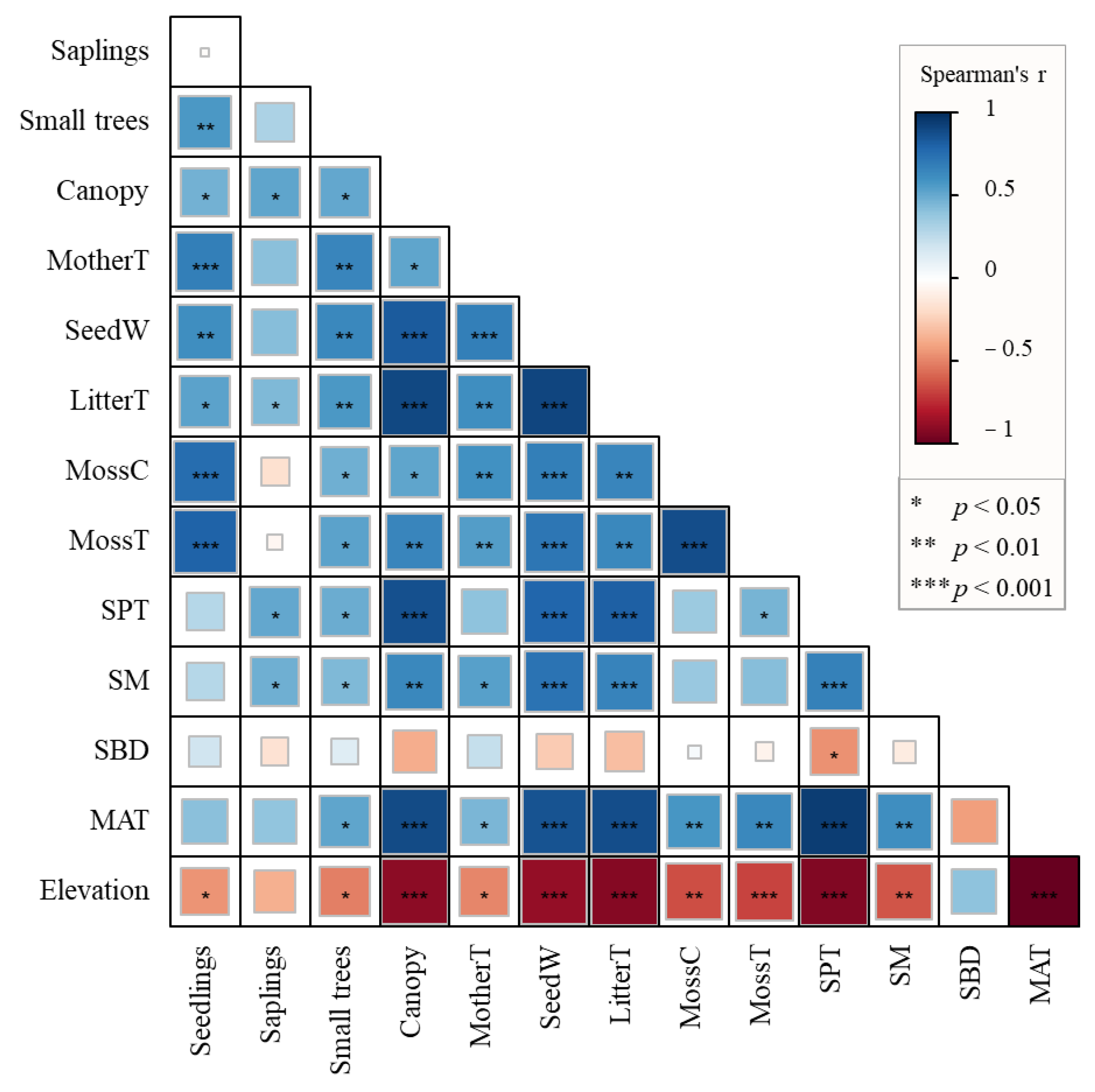
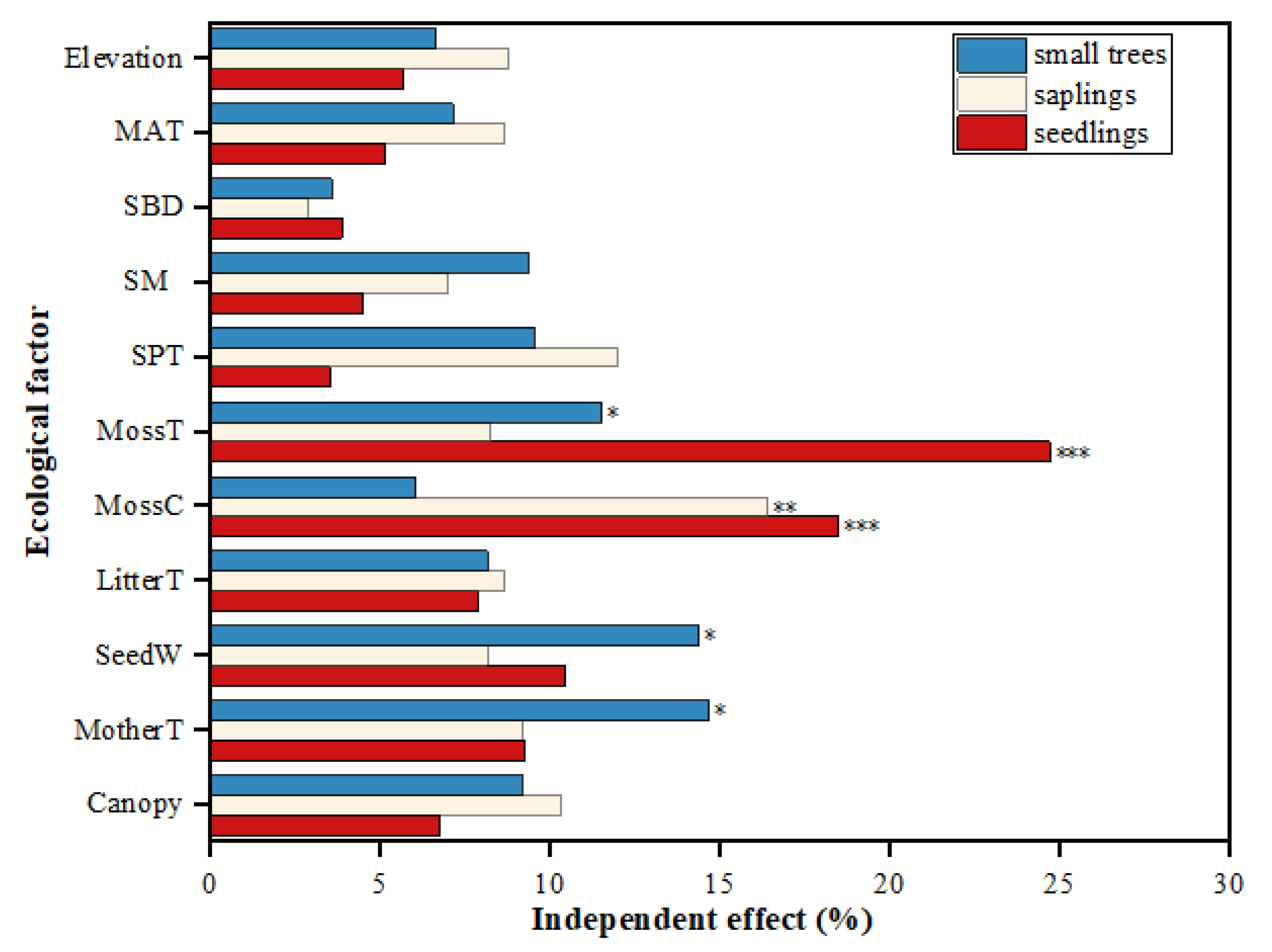
3.2. Factors Influencing the Variations in Natural Regeneration of the Abies Forest
4. Discussion
4.1. Effects of Forest Stand Structure Factors on Natural Regeneration of Abies
4.2. Effects of Forest Floor Attributes on Natural Regeneration of Abies
4.3. Effects of Abiotic Factors on Natural Regeneration of Abies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, F.; Hao, Z.; Ye, J. Effects of bryophytes on plant natural regeneration. Chin. J. Ecol. 2006, 25, 456–460. [Google Scholar]
- Puhlick, J.J.; Laughlin, D.C.; Moore, M.M. Factors influencing ponderosa pine regeneration in the southwestern USA. For. Ecol. Manag. 2012, 264, 10–19. [Google Scholar] [CrossRef]
- Chen, Y.M.; Cao, Y. Response of tree regeneration and understory plant species diversity to stand density in mature Pinus tabulaeformis plantations in the hilly area of the Loess Plateau, China. Ecol. Eng. 2014, 73, 238–245. [Google Scholar] [CrossRef]
- Liang, W.J.; Wei, X. Multivariate path analysis of factors influencing Larix principis-rupprechtii plantation regeneration in northern China. Ecol. Indic. 2021, 129, 107886. [Google Scholar] [CrossRef]
- Zhao, W.W.; Liang, W.J.; Han, Y.Z.; Xi, W. Characteristics and factors influencing the natural regeneration of Larix principis-rupprechtii seedlings in northern China. PeerJ 2021, 9, e12327. [Google Scholar] [CrossRef] [PubMed]
- Sugita, H.; Nagaike, T. Microsites for seedling establishment of subalpine conifers in a forest with moss-type undergrowth on Mt. Fuji, central Honshu, Japan. Ecol. Res. 2005, 20, 678–685. [Google Scholar] [CrossRef]
- Kambo, D.; Danby, R.K. Factors influencing the establishment and growth of tree seedlings at Subarctic alpine treelines. Ecosphere 2018, 9, e02176. [Google Scholar] [CrossRef]
- Bose, A.K.; Weiskittel, A.; Wagner, R.G.; Kuehne, C. Assessing the factors influencing natural regeneration patterns in the diverse, multi-cohort, and managed forests of Maine, USA. J. Veg. Sci. 2016, 27, 1140–1150. [Google Scholar] [CrossRef]
- Ma, J.-M.; Liu, S.-R.; Shi, Z.-M.; Zhang, Y.-D.; Miao, N. Natural regeneration of Abies faxoniana along restoration gradients of subalpine dark coniferous forest in western Sichuan, China. Chin. J. Plant Ecol. 2009, 33, 646–657. [Google Scholar]
- Andersson, C. Distribution of seedlings and saplings of Quercus robur in a grazed deciduous forest. J. Veg. Sci. 1991, 2, 279–282. [Google Scholar] [CrossRef]
- Wang, Z.B.; Yang, H.J.; Wang, D.H.; Zhao, Z. Response of height growth of regenerating trees in a Pinus tabulaeformis Carr. plantation to different thinning intensities. For. Ecol. Manag. 2019, 444, 280–289. [Google Scholar] [CrossRef]
- Wu, Z. Flora Xizangica; Science Press: Beijing, China, 1985. [Google Scholar]
- Ren, Y.; Luo, D.; Fang, J.; Lu, J. Structure and dynamics of Abies georgei var. smithii population in eastern slope of the Sejila Mountains. J. Northwest AF Univeristiy Nat. Sci. Ed. 2021, 49, 59–68. [Google Scholar]
- Guo, Q.Q.; Li, H.E.; Zhang, W.H. Variations in leaf functional traits and physiological characteristics of Abies georgei var. smithii along the altitude gradient in the Southeastern Tibetan Plateau. J. Mt. Sci.-Engl. 2016, 13, 1818–1828. [Google Scholar] [CrossRef]
- Luo, D.; Wang, J.; Ren, Y.; Zhu, D. Fruiting characteristics of Abies georgei var. smithii forest on the eastern slope of the Sejila mountain in Tibet. Sci. Silvae Sin. 2010, 46, 30–35. [Google Scholar]
- He, Z.S.; Wang, L.J.; Jiang, L.; Wang, Z.; Liu, J.F.; Xu, D.W.; Hong, W. Effect of microenvironment on species distribution patterns in the regeneration layer of forest gaps and non-gaps in a subtropical natural forest, China. Forests 2019, 10, 90. [Google Scholar] [CrossRef]
- Shen, C.C.; Nelson, A.S. Natural conifer regeneration patterns in temperate forests across the Inland Northwest, USA. Ann. For. Sci. 2018, 75, 54. [Google Scholar] [CrossRef]
- Johnson, D.J.; Magee, L.; Pandit, K.; Bourdon, J.; Broadbent, E.N.; Glenn, K.; Kaddoura, Y.; Machado, S.; Nieves, J.; Wilkinson, B.E.; et al. Canopy tree density and species influence tree regeneration patterns and woody species diversity in a longleaf pine forest. For. Ecol. Manag. 2021, 490, 119082. [Google Scholar] [CrossRef]
- Norghauer, J.M.; Newbery, D.M. Herbivores differentially limit the seedling growth and sapling recruitment of two dominant rain forest trees. Oecologia 2014, 174, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Cappa, F.M.; Campos, V.E.; Barri, F.R.; Ramos, L.; Campos, C.M. Environmental and biological factors affecting the abundance of Prosopis flexuosa saplings in the central-west Monte of Argentina. For. Ecosyst. 2022, 9, 100010. [Google Scholar]
- Dorota, D.; Przemyslaw, K.; Grazyna, O.; Bolibok, L. Effects of stand features and soil enzyme activity on spontaneous pedunculate oak regeneration in Scots pine dominated stands—Implication for forest management. For. Ecosyst. 2021, 8, 43. [Google Scholar] [CrossRef]
- Akash; Navneet; Bhandari, B.S. Natural regeneration dynamics of tree species along the altitudinal gradient in a subtropical moist deciduous forest of northern India. Curr. Sci. India 2020, 119, 2019–2023. [Google Scholar] [CrossRef]
- Barna, M.; Bosela, M. Tree species diversity change in natural regeneration of a beech forest under different management. For. Ecol. Manag. 2015, 342, 93–102. [Google Scholar] [CrossRef]
- Liang, W.J.; Wei, X. Factors promoting the natural regeneration of Larix principis-rupprechtii plantation in the Lvliang Mountains of central China. PeerJ 2020, 8, e9339. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Properties and vertical distribution of soil on Shergyla mountain in Xizang. Mt. Res. 1997, 15, 228–233. [Google Scholar]
- Zang, J.; Song, M.; Huang, Z.; Panpan, Z. Spatial and temporal distribution of soil animal community diversity at different altitudes in Sejila Mountain (sunny slope), Tibet. J. China Agric. Univ. 2023, 28, 189–199. [Google Scholar]
- Carrer, M.; Soraruf, L.; Lingua, E. Convergent space-time tree regeneration patterns along an elevation gradient at high altitude in the Alps. For. Ecol. Manag. 2013, 304, 1–9. [Google Scholar] [CrossRef]
- Muhamed, H.; Youssef, S.; Mustafa, A.; Suliman, H.; Abdulqader, A.; Mohammed, H.; Michalet, R. Natural regeneration of Pinus brutia Ten. in a recreational public forest in Zawita-Kurdistan region, Iraq. J. For. Res. 2019, 30, 1849–1857. [Google Scholar] [CrossRef]
- Mac Nally, R. Hierarchical partitioning as an interpretative tool in multivariate inference. Aust. J. Ecol. 1996, 21, 224–228. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Zhu, J.; Tao, J.; Zhang, T.; Xi, Y. Effects of community structure on precipitation-use efficiency of grasslands in northern Tibet. J. Veg. Sci. 2017, 28, 281–290. [Google Scholar] [CrossRef]
- Walsh, C.; Nally, R.M. Package ‘hier.part’, R Package Version 1.0-4; R Core Team: Vienna, Austria, 2013. Available online: https://CRAN.R-project.org/package=hier.part (accessed on 8 January 2013).
- Wang, Y.; Zhang, S. The study of regeneration of natura Pinus bungeana in different forest canopy closure. J. Shanxi Norm. Univ. Nat. Sci. Ed. 2008, 22, 83–85. [Google Scholar]
- Luo, D.; Guo, Q.; Xue, H.; Bianba, D.; Pan, G. A research of gap regeneration of virgin fir forest in mount Sejila in Tibet. For. Res. 2002, 15, 564–569. [Google Scholar]
- Balandier, P.; Collet, C.; Miller, J.H.; Reynolds, P.E.; Zedaker, S.M. Designing forest vegetation management strategies based on the mechanisms and dynamics of crop tree competition by neighbouring vegetation. Forestry 2006, 79, 3–27. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Schütz, J.P. Opportunistic methods of controlling vegetation, inspired by natural plant succession dynamics with special reference to natural outmixing tendencies in a gap regeneration. Ann. For. Sci. 2004, 61, 149–156. [Google Scholar] [CrossRef]
- Mao, P.L.; Han, G.X.; Wang, G.M.; Yu, J.B.; Shao, H.B. Effects of age and stand density of mother trees on early Pinus thunbergii seedling establishment in the coastal zone, China. Sci. World J. 2014, 2014, 468036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Matsuzaki, T.; Lee, F.Q.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Ali, A.; Dai, D.; Akhtar, K.; Teng, M.; Yan, Z.; Urbina-Cardona, N.; Mullerova, J.; Zhou, Z.X. Response of understory vegetation, tree regeneration, and soil quality to manipulated stand density in a Pinus massoniana plantation. Glob. Ecol. Conserv. 2019, 20, e00775. [Google Scholar] [CrossRef]
- Hébert, F.; Krause, C.; Plourde, P.Y.; Achim, A.; Pregent, G.; Menetrier, J. Effect of tree spacing on tree level volume growth, morphology, and wood properties in a 25-year-old Pinus banksiana plantation in the boreal forest of Quebec. Forests 2016, 7, 276. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Donath, T.W. Interactions between litter and water availability affect seedling emergence in four familial pairs of floodplain species. J. Ecol. 2005, 93, 807–816. [Google Scholar] [CrossRef]
- Asplund, J.; Hustoft, E.; Nybakken, L.; Ohlson, M.; Lie, M.H. Litter impair spruce seedling emergence in beech forests: A litter manipulation experiment. Scand. J. For. Res. 2018, 33, 332–337. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Hermanutz, L.; Marino, P.M. Feathermoss seedbeds facilitate black spruce seedling recruitment in the forest-tundra ecotone (Labrador, Canada). Oikos 2011, 120, 1263–1271. [Google Scholar] [CrossRef]
- Zamfir, M. Effects of bryophytes and lichens on seedling emergence of alvar plants: Evidence from greenhouse experiments. Oikos 2000, 88, 603–611. [Google Scholar] [CrossRef]
- Collins, S.L. Habitat relationships and survivorship of tree seedlings in Hemlock—Hardwood forest. Can. J. Bot. 1990, 68, 790–797. [Google Scholar] [CrossRef]
- Nakamura, T. Effect of bryophytes on survival of conifer seedlings in sub-alpine forests of central Japan. Ecol. Res. 1992, 7, 155–162. [Google Scholar] [CrossRef]
- Holeksa, J.; Saniga, M.; Szwagrzyk, J.; Dziedzic, T.; Ferenc, S.; Wodka, M. Altitudinal variability of stand structure and regeneration in the subalpine spruce forests of the Pol’ana biosphere reserve, Central Slovakia. Eur. J. For. Res. 2007, 126, 303–313. [Google Scholar] [CrossRef]
- Dessalegn, D.; Beyene, S.; Ram, N.; Walley, F.; Gala, T.S. Effects of topography and land use on soil characteristics along the toposequence of Ele watershed in southern Ethiopia. Catena 2014, 115, 47–54. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J.M.; Kucuk, M. Effects of tree species and topography on soil chemistry, litter quality, and decomposition in Northeast Turkey. Soil. Biol. Biochem. 2005, 37, 1695–1706. [Google Scholar] [CrossRef]
| Elevation m | Canopy % | MotherT Plant hm−2 | SeedW g | LitterT cm | MossC % | MossT cm | SPT cm | SM % | SBD g cm−3 | MAT °C |
|---|---|---|---|---|---|---|---|---|---|---|
| 3730 | 69.40 ± 2.43 a | 600 ± 25 ab | 10.31 ± 0.16 c | 8.38 ± 0.33 a | 45.08 ± 6.49 ab | 5.21 ± 0.700 a | 11.32 ± 0.19 a | 39.41 ± 2.37 a | 0.58 ± 0.03 b | −0.46 ± 0.03 a |
| 3830 | 63.00 ± 3.56 a | 800 ± 85 a | 12.34 ± 0.19 a | 8.56 ± 0.26 a | 53.67 ± 6.55 a | 6.63 ± 0.90 a | 10.45 ± 0.28 b | 44.3 ± 1.01 a | 0.74 ± 0.02 a | −1.16 ± 0.05 b |
| 3930 | 70.61 ± 2.30 a | 900 ± 51 a | 11.35 ± 0.13 b | 8.49 ± 0.28 a | 46.27 ± 6.95 a | 6.70 ± 0.86 a | 9.97 ± 0.33 b | 40.52 ± 2.16 a | 0.72 ± 0.04 a | −1.70 ± 0.07 c |
| 4030 | 50.00 ± 3.20 b | 800 ± 52 a | 9.64 ± 0.12 c | 4.87 ± 0.20 b | 35.80 ± 6.70 b | 4.65 ± 0.65 ab | 9.06 ± 0.36 c | 37.74 ± 0.98 a | 0.80 ± 0.03 a | −2.32 ± 0.17 d |
| 4130 | 49.50 ± 3.67 b | 700 ± 42 a | 8.53 ± 0.26 d | 4.41 ± 0.28 b | 32.07 ± 6.53 b | 3.79 ± 0.68 b | 8.57 ± 0.36 c | 38.73 ± 0.56 a | 0.86 ± 0.01 a | −2.95 ± 0.47 e |
| 4230 | 40.00 ± 2.69 c | 500 ± 34 b | 7.70 ± 0.28 d | 3.36 ± 0.21 c | 24.33 ± 3.92 bc | 3.59 ± 0.80 bc | 7.35 ± 0.29 d | 37.76 ± 0.62 a | 0.78 ± 0.02 a | −3.59 ± 0.18 f |
| 4330 | 18.00 ± 1.26 d | 200 ± 17 b | 6.67 ± 0.40 e | 2.70 ± 0.21 c | 15.20 ± 2.65 c | 2.12 ± 0.44 c | 5.91 ± 0.24 e | 24.82 ± 2.76 b | 0.70 ± 0.03 a | −4.22 ± 0.27 g |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Whole Model | Variable | Seedlings | Saplings | Small Trees | |||
|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | ||
| Constant | −0.907 | 0.382 | 0.513 | 0.617 | −1.211 | 0.249 | |
| Canopy | 0.575 | 0.576 | 2.275 | 0.042 | −0.479 | 0.640 | |
| MotherT | 0.732 | 0.478 | 1.847 | 0.089 | 1.058 | 0.311 | |
| SeedW | 1.022 | 0.327 | 1.844 | 0.090 | 0.726 | 0.482 | |
| MossC | −0.113 | 0.912 | −3.434 | 0.005 | −0.802 | 0.438 | |
| MossT | 2.285 | 0.041 | −0.244 | 0.811 | 1.356 | 0.200 | |
| SPT | 0.274 | 0.789 | −0.951 | 0.361 | 1.171 | 0.264 | |
| SM | −2.238 | 0.045 | −1.353 | 0.201 | −0.773 | 0.454 | |
| BD | 1.707 | 0.114 | −0.122 | 0.905 | 1.065 | 0.308 | |
| Model statistics | |||||||
| R2 | 0.810 | 0.799 | 0.648 | ||||
| Adjusted R2 | 0.683 | 0.665 | 0.413 | ||||
| F | 6.386 | 5.962 | 2.756 | ||||
| p | 0.002 | 0.003 | 0.055 | ||||
| Best Model | Variable | t | p | t | p | t | p |
| Constant | −1.848 | 0.080 | 0.052 | 0.959 | 0.376 | 0.711 | |
| Canopy | 5.540 | 0.000 | 4.172 | 0.001 | |||
| MotherT | |||||||
| MossC | −4.526 | 0.000 | |||||
| MossT | 6.528 | 0.000 | |||||
| Model statistics | |||||||
| R2 | 0.692 | 0.651 | 0.478 | ||||
| Adjusted R2 | 0.675 | 0.612 | 0.451 | ||||
| F | 42.617 | 16.771 | 17.405 | ||||
| p | 0.000 | 0.000 | 0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Hu, R.; Wu, Y.; Shaaban, M.; Zhang, T.; Pan, G.; Lu, J.; Jiang, Y. Elevation-Dependent Natural Regeneration of Abies georgei var. smithii Forest in Southeastern Tibet. Forests 2024, 15, 142. https://doi.org/10.3390/f15010142
Wang R, Hu R, Wu Y, Shaaban M, Zhang T, Pan G, Lu J, Jiang Y. Elevation-Dependent Natural Regeneration of Abies georgei var. smithii Forest in Southeastern Tibet. Forests. 2024; 15(1):142. https://doi.org/10.3390/f15010142
Chicago/Turabian StyleWang, Ruihong, Ronggui Hu, Yupeng Wu, Muhammad Shaaban, Tao Zhang, Gang Pan, Jie Lu, and Yanbin Jiang. 2024. "Elevation-Dependent Natural Regeneration of Abies georgei var. smithii Forest in Southeastern Tibet" Forests 15, no. 1: 142. https://doi.org/10.3390/f15010142
APA StyleWang, R., Hu, R., Wu, Y., Shaaban, M., Zhang, T., Pan, G., Lu, J., & Jiang, Y. (2024). Elevation-Dependent Natural Regeneration of Abies georgei var. smithii Forest in Southeastern Tibet. Forests, 15(1), 142. https://doi.org/10.3390/f15010142








