Nutrient Element Stocks and Dynamic Changes in Stump–Root Systems of Eucalyptus urophylla × E. grandis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Setup and Field Survey
2.3. Sample Collection
2.4. Nutrient Element Determination
2.5. Statistics and Data Analysis
3. Results
3.1. Distribution of Elemental Stocks in Stump–Root Systems
3.2. Residual Rates of Elemental Stocks in Stump–Root Systems
3.2.1. Dynamics of Residual Rates of Elemental Stock
3.2.2. Fitting of Residual Rates of Elemental Stock
3.3. Relationship between Elemental Stocks and Stump–Root System Factors
4. Discussion
4.1. Nutrient Element Stocks in Stump–Root Systems
4.2. Residual Rates of Nutrient Stocks in Stump–Root Systems
4.3. Factors Influencing Elemental Stocks in Stump–Root Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marañón-Jiménez, S.; Castro, J. Effect of decomposing post-fire coarse woody debris on soil fertility and nutrient availability in a Mediterranean ecosystem. Biogeochemistry 2013, 112, 519–535. [Google Scholar] [CrossRef]
- Dai, Z.H.; Trettin, C.C.; Burton, A.J.; Jurgensen, M.F.; Page-Dumroese, D.S.; Forschler, B.T.; Schilling, J.S.; Lindner, D.L. Coarse woody debris decomposition assessment tool: Model development and sensitivity analysis. PLoS ONE 2021, 16, 0251893. [Google Scholar] [CrossRef]
- Didion, M.; Abegg, M. Tree stumps—An important but undervalued dead wood pool. Ann. For. Sci. 2022, 79, 34. [Google Scholar] [CrossRef]
- Woodall, C.; Rondeux, J.; Verkerk, H.; Ståhl, G. Estimating dead wood during national forest inventories: A review of inventory methodologies and suggestions for harmonization. Environ. Manag. 2009, 44, 624–631. [Google Scholar] [CrossRef]
- Dossa, G.; Schaefer, D.; Zhang, J.L.; Tao, J.P.; Cao, K.F.; Corlett, R.T.; Cunningham, A.B.; Xu, J.C.; Cornelissen, J.; Harrison, R.D. The cover uncovered: Bark control over wood decomposition. J. Ecol. 2018, 106, 2147–2160. [Google Scholar] [CrossRef]
- Shorohova, E.; Kapitsa, E.; Kuznetsov, A.; Kuznetsova, S.; Lopes De Gerenyu, V.; Kaganov, V.; Kurganova, I. Coarse woody debris density and carbon concentration by decay classes in mixed montane wet tropical forests. Biotropica 2022, 54, 635–644. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Laiho, R.; Shorohova, E.; Kapitsa, E.; Vanha-Majamaa, I. Carbon and nitrogen release from decomposing Scots pine, Norway spruce and silver birch stumps. For. Ecol. Manag. 2010, 259, 390–398. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Laiho, R.; Shorohova, E.; Kapitsa, E.; Vanha-Majamaa, I. Phosphorus and base cation accumulation and release patterns in decomposing Scots pine, Norway spruce and silver birch stumps. For. Ecol. Manag. 2010, 260, 1478–1489. [Google Scholar] [CrossRef]
- Deng, X. Stump Biomass of Eucalyptus Plantations and Physical, Chemical and Structural Component Characteristics of Stumps. Master’s Thesis, Guangxi University, Nanning, China, 2022. [Google Scholar]
- Deng, X.; Liang, X.; Shen, L.; Liu, H.; Yang, M.; Zeng, M.; Liang, M.; Cheng, F. Decomposition and nutrient dynamics of stumps and coarse roots of Eucalyptus plantations in southern China. Ann. For. Sci. 2023, 80, 30. [Google Scholar] [CrossRef]
- Debeljak, M. Coarse woody debris in virgin and managed forest. Ecol. Indic. 2006, 6, 733–742. [Google Scholar] [CrossRef]
- Gora, E.M.; Sayer, E.J.; Turner, B.L.; Tanner, E. Decomposition of coarse woody debris in a long-term litter manipulation experiment: A focus on nutrient availability. Funct. Ecol. 2018, 32, 1128–1138. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Cui, X.H.; Fan, B.; Lin, H. Application of ground penetrating radar for coarse root detection and quantification: A review. Plant Soil 2013, 362, 1–23. [Google Scholar] [CrossRef]
- Resh, S.C.; Battaglia, M.; Worledge, D.; Ladiges, S. Coarse root biomass for Eucalyptus plantations in Tasmania, Australia: Sources of variation and methods for assessment. Trees 2003, 17, 389–399. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Hope, G.D. Changes in soil properties, tree growth, and nutrition over a period of 10 years after stump removal and scarification on moderately coarse soils in interior British Columbia. For. Ecol. Manag. 2007, 242, 625–635. [Google Scholar] [CrossRef]
- Carmona, M.R.; Armesto, J.J.; Aravena, J.C.; Pérez, C.A. Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile. For. Ecol. Manag. 2002, 164, 265–275. [Google Scholar] [CrossRef]
- Sucre, E.B.; Fox, T.R. Decomposing stumps influence carbon and nitrogen pools and fine-root distribution in soils. For. Ecol. Manag. 2009, 258, 2242–2248. [Google Scholar] [CrossRef]
- Yang, H.; Ciais, P.; Chave, J.; Huang, Y.; Ballantyne, A.P.; Yu, K.; Berzaghi, F.; Wigneron, J. Coarse woody debris are buffering mortality-induced carbon losses to the atmosphere in tropical forests. Environ. Res. Lett. 2021, 16, 011006. [Google Scholar] [CrossRef]
- Idol, T.W.; Figler, R.A.; Pope, P.E.; Ponder, F. Characterization of coarse woody debris across a 100 year chronosequence of upland oak-hickory forests. For. Ecol. Manag. 2001, 149, 153–161. [Google Scholar] [CrossRef]
- Benoist, A.; Houle, D.; Bradley, R.L.; Bellenger, J. Evaluation of biological nitrogen fixation in coarse woody debris from Eastern Canadian boreal forests. Soil Biol. Biochem. 2022, 165, 108531. [Google Scholar] [CrossRef]
- Ortiz, C.A.; Hammar, T.; Ahlgren, S.; Hansson, P.; Stendahl, J. Time-dependent global warming impact of tree stump bioenergy in Sweden. For. Ecol. Manag. 2016, 371, 5–14. [Google Scholar] [CrossRef]
- Keyser, T.L.; Loftis, D.L. Stump sprouting of 19 upland hardwood species 1 year following initiation of a shelterwood with reserves silvicultural system in the southern Appalachian Mountains, USA. New For. 2015, 46, 449–464. [Google Scholar] [CrossRef]
- Yue, Y.; Men, X.L.; Sun, Z.H.; Chen, X.W. Effects of Larix olgensis henry stumps and coarse roots on phosphorus fractions and availability in plantation microsite soils. Forests 2022, 13, 2166. [Google Scholar] [CrossRef]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Mu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef]
- Herrmann, S.; Bauhus, J. Effects of moisture, temperature and decomposition stage on respirational carbon loss from coarse woody debris (CWD) of important European tree species. Scand. J. For. Res. 2013, 28, 346–357. [Google Scholar] [CrossRef]
- de Souza Kulmann, M.S.; de Jesus Eufrade-Junior, H.; Dick, G.; Schumacher, M.V.; de Azevedo, G.B.; Azevedo, G.T.D.O.S.; Guerra, S.P.S. Belowground biomass harvest influences biomass production, stock, export and nutrient use efficiency of second rotation Eucalyptus plantations. Biomass Bioenergy 2022, 161, 106476. [Google Scholar] [CrossRef]
- Eufrade-Junior HD, J.; Leonello, E.C.; Spadim, E.R.; Rodrigues, S.A.; Azevedo, G.B.D.; Guerra, S.P.S. Stump and coarse root biomass from eucalypt forest plantations in a commercial-scale operation for bioenergy. Biomass Bioenergy 2020, 142, 1–7. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenço, A.; Miranda, I.; Pereira, H. Chemical and fuel properties of stumps biomass from Eucalyptus globulus plantations. Ind. Crops Prod. 2012, 39, 12–16. [Google Scholar] [CrossRef]
- Gielen, S.; IBatlle, J.V.; Vincke, C.; Van Hees, M.; Vandenhove, H. Concentrations and distributions of Al, Ca, Cl, K, Mg and Mn in a Scots pine forest in Belgium. Ecol. Model. 2016, 324, 1–10. [Google Scholar] [CrossRef]
- Prieto, I.; Querejeta, J.I. Simulated climate change decreases nutrient resorption from senescing leaves. Glob. Chang. Biol. 2020, 26, 1795–1807. [Google Scholar] [CrossRef]
- Ågren, G.I.; Weih, M. Plant stoichiometry at different scales: Element concentration patterns reflect environment more than genotype. New Phytol. 2012, 194, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Addo-Danso, S.D.; Prescott, C.E.; Smith, A.R. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. For. Ecol. Manag. 2016, 359, 332–351. [Google Scholar] [CrossRef]
- Kaarakka, L.; Vaittinen, J.; Marjanen, M.; Hellsten, S.; Kukkola, M.; Saarsalmi, A.; Palviainen, M.; Helmisaari, H. Stump harvesting in Picea abies stands: Soil surface disturbance and biomass distribution of the harvested stumps and roots. For. Ecol. Manag. 2018, 425, 27–34. [Google Scholar] [CrossRef]
- Trofymow, J.A.; Moore, T.R.; Titus, B.; Prescott, C.; Morrison, I.; Siltanen, M.; Smith, S.; Fyles, J.; Wein, R.; Camiré, C.; et al. Rates of litter decomposition over 6 years in Canadian forests: Influence of litter quality and climate. Can. J. For. Res. 2002, 32, 789–804. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Ravi Kumar, G.; Rao, J.K. Evaluation of triacid and dry ashing procedures for determining potassium, calcium, magnesium, iron, zinc, manganese, and copper in plant materials. Commun. Soil Sci. Plant Anal. 2002, 33, 95–102. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Warman, P.R. Comparison of three digestion methods for the recovery of 17 plant essential nutrients and trace elements from six composts. Compos. Sci. Util. 2002, 10, 197–203. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G. Transformation of a Podocarpus falcatus dominated natural forest into a monoculture Eucalyptus globulus plantation at Munesa, Ethiopia: Soil organic C, N and S dynamics in primary particle and aggregate-size fractions. Agric. Ecosyst. Environ. 2005, 106, 89–98. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L. Decomposition and nutrient release from Norway spruce coarse roots and stumps—A 40-year chronosequence study. For. Ecol. Manag. 2015, 358, 1–11. [Google Scholar] [CrossRef]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of soil erosion in biogeochemical cycling of essential elements: Carbon, nitrogen, and phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Kappes, H.; Catalano, C.; Topp, W. Coarse woody debris ameliorates chemical and biotic soil parameters of acidified broad-leaved forests. Appl. Soil Ecol. 2007, 36, 190–198. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Morris, D.M.; Luckai, N.J.; Reid, D.E.B. The influence of coarse woody debris on soil carbon and nutrient pools 15 years after clearcut harvesting in black spruce—Dominated stands in northwestern Ontario, Canada. Écoscience 2014, 21, 11–20. [Google Scholar] [CrossRef]
- Johnson, D.W.; Todd, D.E., Jr.; Trettin, C.F.; Mulholland, P.J. Decadal Changes in Potassium, Calcium, and Magnesium in a Deciduous Forest Soil. Soil Sci. Soc. Am. J. 2008, 72, 1795–1805. [Google Scholar] [CrossRef]
- Shorohova, E.; Kapitsa, E.; Ruokolainen, A.; Romashkin, I.; Kazartsev, I. Types and rates of decomposition of Larix sibirica trees and logs in a mixed European boreal old-growth forest. For. Ecol. Manag. 2019, 439, 173–180. [Google Scholar] [CrossRef]
- Marañón-Jimenez, S.; Castro, J.; Fernández-Ondoño, E.; Zamora, R. Charred wood remaining after a wildfire as a reservoir of macro- and micronutrients in a Mediterranean pine forest. Int. J. Wildland Fire 2013, 22, 681–695. [Google Scholar] [CrossRef]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Krüger, D.; Bauhus, J.; Hofrichter, M. Dynamics of fungal community composition, decomposition and resulting deadwood properties in logs of Fagus sylvatica, Picea abies and Pinus sylvestris. For. Ecol. Manag. 2016, 382, 129–142. [Google Scholar] [CrossRef]
- Deng, X.; Cheng, F.; Li, M.; He, P.; Shen, L.; Liu, H. Effect of different decay classes of Eucalyptus stump substrates on microbial resource limitation and carbon-use efficiency. Plant Soil 2022, 478, 651–669. [Google Scholar] [CrossRef]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Krüger, D.; Bässler, C.; Bauhus, J.; Hofrichter, M. Patterns of laccase and peroxidases in coarse woody debris of Fagus sylvatica, Picea abies and Pinus sylvestris and their relation to different wood parameters. Eur. J. For. Res. 2016, 135, 109–124. [Google Scholar] [CrossRef]
- Garavaglia, S.; Teresa Cambria, M.; Miglio, M.; Ragusa, S.; Iacobazzi, V.; Palmieri, F.; D’Ambrosio, C.; Scaloni, A.; Rizzi, M. The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J. Mol. Biol. 2004, 342, 1519–1531. [Google Scholar] [CrossRef]
- Hafner, S.D.; Groffman, P.M. Soil nitrogen cycling under litter and coarse woody debris in a mixed forest in New York State. Soil Biol. Biochem. 2005, 37, 2159–2162. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Ascher-Jenull, J.; Bardelli, T.; Fornasier, F.; Fravolini, G.; Arfaioli, P.; Ceccherini, M.T.; Pietramellara, G.; Lamorski, K.; Sławiński, C.; et al. Physico-chemical and microbiological evidence of exposure effects on Picea abies—Coarse woody debris at different stages of decay. For. Ecol. Manag. 2017, 391, 376–389. [Google Scholar] [CrossRef]
- Alvarez, E.; Fernández Marcos, M.L.; Torrado, V.; Fernández Sanjurjo, M.J. Dynamics of macronutrients during the first stages of litter decomposition from forest species in a temperate area (Galicia, NW Spain). Nutr. Cycl. Agroecosystems 2008, 80, 243–256. [Google Scholar] [CrossRef]
- Herrmann, S.; Bauhus, J. Nutrient retention and release in coarse woody debris of three important central European tree species and the use of NIRS to determine deadwood chemical properties. For. Ecosyst. 2018, 5, 22. [Google Scholar] [CrossRef]
- Klockow, P.A.; D’Amato, A.W.; Bradford, J.B.; Fraver, S. Nutrient concentrations in coarse and fine woody debris of Populus tremuloides Michx.-dominated forests, northern Minnesota, USA. Silva Fenn. 2014, 48, 962. [Google Scholar] [CrossRef]
- Saunders, M.R.; Fraver, S.; Wagner, R.G. Nutrient concentration of down woody debris in mixedwood forests in central Maine, USA. Silva Fenn. 2011, 45, 197–210. [Google Scholar] [CrossRef]
- Dhiedt, E.; De Keersmaeker, L.; Vandekerkhove, K.; Verheyen, K. Effects of decomposing beech (Fagus sylvatica) logs on the chemistry of acidified sand and loam soils in two forest reserves in Flanders (northern Belgium). For. Ecol. Manag. 2019, 445, 70–81. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen JH, C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.; Scarff, F.; Weedon, J.; Wirth, C.; Zanne, A. Plant traits and wood fates across the globe: Rotted, burned, or consumed. Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Błońska, E.; Kempf, M.; Lasota, J. Woody debris as a substrate for the growth of a new generation of forest trees. For. Ecol. Manag. 2022, 525, 120566. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Cesarano, G.; Sarker, T.C.; Saulino, L.; Saracino, A.; Idbella, M.; Agrelli, D.; Ascoli, R.; Rita, A.; et al. Decomposition of woody debris in Mediterranean ecosystems: The role of wood chemical and anatomical traits. Plant Soil 2021, 460, 263–280. [Google Scholar] [CrossRef]
- Filipiak, M.; Sobczyk, L.; Weiner, J. Fungal Transformation of Tree Stumps into a Suitable Resource for Xylophagous Beetles via Changes in Elemental Ratios. Insects 2016, 7, 13. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Katsumata, S.; Mori, A.S.; Osono, T.; Takeda, H. Accumulation and decay dynamics of coarse woody debris in a Japanese old-growth subalpine coniferous forest. Ecol. Res. 2014, 29, 257–269. [Google Scholar] [CrossRef]
- Romashkin, I.; Shorohova, E.; Kapitsa, E.; Galibina, N.; Nikerova, K. Substrate quality regulates density loss, cellulose degradation and nitrogen dynamics in downed woody debris in a boreal forest. For. Ecol. Manag. 2021, 491, 119143. [Google Scholar] [CrossRef]
- Fukasawa, Y. Fungal succession and decomposition of Pinus densiflora snags. Ecol. Res. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, R.; Paletto, A.; Agnelli, A.E.; Lagomarsino, A.; De Meo, I. Microbial communities associated with decomposing deadwood of downy birch in a natural forest in Khibiny Mountains (Kola Peninsula, Russian Federation). For. Ecol. Manag. 2020, 455, 117643. [Google Scholar] [CrossRef]
- Gorgolewski, A.; Rudz, P.; Jones, T.; Basiliko, N.; Caspersen, J. Assessing Coarse Woody Debris Nutrient Dynamics in Managed Northern Hardwood Forests Using a Matrix Transition Model. Ecosystems 2020, 23, 541–554. [Google Scholar] [CrossRef]
- Hellsten, S.; Helmisaari, H.; Melin, Y.; Skovsgaard, J.P.; Kaakinen, S.; Kukkola, M.; Saarsalmi, A.; Petersson, H.; Akselsson, C. Nutrient concentrations in stumps and coarse roots of Norway spruce, Scots pine and silver birch in Sweden, Finland and Denmark. For. Ecol. Manag. 2013, 290, 40–48. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Osono, T.; Takeda, H. Dynamics of physicochemical properties and occurrence of fungal fruit bodies during decomposition of coarse woody debris of Fagus crenata. J. For. Res. 2009, 14, 20–29. [Google Scholar] [CrossRef]
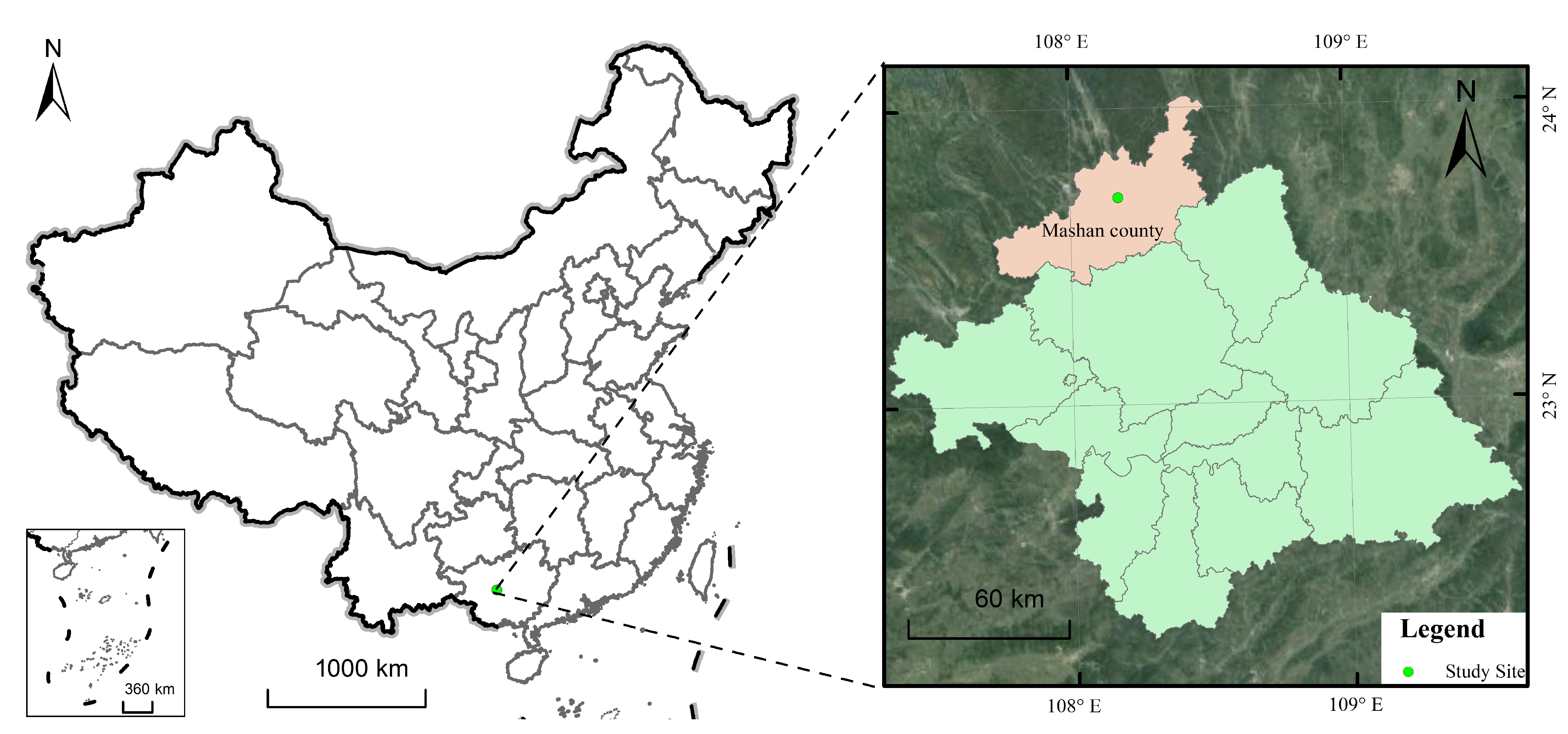

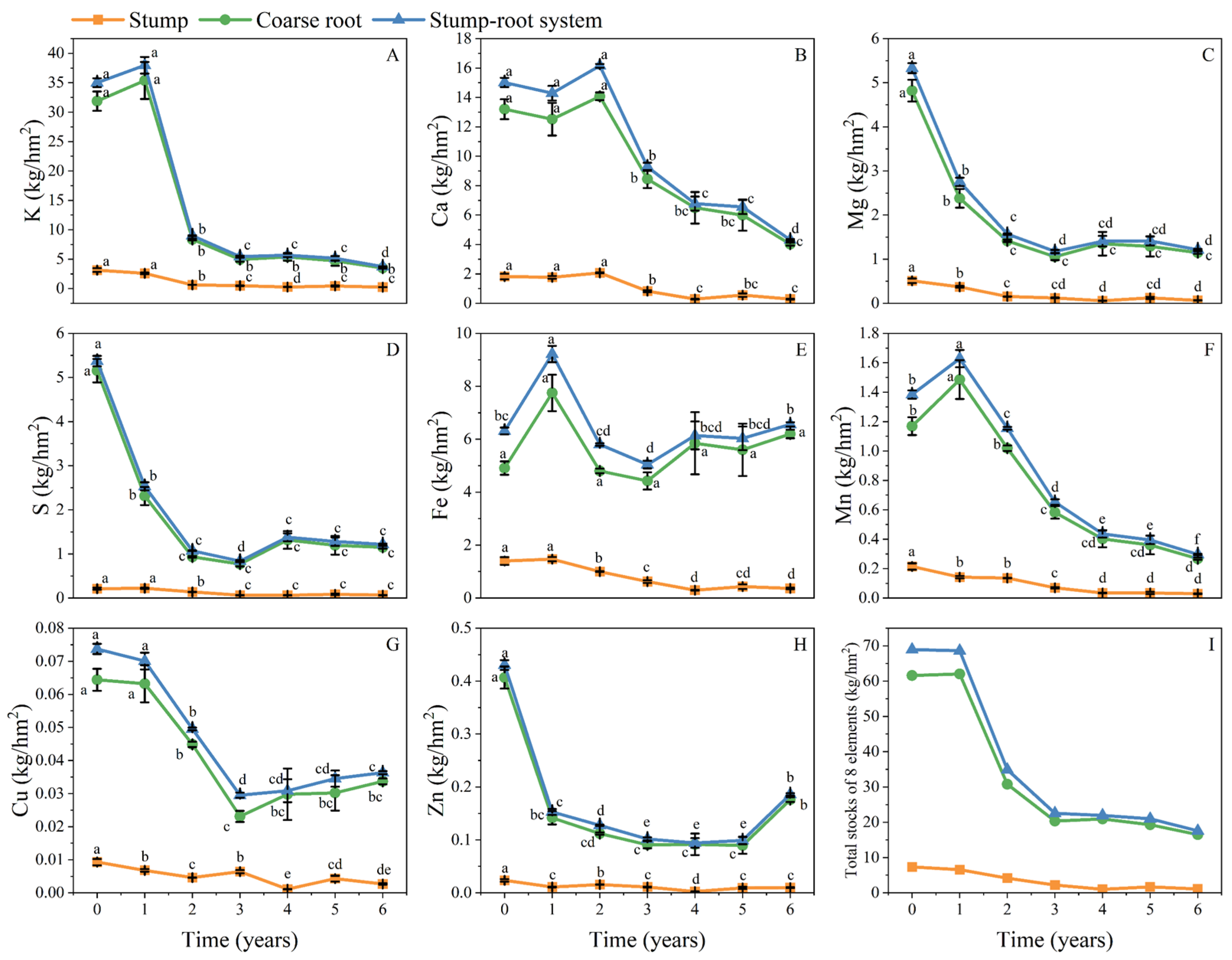
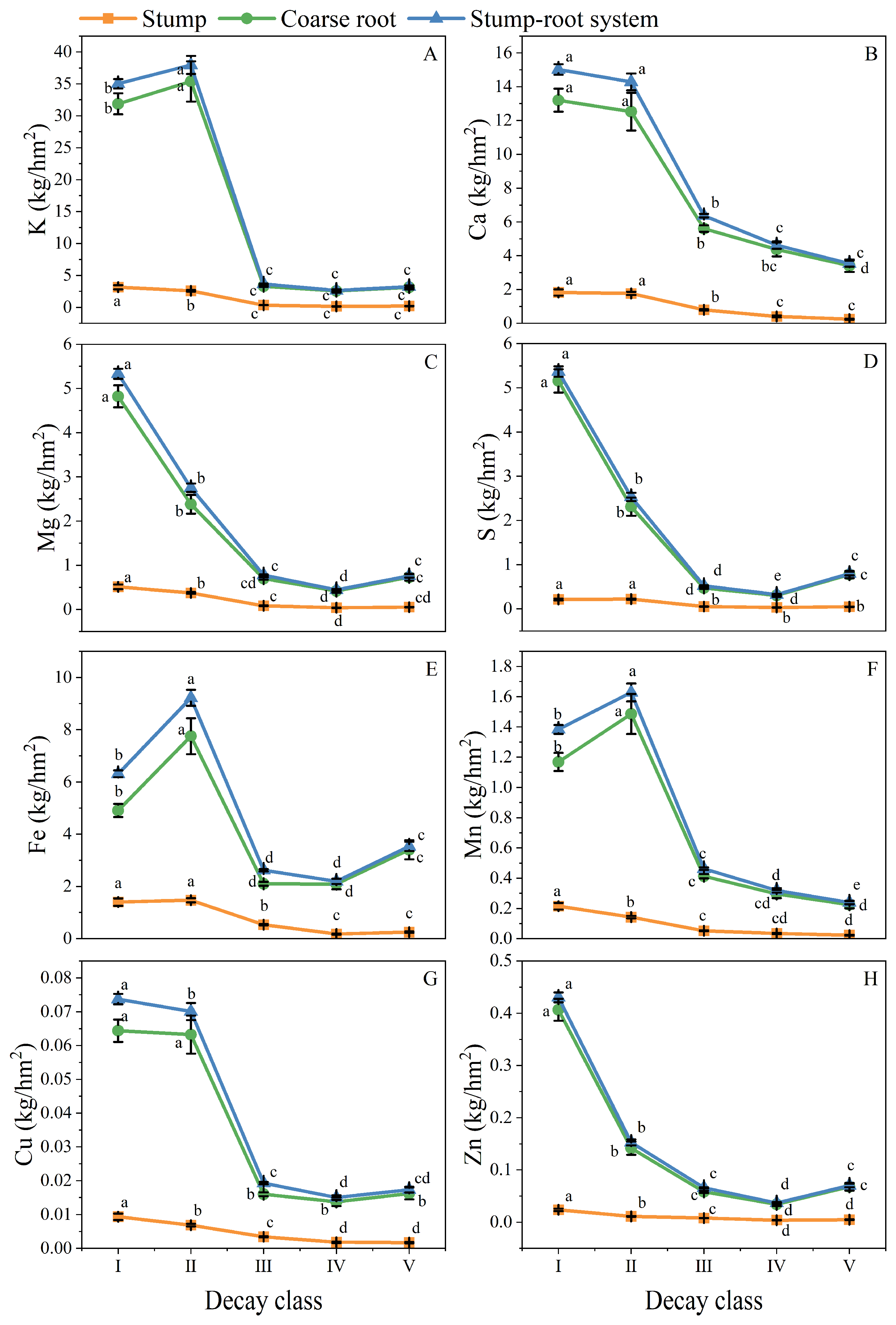

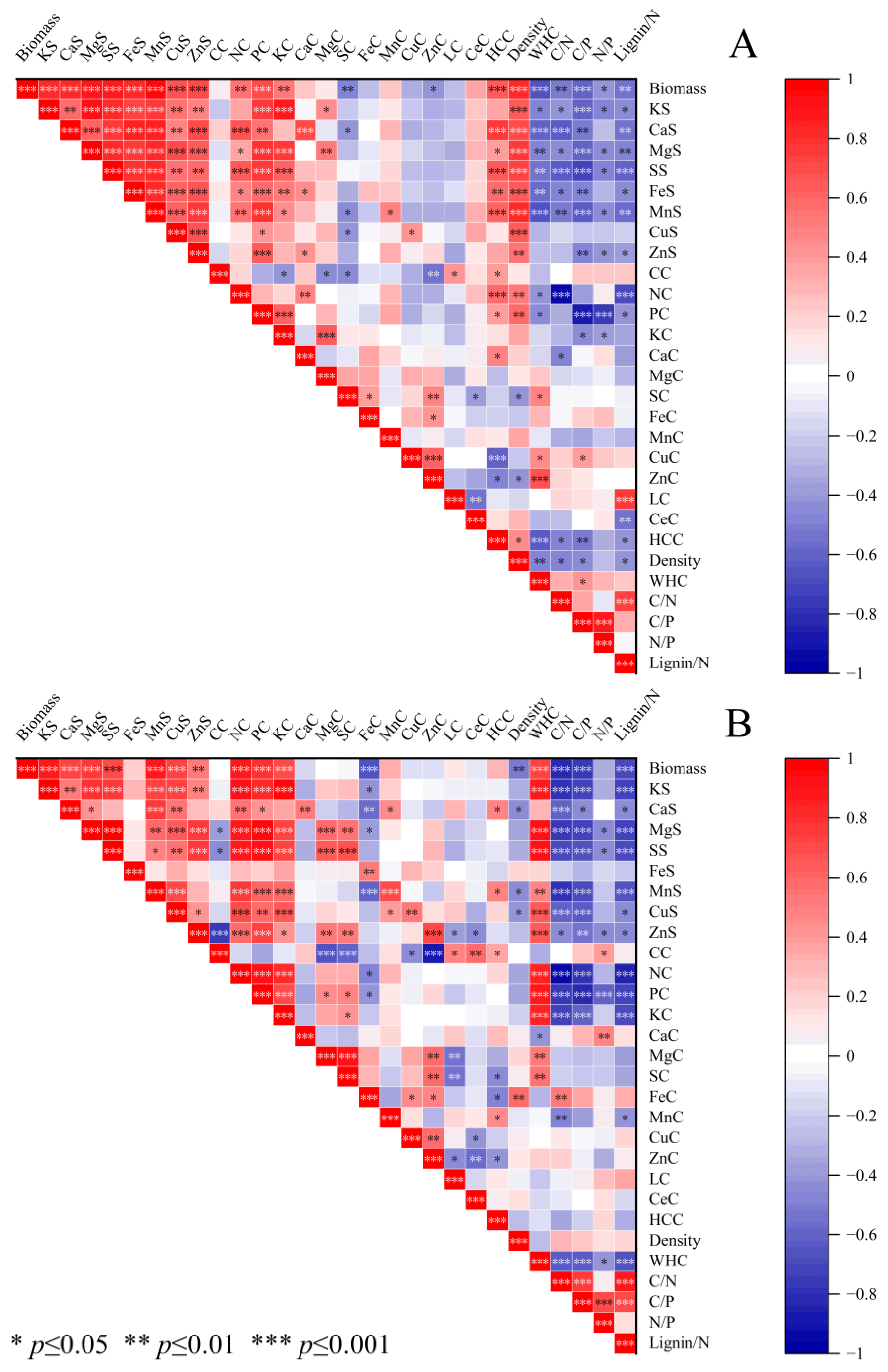
| Decomposition Time (Year) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Year of replanting | - | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 |
| DBH (cm) | - | 4.5 | 8 | 13.2 | 13.6 | 15.3 | 16.7 |
| TH (m) | - | 4.7 | 10.5 | 13.5 | 17.5 | 18.5 | 20.5 |
| Density of plantation (tree·ha−1) | - | 1667 | 1667 | 1667 | 1667 | 1667 | 1667 |
| NSH | 1506 | 1637 | 1350 | 1343 | 1037 | 1081 | 1332 |
| Distribution by decay classes of stumps | I | II | III, IV | III, IV | IV, V | IV, V | V |
| Diameter of stump at 10 cm above ground (cm) | 22.9 | 20.9 | 22.4 | 24.9 | 24.5 | 22.6 | 21.6 |
| Element | Type | Regression Equation | R2 | K | T0.5 (Years) | T0.95 (Years) |
|---|---|---|---|---|---|---|
| K | Stump | y = 118.904e−0.613t | 0.668 | 0.613 | 1.413 | 5.167 |
| Coarse root | y = 132.837e−0.566t | 0.682 | 0.566 | 1.725 | 5.789 | |
| Ca | Stump | y = 142.448e−0.305t | 0.584 | 0.305 | 3.427 | 9.823 |
| Coarse root | y = 128.042e−0.211t | 0.516 | 0.211 | 4.463 | 14.199 | |
| Mg | Stump | y = 102.824e−0.490t | 0.837 | 0.49 | 1.472 | 6.114 |
| Coarse root | y = 77.415e−0.375t | 0.613 | 0.375 | 1.848 | 7.989 | |
| S | Stump | y = 116.399e−0.313t | 0.637 | 0.313 | 2.703 | 9.572 |
| Coarse root | y = 81.062e−0.518t | 0.495 | 0.518 | 0.934 | 5.382 | |
| Fe | Stump | y = 124.305e−0.318t | 0.576 | 0.318 | 2.868 | 9.421 |
| Coarse root | - | - | - | - | - | |
| Mn | Stump | y = 105.329e−0.358t | 0.831 | 0.358 | 2.083 | 8.369 |
| Coarse root | y = 146.183e−0.306t | 0.64 | 0.306 | 3.509 | 9.791 | |
| Cu | Stump | y = 89.853e−0.205t | 0.276 | 0.205 | 3.413 | 14.615 |
| Coarse root | y = 115.900e−0.246t | 0.282 | 0.246 | 2.866 | 12.179 | |
| Zn | Stump | y = 80.906e−0.180t | 0.248 | 0.18 | 2.678 | 16.644 |
| Coarse root | y = 54.487e−0.138t | 0.039 | 0.138 | 5.022 | 21.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Liang, X.; Liu, H.; Deng, X.; Cheng, F. Nutrient Element Stocks and Dynamic Changes in Stump–Root Systems of Eucalyptus urophylla × E. grandis. Forests 2024, 15, 1. https://doi.org/10.3390/f15010001
Xie Z, Liang X, Liu H, Deng X, Cheng F. Nutrient Element Stocks and Dynamic Changes in Stump–Root Systems of Eucalyptus urophylla × E. grandis. Forests. 2024; 15(1):1. https://doi.org/10.3390/f15010001
Chicago/Turabian StyleXie, Zhushan, Xiang Liang, Haiyu Liu, Xiangsheng Deng, and Fei Cheng. 2024. "Nutrient Element Stocks and Dynamic Changes in Stump–Root Systems of Eucalyptus urophylla × E. grandis" Forests 15, no. 1: 1. https://doi.org/10.3390/f15010001






