Assessment of Various Iron Extraction Treatments on Waterlogged Archaeological Oak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waterlogged Archaeological Wood Samples
2.2. Impregnation Process
2.3. Chemical Extraction Methods
2.4. Analytical Protocol
2.4.1. Atomic Absorption Spectroscopy
2.4.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.4.3. Colorimetric Assessments
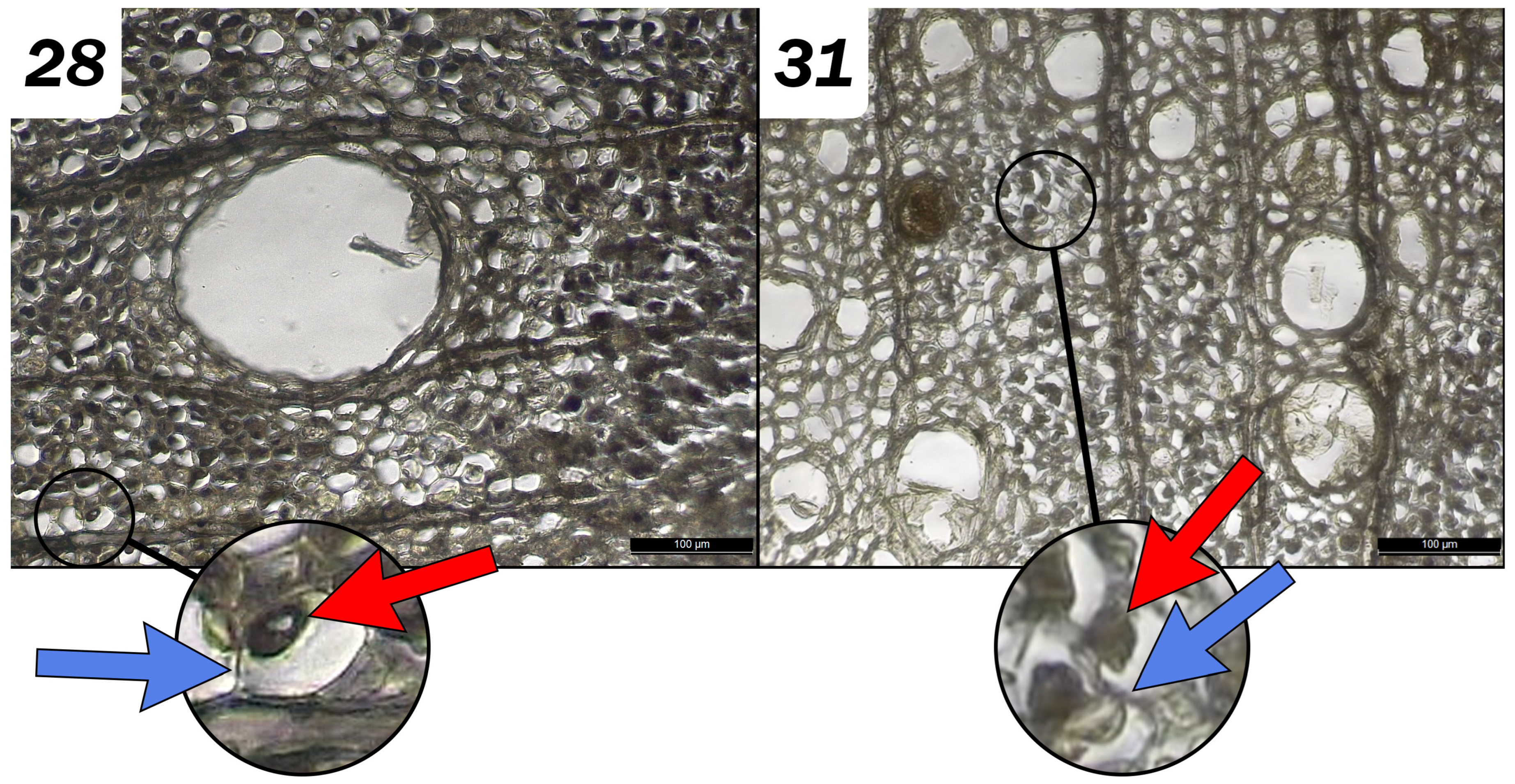
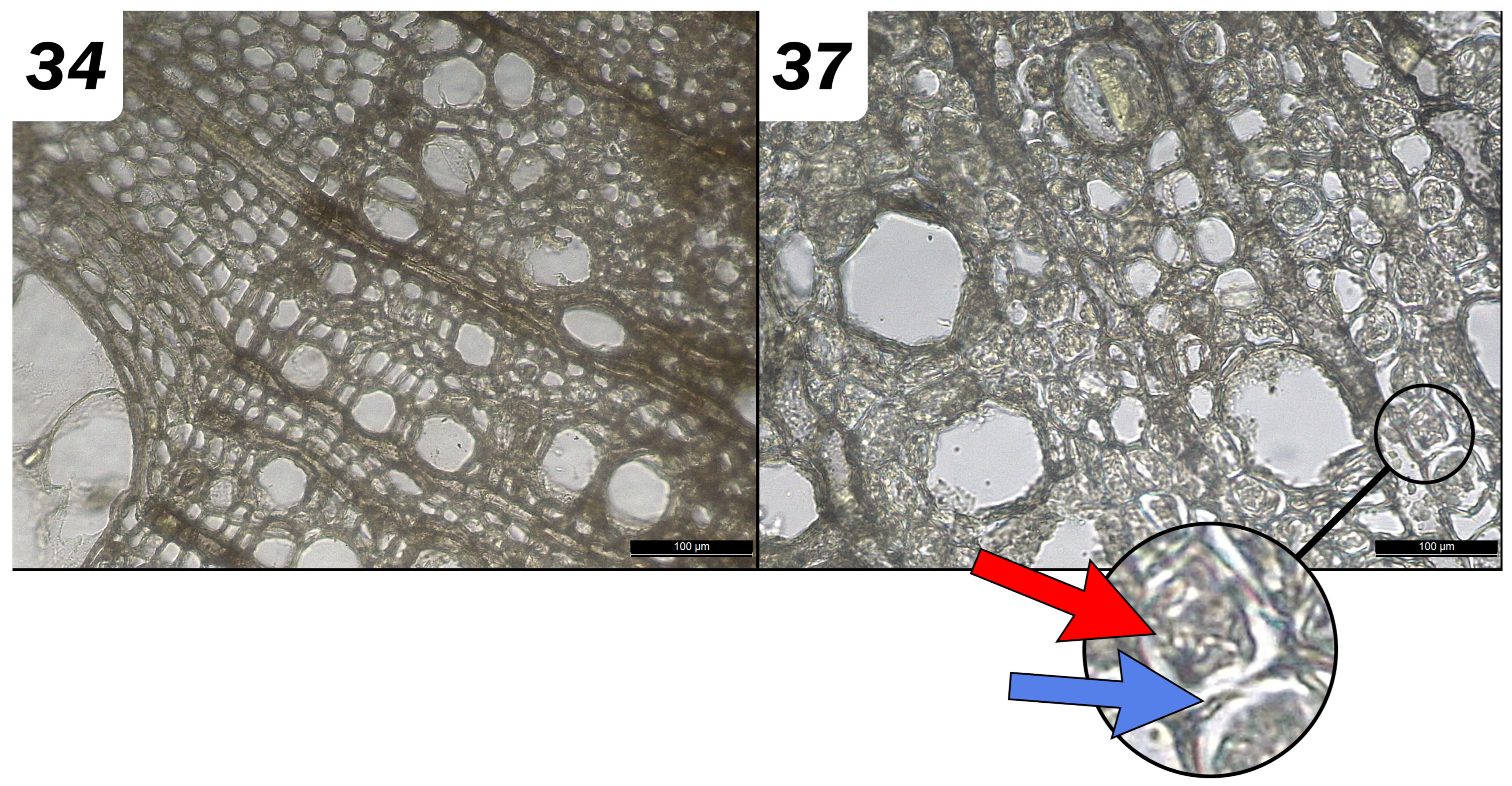
2.4.4. Anatomical and Micromorphological Observations
3. Results
3.1. Efficiency of Iron Removal Treatments: Iron Impregnation and Titration
3.2. Impacts of the Chemical Extraction Treatments
3.2.1. Color Evolution of Samples before and after Treatment
3.2.2. Effects of the Chemical Extractions on Micromorphology: Diagnostic Evaluation
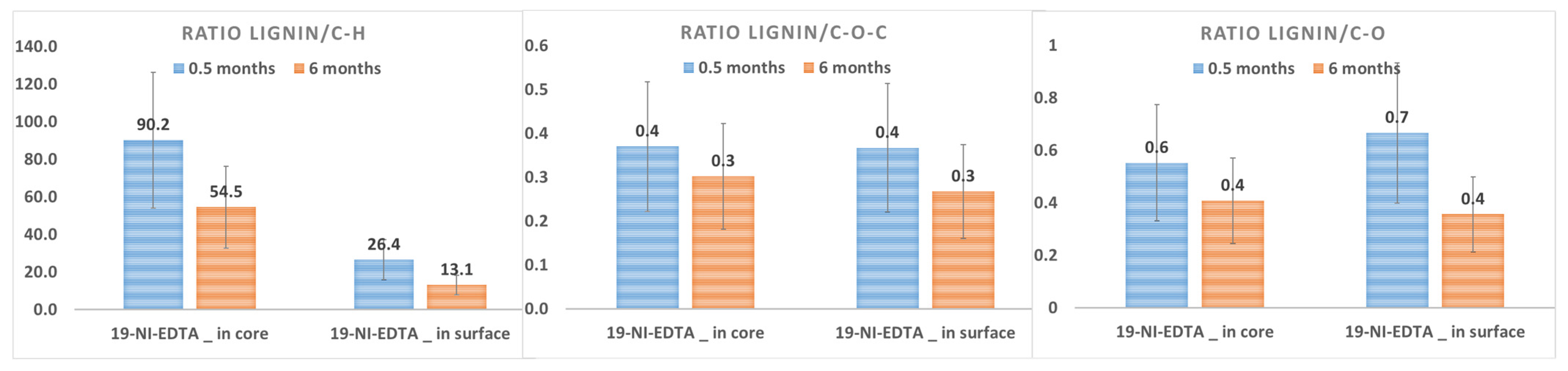
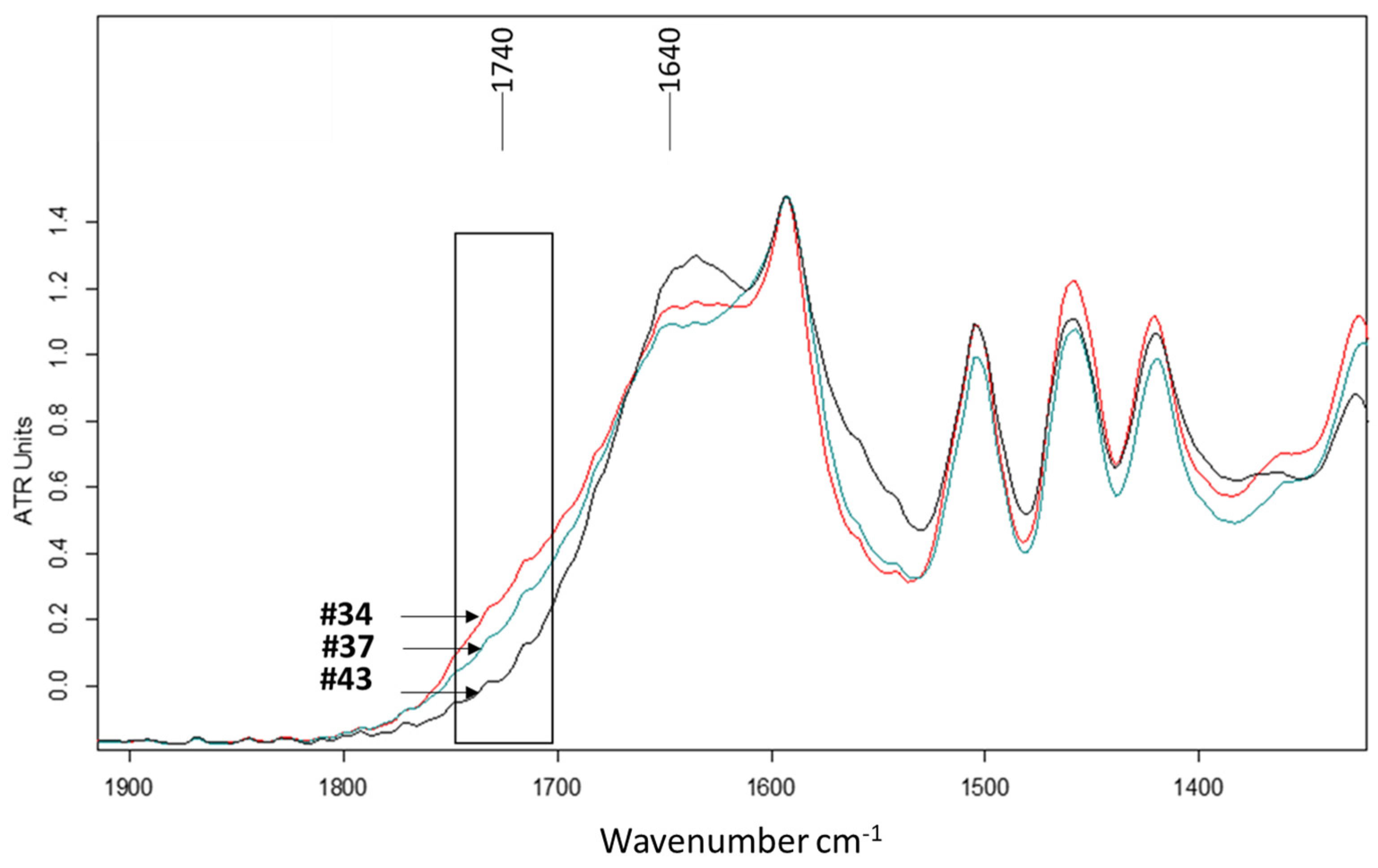
3.2.3. Chemical Impacts on Lignin and Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandström, M.; Fors, Y.; Jalilehvand, F.; Damian, E.; Gelius, U. Analyses of Sulfur and Iron in Marine Archaeological Wood. In Proceedings of the 9th ICOM Conservation Group on Wet Organic Archaeological Naterials, Copenhagen, Denmark, 4 July 2004; Hoffmann, P., Spriggs, J.A., Straetkvern, K., Gregory, D., Eds.; The International Council of Museums, Committee for Conservation Working Group on Wet Organic Archaeological Materials: Copenhagen, Denmark, 2004; pp. 181–202. [Google Scholar]
- Almkvist, G.; Persson, I. Extraction of iron compounds from wood from the Vasa. Holzforschung 2006, 60, 678–684. [Google Scholar] [CrossRef]
- Fors, Y.; Jalilehvand, F.; Risberg, E.D.; Björdal, C.; Phillips, E.; Sandström, M. Sulfur and iron analysis of marine archaeological wood in shipwrecks from the Baltic Sea and Scandinavian waters. J. Archaeol. Sci. 2012, 39, 2521–2532. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, D.; Zhang, Z.; Ma, Q. Characterization of degradation and iron deposit of the wood of Nanhai I shipwreck. Herit. Sci. 2022, 10, 202. [Google Scholar] [CrossRef]
- Pelé, C.; Moulhérat, C.; Lemoine, G.; Labroche, S.; Guilminot, E.; Baron, G. Extraction of iron compounds from marine organic objects. In Proceedings of the 12th ICOM Wet Organic Archaeological Materials Conference (WOAM), Istanbul, Turkey, 13–17 May 2013. [Google Scholar]
- Pelé, C.; Lemoine, G.; Labroche, S.; Guilminot, E.; Baron, G. Iron removal from waterlogged wood: Extraction by electrophoresis and chemical treatments. Stud. Conserv. 2015, 60, 155–171. [Google Scholar] [CrossRef]
- Pecoraro, E. New Studies about the Effects of Selected Treatments on the Chemical and Dynamic Mechanical Properties of Waterlogged Archaeological Wood. Ph.D. Thesis, Università degli Studi Firenze, Florence, Italy, 2016. [Google Scholar]
- Pecoraro, E.; Pelé-Meziani, C.; Macchioni, N.; Lemoine, G.; Guilminot, E.; Pizzo, B. Effects of iron removal treatments on the chemical and viscoelastic properties of waterlogged wood. J. Cult. Herit. 2022, 56, 149–158. [Google Scholar] [CrossRef]
- Pecoraro, E.; Pelé-Meziani, C.; Macchioni, N.; Lemoine, G.; Guilminot, E.; Shen, D.; Pizzo, B. The removal of iron from waterlogged archaeological wood: Efficacy and effects on the room temperature wood properties. Wood Mater. Sci. Eng. 2023, 18, 672–689. [Google Scholar] [CrossRef]
- Mouchard, J.; Guitton, D.; Monteil, M.; Favreau, X.; Ménez, N.; Yacger, M. Le Port Romain du Quartier de Saint-Lupien à Rezé/Ratiatum (Loire-Atlantique): Origine et Evolution/The Roman Port Neighborhood of Saint-Lupien in Rezé/Ratiatum (Loire-Atlantique): Origin and Evolution. Gallia; Archéologie de la Gaulle soutenu par le Ministère de la Culture: Paris, France, 2020; pp. 67–97. [Google Scholar]
- Arthuis, R.; Guitton, D.; Monteil, M.; Mouchard, J.; de Peretti, O. Rezé–Saint-Lupien, Fouille Programmée (2009); Edition Electronique; Ministère de la Culture: Paris, France, 2020. Available online: http://journals.openedition.org/adlfi/36713 (accessed on 31 July 2023).
- Bingham, J. A Quantitative and Nondestructive Method for Determining the Degradation of Waterlogged Archaeological Wood. Ph.D. Thesis, The Faculty of the Department of Chemistry, East Carolina University, Greenville, NC, USA, 2013. [Google Scholar]
- Gregory, D.; Jensen, P.; Matthiesen, H.; Straetkvern, K. The correlation between bulk density and shock resistance of waterlogged archaeological wood using the Pilodyn. Stud. Conserv. 2007, 52, 289–298. [Google Scholar] [CrossRef]
- Tran, Q.K.; Remazeilles, C.; Guilminot, É. Prévention de l’Acidification des Objets Archéologiques Humides Issus de Fouilles sous-Marines par Extraction des Composés Soufrés; Programme National de Recherche sur la Connaissance et la Conservation des Matériaux du Patrimoine Culturel: Paris, France, 2008.
- Monachon, M.; Albelda-Berenguer, M.; Pelé, C.; Cornet, E.; Guilminot, E.; Rémazeilles, C.; Joseph, E. Characterization of model samples simulating degradation processes induced by iron and sulfur species on waterlogged wood. Microchem. J. 2020, 155, 104756. [Google Scholar] [CrossRef]
- Bourdoiseau, J.-A. Rôle des Espèces Sulfures sur le Comportement d’un Acier non Allié en Milieu de Stockage des Déchets Radioactifs de Type C: Interaction Sulfures/Produits de Corrosion. Ph.D. Thesis, Université de la Rochelle, La Rochelle, France, 2011. [Google Scholar]
- Pizzo, B.; Pecoraro, E.; Macchioni, N. A new method to quantitatively evaluate the chemical composition of waterlogged wood by means of Attenuated Total Reflectance Fourier Transform Infrared (ATR-FT-IR) measurements carried out on wet material. Appl. Spectrosc. 2013, 67, 553–562. [Google Scholar] [CrossRef]
- Pizzo, B.; Pecoraro, E.; Alves, A.; Macchioni, N.; Rodrigues, J.-C. Quantitative evaluation of attenuated total reflectance infrared (ATR-FTIR) spectroscopy to the chemical composition of decayed wood preserved in waterlogged conditions. Talanta 2015, 131, 14–20. [Google Scholar] [CrossRef]
- High, K.E.; Penkman, K.E.H. A review of analytical methods for assessing preservation in waterlogged archaeological wood and their application in practice. Herit. Sci. 2020, 8, 83. [Google Scholar] [CrossRef]
- Picollo, M.; Cavallo, E.; Macchioni, N.; Pignatelli, O.; Pizzo, B.; Santoni, I. Spectral characterization of ancient wooden artefacts with the use of traditional IR techniques and ATR device: A methodological approach. In Proceedings of the 9th Conference of the Infrared and Raman Users’ Group, Buenos Aires, Argentina, 3–6 March 2010; Morana RTD: Ljubljana, Slovenia, 2010; pp. 23–28. [Google Scholar]
- Keey, R. Colour Development on Drying. Maderas Cie. Tecnol. 2005, 7, 3–16. [Google Scholar] [CrossRef]
- Macchioni, N.; Capretti, C.; Sozzi, L.; Pizzo, B. Grading the decay of waterlogged archaeological wood according to anatomical characterization. The case of the Fiavé site (N-E Italy). Int. Biodeterior. Biodegrad. 2013, 84, 54–64. [Google Scholar] [CrossRef]
- Sandström, M.; Jalilehvand, F.; Damian, E.; Fors, Y.; Gelius, U.; Jones, M.; Salomé, M.V. Sulfur accumulation in the timbers of King Henry VIII’s warship Mary Rose: A pathway in the sulfur cycle of conservation concern. Proc. Natl. Acad. Sci. USA 2005, 102, 14165–14170. [Google Scholar] [CrossRef]
- Fords, Y.; Richards, V. The effect of the ammonia neutralizing treatment on marine archaeological Vasa wood. Stud. Conserv. 2010, 55, 41–54. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchiou, M.; Douni, I.; Paspaliaris, I.; Kontopoulos, A. Dissolution of hematite in acidic oxalate solutions: The effect of ferrous ions addition. Hydrometallurgy 1996, 43, 219–230. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 16, 3835–3843. [Google Scholar] [CrossRef]
- Kaneko, S.; Yoshitake, K.; Itakura, S.; Tanaka, H.; Enoki, A. Relationship between production of hydroxyl radicals and degradation of wood, crystalline cellulose, and a lignin-related compound or accumulation of oxalic acid in cultures of brown-rot fungi. J. Wood Sci. 2005, 51, 262–269. [Google Scholar] [CrossRef]
- Beck-Anderson, J. Production, Function, and Neutralization of Oxalic Acid Produced by the Dry Rot Fungus and Other Brown-Rot Fungi; International Research Group on Wood Preservation: Stockholm, Sweden, 1987. [Google Scholar]
- Shimada, M.; Akamatsu, Y.; Ohta, A.; Takahashi, M. Biochemical Relationships between Biodegradation of Cellulose and Formation of Oxalic Acid in Brown-Rot Wood Decay; International Research Group on Wood Preservation: Stockholm, Sweden, 1991; Doc No. IRG/WP/1472. [Google Scholar]
- Nowack, B.; Sigg, L. Adsorption of EDTA and Metal-EDTA Complexes onto Goethite. J. Colloid Interface Sci. 1995, 177, 106–121. [Google Scholar] [CrossRef]
- Belattar, S. Réactivités Thermiques et Photochimiques du Fer en Solution Aqueuse vis-à-vis de Molécules Organiques en Phase Homogène et Hétérogène; Mentouri-Constantine University: Constantine, Algeria, 2009; pp. 20–41. [Google Scholar]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Bondietti, G.; Sinniger, J.; Stumm, W. The reactivity of Fe(III) (hydroxides: Effects of ligands in inhibiting the dissolution. Colloids Surf. A Physicochem. Eng. Asp. 1993, 79, 157–167. [Google Scholar] [CrossRef]
- Abrahamson, H.B.; Rezvani, A.B.; Brushmiller, J.G. Photochemical and spectroscopic studies of complexes of iron(III) with citric acid and other carboxylic acids. Inorg. Chim. Acta 1994, 226, 117–127. [Google Scholar] [CrossRef]
- Abida, O. Impact des Complexes de Fer et de la Lumière Solaire sur le Devenir de Polluants de l’Environnement Aquatique. Ph.D. Thesis, Université Blaise Pascal de Clermont-Ferrand, Clermont-Ferrand, France, 2005. [Google Scholar]
- Jean, L. Mobilisation du Chrome et du Nickel à Partir de Sols Contaminés, en Présence de Complexants: Transfert et Accumulation de ces Métaux chez Datura innoxia. Ph.D. Thesis, Université de Limoges, Limoges, France, 2007. [Google Scholar]
- Sandström, T.P.A.; Hall-Roth, I.; Karlsson, A. Salt precipitation on Vasa Timbers; an introduction to a problem. In Proceedings of the 8th ICOM Group on Wet Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; Committee for Conservation Working Group on Wet Organic Archaeological Materials: Paris, France, 2001; pp. 54–55. [Google Scholar]
- Königsberger, L.C.; Königsberger, E.; May, P.M.; Hefter, G.T. Complexation of iron(III) and iron(II) by citrate. Implications for iron speciation in blood plasma. J. Inorg. Biochem. 2000, 78, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses; VCH Verlagsgesellschaft GmbH: Weinheim, Germany, 1996. [Google Scholar]
- Stumm, W. Reactivity at the mineral-water interface: Dissolution and inhibition. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 143–166. [Google Scholar] [CrossRef]
- Taxiarchou, M.; Panias, D.; Douni, I.; Paspaliaris, I.; Kontopoulos, A. Removal of iron from silica by leaching with oxalic acid. Hydrometallurgy 1997, 46, 215–227. [Google Scholar] [CrossRef]
- Sung, O.L.; Tran, T.; Jung, B.H.; Kim, S.J.; Kim, M.J. Dissolution of iron oxide using oxalic acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar]
- Cornell, R.M.; Schindler, P.W. Photochemical dissolution of goethite in acid/oxalate solution. Clays Clay Miner. 1987, 35, 347–352. [Google Scholar] [CrossRef]
- Henrik-Klemens, A.; Bengtsson, F.; Björdal, C.G. Raman spectroscopic investigation of iron-tannin precipitates in waterlogged archaeological oak. Stud. Conserv. 2021, 67, 237–247. [Google Scholar] [CrossRef]
- Blesa, M.A.; Rueda, E.H.; Ballesteros, M.C.; Grassi, R.L. Dithionite as a dissolving reagent for goethite in the presence of EDTA and citrate. Application to soils analysis. Clays Clay Miner. 1992, 40, 575–585. [Google Scholar]
- Gelbrich, J.; Mai, C.; Militz, H. Chemical changes in wood degradred by bacteria. Int. Biodeterior. Biodegrad. 2008, 61, 24–32. [Google Scholar] [CrossRef]
- Broda, M.; Hill, C.A.S. Conservation of waterlogged wood-past, present and future perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]





| Chemical Solutions | Concentrations | pH |
|---|---|---|
| EDTA | 3.7%w/w | 5.0 |
| DTPA | 0.010 mol/L | 7.0 |
| Citric acid | 1.0%w/w | 2.2 |
| Oxalic acid | 1.0%w/w | 1.0 |
| Sodium persulfate | 0.37%w/v | 6.0 |
| Sodium dithionite | 2.0%w/w | 6.5 |
| Sample n° | Impregnation | Pre-Treatment | Treatment |
|---|---|---|---|
| #40-41-42 | No | - | - |
| #19-20-21 | No | - | EDTA |
| #13-14-15 | No | Sodium dithionite | EDTA |
| #16-17-18 | No | Sodium persulfate | EDTA |
| #43-44-45 | Yes | - | - |
| #1-2-3 | Yes | - | EDTA |
| #4-5-6 | Yes | Sodium dithionite | EDTA |
| #7-8-9 | Yes | Sodium persulfate | EDTA |
| #10-11-12 | Yes | - | DTPA |
| #22-23-24 | Yes | Sodium dithionite | DTPA |
| #25-26-27 | Yes | Sodium persulfate | DTPA |
| #28-29-30 | Yes | - | Citric acid |
| #31-32-33 | Yes | Sodium persulfate | Citric acid |
| #34-35-36 | Yes | - | Oxalic acid |
| #36-37-38 | Yes | Sodium persulfate | Oxalic acid |
| Chemical Treatments | Iron Extracted in Solution (mgFe/g Wood) | Iron Remained in Wood (mgFe/g Wood) | Total Iron in Wood before Treatment (mgFe/g Wood) | % of Iron Extracted |
|---|---|---|---|---|
| EDTA | 6.3 | 1.5 | 7.8 | 80% |
| Sodium dithionite–EDTA | 6.6 | 1.4 | 8.0 | 82% |
| Sodium persulfate–EDTA | 5.9 | 1.8 | 7.7 | 76% |
| DTPA | 1.3 | 8.5 | 9.8 | 13% |
| Sodium dithionite–DTPA | 2.3 | 8.5 | 10.8 | 21% |
| Sodium persulfate–DTPA | 3.2 | 5.4 | 8.6 | 37% |
| Citric acid | 5.0 | 3.8 | 8.8 | 57% |
| Sodium persulfate–citric acid | 6.0 | 1.6 | 7.6 | 79% |
| Oxalic acid | 0.6 | 6.8 | 7.2 | 8% |
| Sodium persulfate–oxalic acid | 1.8 | 6.5 | 8.3 | 22% |
| ΔL* (D65) | Δa* (D65) | Δb* (D65) | ΔE | ||
|---|---|---|---|---|---|
| #1 | EDTA | 20.64 | 0.90 | 2.59 | 21 |
| #4 | Sodium dithionite–EDTA | 17.28 | 1.99 | 4.60 | 18 |
| #7 | Sodium persulfate–EDTA | 23.40 | 3.12 | 5.84 | 24 |
| #19 | Non-impregnated–EDTA | 4.71 | 3.05 | 4.97 | 7 |
| #13 | Non-impregnated–Sodium dithionite–EDTA | 7.02 | 3.44 | 7.17 | 11 |
| #16 | Non-impregnated–Sodium persulfate–EDTA | 5.08 | 4.38 | 6.53 | 9 |
| #10 | DTPA | 4.81 | −1.25 | −2.42 | 6 |
| #22 | Sodium dithionite–DTPA | 4.37 | −1.25 | −1.96 | 5 |
| #25 | Sodium persulfate–DTPA | 8.36 | −1.67 | −1.38 | 9 |
| #28 | Citric acid | 13.24 | −2.41 | −3.23 | 14 |
| #31 | Sodium persulfate–Citric acid | 17.09 | −0.34 | 1.20 | 17 |
| #34 | Oxalic acid | 21.66 | 5.30 | 11.51 | 25 |
| #37 | Sodium persulfate–Oxalic acid | 30.95 | 8.68 | 19.22 | 37 |
| Corpus: Archaeological Oak Samples | Degradation Level: Slightly Spongy | Impregnation: Defined by Previous Studies (Monachon et al., 2020) | Concentration: Defined by Main Treatments Used in the Field of Conservation—Restoration | Duration Treatment: 4 h in Pre-Treatment, 5 Days in Chelating or Acidic Solutions | ||||
|---|---|---|---|---|---|---|---|---|
| Diagnostic | EDTA | DTPA | Citric acid | Oxalic acid | ||||
| Chemicals alone | Lumens seem lighter Disintegrated material removed Secondary wall seems washed away |  | Very dark Fiber collapsed |  | A strong sulfur odor Core is darker Some portions of walls have collapsed |  | Very light and yellowing, a few black spots Some wall areas have collapsed but remain in position |  |
| With Na Dithionite | Iron spots remain Small fungal hyphae |  | Very dark, Intensification of collapse, Nonhomogeneous Hyphae |  | X toxic emanation | X toxic emanation | ||
| With Na Persulfate | Non-homogeneous Secondary walls partially degraded Cellular lumens partially empty Black-colored areas Spores and hyphae |  | Very dark Intensification of collapse Nonhomogeneous Hyphae |  | Tissue appears very degraded Much lighter Hyphae |  | Light and yellowed (less than without pre-treatment) A few black spots Some wall areas have collapsed but remain in position |  |
| Colorimetry | EDTA | DTPA | Citric acid | Oxalic acid | ||||
| Chemicals alone | Color is homogeneous Lighter color |  | Lower evolution |  | Color is homogeneous Lighter color |  | Color is homogeneous Lighter color Yellowing surface |  |
| With Na Dithionite | No significant change |  | No significant change |  | X toxic emanation | X toxic emanation | ||
| With Na Persulfate | No significant change |  | Slightly increase the change |  | Slightly increase the change |  | Slightly increase the change |  |
| Extraction rate | EDTA | DTPA | Citric acid | Oxalic acid | ||||
| Chemicals alone | 80% |  | 13% |  | 57% |  | 8% |  |
| With Na Dithionite | 82% |  | 21% |  | X toxic emanation | X toxic emanation | ||
| With Na Persulfate | 76% |  | 37% |  | 79% |  | 22% |  |
| No interest to use pre-treatment | Pre-treatment improves extraction | Pre-treatment improves extraction | Pre-treatment improves extraction | |||||
| Chemical impacts | EDTA | DTPA | Citric acid | Oxalic acid | ||||
| Chemicals alone | 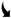 lignin/C-H lignin/C-H |  | 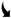 lignin/C-H lignin/C-H |  | 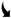 lignin/C-H lignin/C-H |  | 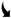 lignin/C-H lignin/C-H |  |
| Rinsing | ||||||||
| With Na Dithionite | 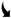 lignin/C-H lignin/C-H |  | 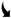 lignin/C-H lignin/C-H |  | X toxic emanation | X toxic emanation | ||
| Rinsing |  | 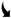 lignin/C-O-C and C-O lignin/C-O-C and C-O |  | |||||
| With Na Persulfate | 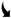 lignin/C-H lignin/C-H |  |  lignin/C-H lignin/C-H |  lignin/C-H lignin/C-H |  lignin/C-H lignin/C-H | |||
| Rinsing | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelé-Meziani, C.; Macchioni, N.; Sozzi, L.; Guilminot, E.; Lemoine, G.; Pizzo, B.; Mevellec, J.Y.; Pecoraro, E.; Monachon, M. Assessment of Various Iron Extraction Treatments on Waterlogged Archaeological Oak. Forests 2023, 14, 1834. https://doi.org/10.3390/f14091834
Pelé-Meziani C, Macchioni N, Sozzi L, Guilminot E, Lemoine G, Pizzo B, Mevellec JY, Pecoraro E, Monachon M. Assessment of Various Iron Extraction Treatments on Waterlogged Archaeological Oak. Forests. 2023; 14(9):1834. https://doi.org/10.3390/f14091834
Chicago/Turabian StylePelé-Meziani, Charlène, Nicola Macchioni, Lorena Sozzi, Elodie Guilminot, Gwenaël Lemoine, Benedetto Pizzo, Jean Yves Mevellec, Elisa Pecoraro, and Mathilde Monachon. 2023. "Assessment of Various Iron Extraction Treatments on Waterlogged Archaeological Oak" Forests 14, no. 9: 1834. https://doi.org/10.3390/f14091834
APA StylePelé-Meziani, C., Macchioni, N., Sozzi, L., Guilminot, E., Lemoine, G., Pizzo, B., Mevellec, J. Y., Pecoraro, E., & Monachon, M. (2023). Assessment of Various Iron Extraction Treatments on Waterlogged Archaeological Oak. Forests, 14(9), 1834. https://doi.org/10.3390/f14091834








