Weed Coexistence in Eucalyptus Hybrid Stands Decreases Biomass and Nutritional Efficiency Mid-Rotation
Abstract
:1. Introduction
2. Materials and Methods
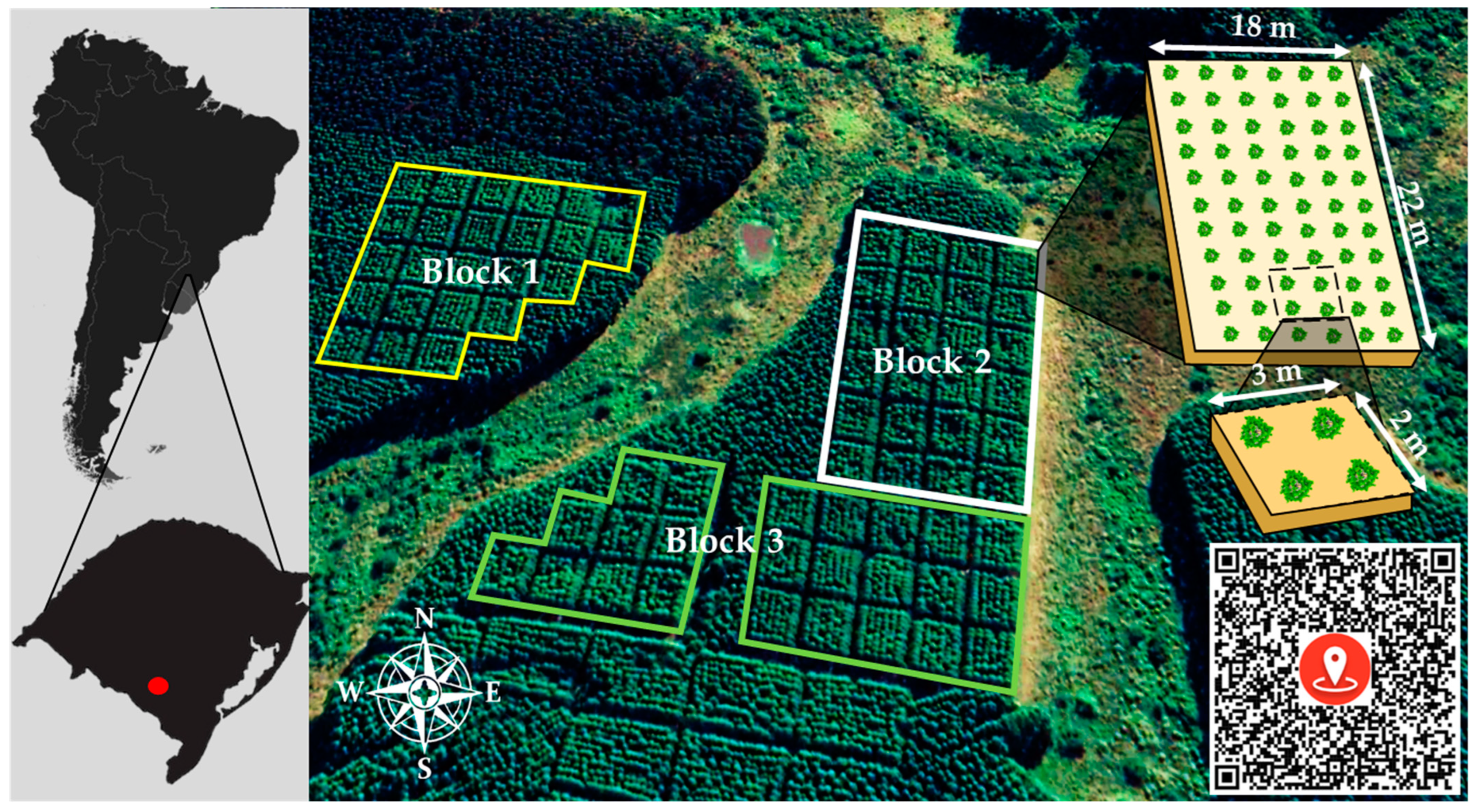
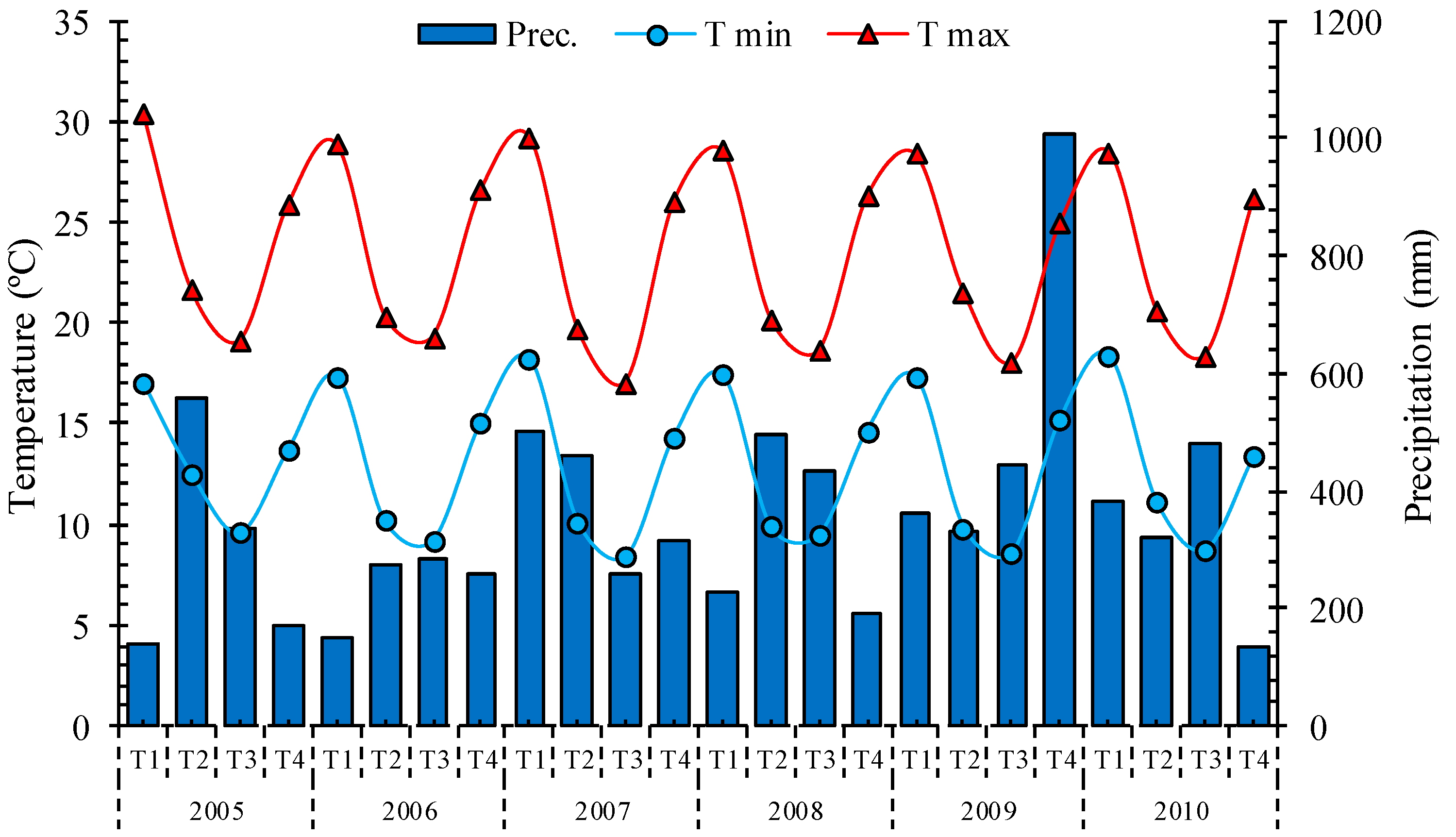
2.1. Study Area
2.2. Implantation and Experimental Design
2.3. Eucalyptus Biomass and Weed Species
2.4. Chemical Analysis
2.5. Statistical Analyses
3. Results
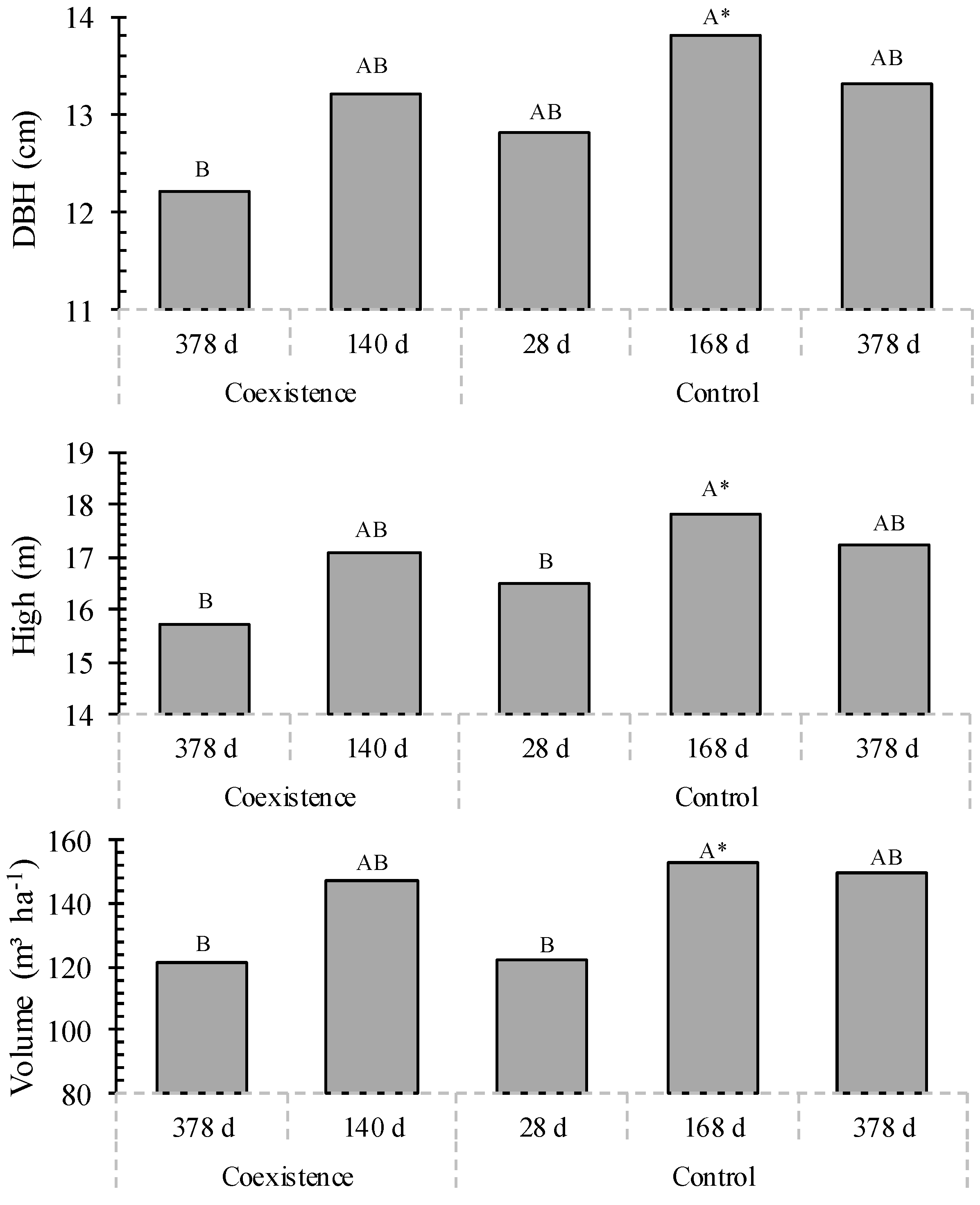
3.1. Forest Inventory
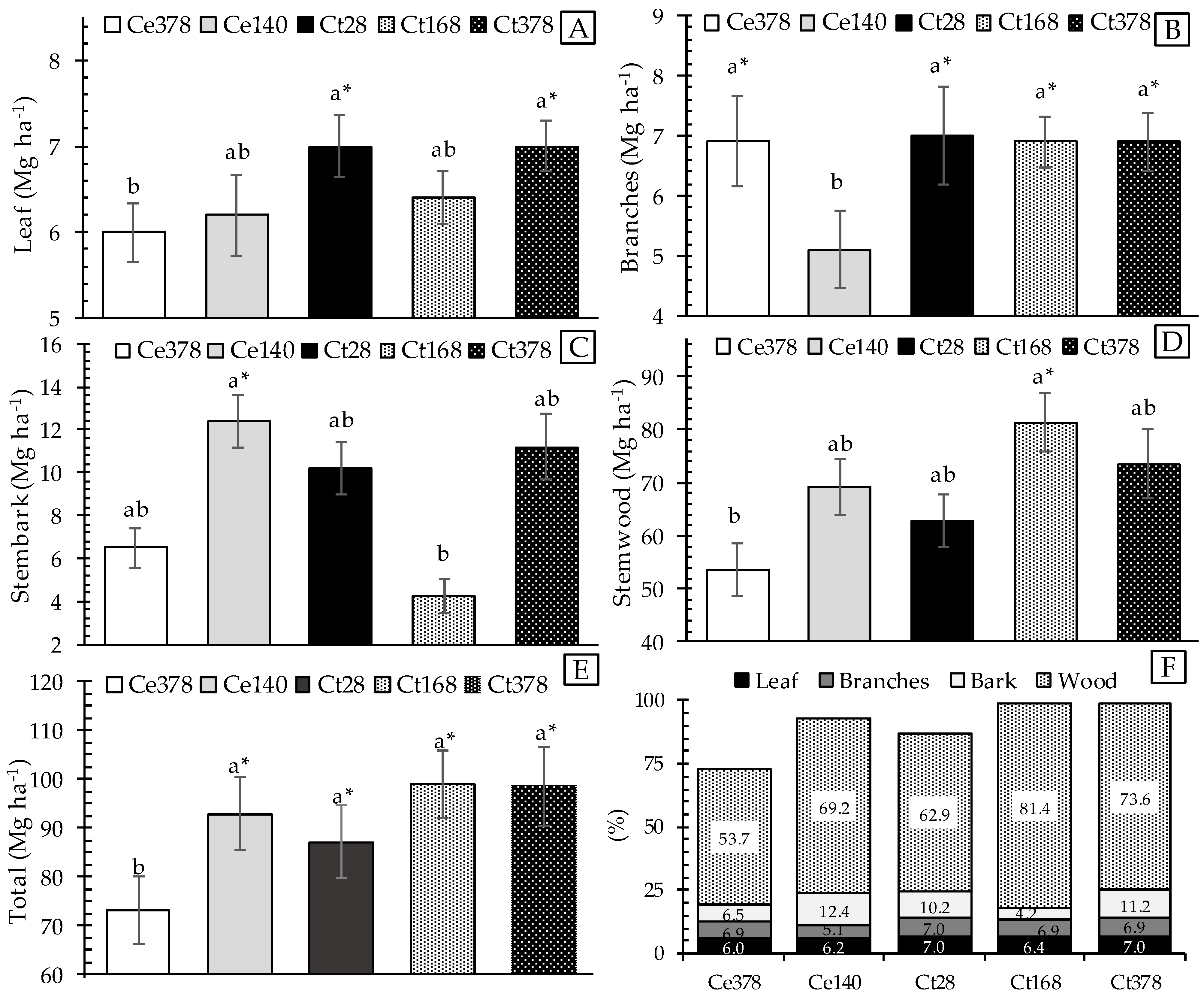
3.2. Forest Biomass
3.3. Nutrient Concentrations
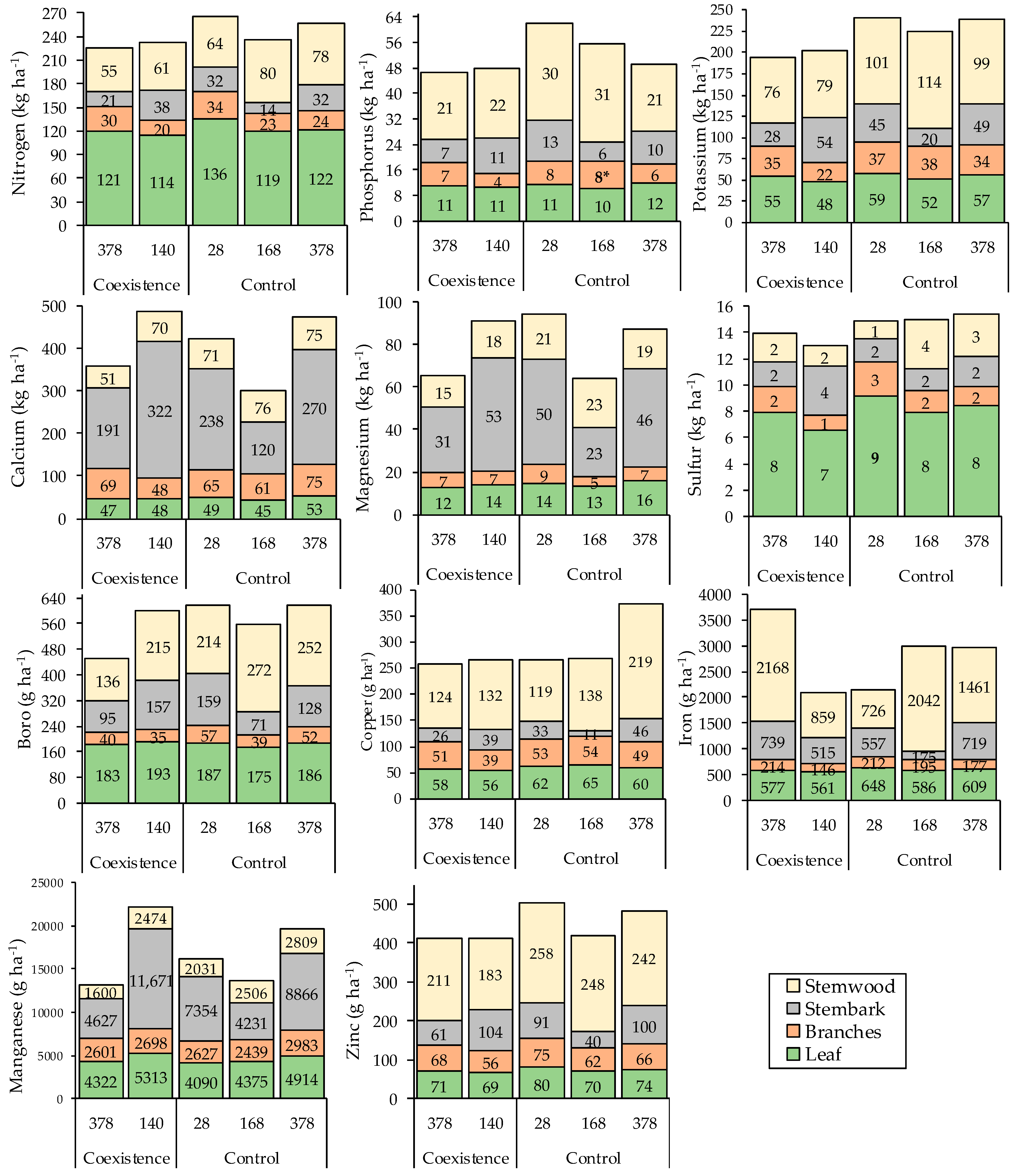
3.4. Nutrient Stock
3.5. Nutrient Utilization Efficiency
4. Discussion
4.1. Forest Inventory
4.2. Forest Biomass
4.3. Nutrient Concentration
4.4. Nutrient Stock
4.5. Nutrient Utilization Efficiency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- IBÁ. Indústria Brasileira de Árvores. Anuário Estatístico IBÁ 2022. 93p. Available online: https://www.iba.org/datafiles/publicacoes/relatorios/relatorioiba2021-compactado.pdf (accessed on 15 February 2023).
- Carrero, O.; Luiz, J.; Allen, L.; Cecilia, M.; Ladeira, M. Productivity gains from weed control and fertilization of short-rotation eucalyptus plantations in the Venezuelan Western Llanos. For. Ecol. Manag. 2018, 430, 566–575. [Google Scholar] [CrossRef]
- Maciel, J.C.; Duque, T.S.; Ferreira, E.A.; Zanuncio, J.C.; Plata-Rueda, A.; Silva, V.P.; Silva, D.V.; Fernandes, B.C.C.; Barros Júnior, A.P.; Dos Santos, J.B. Growth, Nutrient Accumulation, and Nutritional Efficiency of a Clonal Eucalyptus Hybrid in Competition with Grasses. Forests 2022, 13, 1157. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, G.; Xie, Z.; Yu, J.; Jiang, D.; Huang, Z. Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations. Sustainability 2020, 12, 3669. [Google Scholar] [CrossRef]
- Graat, Y.; Bacha, A.L.; Nepomuceno, M.P.; Alves, P.L.C.A. Initial Development of Eucalyptus According to Different Desiccation Periods of Signalgrass. Planta Daninha 2018, 36, e018145168. [Google Scholar] [CrossRef]
- Machado, A.F.L.; Ferreira, L.R.; Santos, L.D.T.; Ferreira, F.A. Interferência das plantas daninhas na cultura do eucalipto. In Manejo Integrado de Plantas Daninhas na Cultura do Eucalipto; Ferreira, L.R., Machado, A.F.L., Ferreira, F.A., Santos, L.D.T., Eds.; UFV: Viçosa, Brazil, 2010; 140p. [Google Scholar]
- Ferreira, E.A.; Silva, E.B.; Carvalho, F.P.; Silva, D.V.; Santos, J.B. Crescimento e análise nutricional de plantas daninhas em competição com pinhão-manso. Enciclopédia Biosf. 2013, 9, 3788. [Google Scholar]
- Pitelli, R.A. O termo planta-daninha. Planta Daninha 2015, 33. [Google Scholar] [CrossRef]
- Ferreira, G.L.; Saraiva, D.T.; Queiroz, G.P.; Silva, D.V.; Pereira, G.A.M.; Ferreira, L.R.; Oliveira Neto, S.N.; Mattiello, E.M. Eucalypt growth submitted to management of Urochloa spp. Planta Daninha 2016, 34, 99–107. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds—Weedscience.org. Available online: http://www.weedscience.org/ (accessed on 15 January 2023).
- Mancuso, M.A.C.; Negrisoli, E.; Perim, L. Efeito residual de herbicidas no solo (“Carryover”). Rev. Bras. Herbic. 2011, 10, 151–164. [Google Scholar] [CrossRef]
- Tiburcio, R.A.S.; Ferreira, F.A.; Ferreira, L.R.; Machado, M.S.; Machado, A.F.L. Controle de plantas daninhas e seletividade do flumioxazin para eucalipto. Cerne 2012, 18, 523–531. [Google Scholar] [CrossRef]
- Santos, S.A.; Tuffi-Santos, L.D.; Alfenas, A.C.; Faria, A.T.; Sant’anna-Santos, B.F. Differential Tolerance of Clones of Eucalyptus grandis Exposed to Drift of the Herbicides Carfentrazone-Ethyl and Glyphosate. Planta Daninha 2019, 37, e019175977. [Google Scholar] [CrossRef]
- Kaneko, J.A.; Lima, S.F.; Lima, A.P.L.; Martins, S.M.; Santos, D.M.C.L. Fitossociologia de plantas daninhas em eucalipto clonal com diferentes espaçamentos. In Aspectos Conceituais Sobre as Ciências da Terra; Catapan, E.A., Ed.; Brazilian Journals Editora: São José dos Pinhais, Paraná, Brazil, 2019; 65p. [Google Scholar]
- Faustino, L.A.; Queiroz, G.P.; Pereira, G.A.M.; Ferreira, E.A.; Ferreira, L.R. Aspectos fisiológicos do eucalipto em convivência com três espécies de plantas daninhas. Ver. Inst. Flor. 2017, 29, 145–155. [Google Scholar] [CrossRef]
- Porto, M. Os campos sulinos: Sustentabilidade e manejo. Ciência Ambiente 2002, 24, 119–138. [Google Scholar]
- Alvares, C.A.; Stape, L.; de Moraes Goncalves, L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Agritempo. Dados meteorológicos—Candiota. Campinas. 2023. Available online: http://www.agritempo.gov.br (accessed on 15 January 2023).
- dos Santos, H.G.; Jacomine, P.K.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; De Araujo Filho, J.C.; De Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. [Google Scholar]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.F.S.; Melo, W.J. Análise Química De Tecido Vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Silva, F.C., Ed.; Embrapa Informação Tecnológica: Brasilia, Brazil, 2009; pp. 191–234. [Google Scholar]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 20.0; IBM Corporation: Armonk, NY, USA, 2011.
- Londero, E.K.; Schumacher, M.V.; Ramos, L.O.O.; Ramiro, G.A.; Szymczak, D.A. Influência de diferentes períodos de controle e convivência de plantas daninhas em eucalipto. Cerne 2012, 18, 441–447. [Google Scholar] [CrossRef]
- Toledo, R.E.B. Faixas e Períodos de Controle de Plantas Daninhas e Seus Reflexos no Crescimento do Eucalipto. Ph.D. Thesis, University of São Paulo, Piracicaba, Brazil, 2002; 130p. [Google Scholar]
- Toledo, R.E.B.; Alves, P.L.d.C.A.; Valle, C.F.d.; Alvarenga, S.F. Manejo de Brachiaria decumbens e seu reflexo no desenvolvimento de Eucalyptus grandis. Sci. For. 1999, 55, 129–144. [Google Scholar]
- Inail, M.A.; Hardiyanto, E.B.; Mendham, D.S.; Thaher, E. Growth Response to Weed Control and Fertilisation in Mid-Rotation Plantations of Eucalyptus pellita in South Sumatra, Indonesia. Forests 2021, 12, 1653. [Google Scholar] [CrossRef]
- Tarouco, C.P.; Agostinetto, D.; Panozzo, L.E.; Santos, L.S.D.; Vignolo, G.K.; de Oliveira Ramos, L.O. Períodos de interferência de plantas daninhas na fase inicial de crescimento do eucalipto. Pesqui. Agropecuária Bras. 2009, 44, 1131–1137. [Google Scholar] [CrossRef]
- Marchi, S.R. Efeitos de Períodos de Controle das Plantas Daninhas no Crescimento Inicial e Composição Mineral de Eucalyptus Grandis Hill ex Maiden. Master’s Thesis, Universidade Estadual de São Paulo, Jaboticabal, Brazil, 1987; 98p. [Google Scholar]
- Schumacher, M.V.; Caldeira, M.V.W. Quantificação de biomassa em povoamentos de Eucalyptus saligna Sm. com diferentes idades. Biomassa Energ. 2004, 1, 381–391. [Google Scholar]
- Guimarães, C.C.; Schumacher, M.V.; Momolli, D.R.; Souza, H.P.; Ludvichak, A.A.; Malheiros, A.C. Biomass Production and Nutritional Efficiency in Eucalyptus Genotypes in the Pampa Biome. J. Exp. Agric. Int. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Ludvichak, A.A.; Schumacher, M.V.; Viera, M.; Santos, K.F.; Momolli, D.M. Growth, biomass and nutrient stock in mixed-species planting of hybrid Eucalyptus urograndis and Acacia mearnsii in Southern Brazil. New For. 2022, 53, 203–219. [Google Scholar] [CrossRef]
- Salvador, S.M.; Ludvichak, A.A.; Momolli, D.R.; Santos, K.F.; Consensa, C.B.; Schumacher, M.V.; Stahl, J. Removal of nutrients due to biomass harvest of Eucalyptus urograndis in different soils: Macronutrients. Rev. Ambient. Água 2021, 16, e2671. [Google Scholar] [CrossRef]
- Schumacher, M.V.; Witschoreck, R.; Calil, F.N. Biomassa em povoamentos de Eucalyptus spp. de pequenas propriedades rurais em Vera Cruz, RS. Ciência Florest. 2011, 21, 17–22. [Google Scholar] [CrossRef]
- Cunha, G.M.; Gama-Rodrigues, A.C.; Costa, G.S. Ciclagem de nutrientes em Eucalyptus grandis W. Hill ex Maiden no Norte Fluminense. Rev. Árvore 2005, 29, 353–363. [Google Scholar] [CrossRef]
- Santos, K.F.; Ludvichak, A.A.; Queiroz, T.B.; Schumacher, M.V.; Araújo, E.F. Biomass production and nutrient content in different Eucalyptus genotypes in Pampa Gaúcho, Brazil. Rev. Bras. Ciências Agrárias 2019, 14, e6575. [Google Scholar] [CrossRef]
- Bellote, A.F.J.; da Silva, H.D. Técnicas de amostragem e avaliações nutricionais in plantios de Eucalyptus spp. In Nutrição e fertilização florestal; Gonçalves, J.L.M., Benedetti, V., Eds.; IPEF: Piracicaba, Brazil, 2000; 427p. [Google Scholar]
- Macedo, R.L.G.; Soares, R.V.; Soares, A.R. “Status” nutricional de Eucalyptus (na fase juvenil) introduzidos na baixada cuiabana, MT. Cerne 1996, 2, 110–123. [Google Scholar]
- Ferreira, J.L.S.; Calil, F.N.; de Melo, C.; Neto, S. Nutrient stock in the forest component in a crop-livestock-forest integration system in Central Brazil. Rev. Ibero Am. Ciências Ambient. 2021, 12, 86–97. [Google Scholar] [CrossRef]
- Medeiros, P.L.; Pimenta, A.S.; da Silva, G.G.C.; de Oliveira, E.M.M.; Silva Júnior, D.N.; Souza, G.L.F. Efficiency of micronutrients and sodium use of a Eucalyptus clone as a function of planting density in short-rotation cropping. Aust. For. 2021, 84, 73–81. [Google Scholar] [CrossRef]
- Marchi, S.R. Efeito dos Períodos de Convivência e de Controle das Plantas Daninhas Sobre o Crescimento Inicial e a Composição Mineral de Eucalyptus Grandis W. Hill ex Maiden. Master’s Thesis, Faculdade de Ciências Agrárias e Veterinárias do Câmpus de Jaboticabal, Universidade Estadual Paulista “Julio de Mesquita Filho”, Jaboticabal, Brazil, 1996. [Google Scholar]
- Pitelli, R.A.; Marchi, S.R. Interferência das plantas invasoras nas áreas de reflorestamento. In Seminário Técnico Sobre Planta Daninhas e o Uso de Herbicidas em Reflorestamento; Anais: Belo Horizonte, Brazil, 1991; pp. 110–123. [Google Scholar]
- Schumacher, M.V.; Poggiani, F. Produção de biomassa e remoção de nutrientes em povoamentos de Eucalyptus camaldulensis Dehnh, Eucalyptus grandis Hill ex Maiden e Eucalyptus torelliana f. Muell, plantados em Anehmbi, SP. Ciência Florest. 1993, 3, 9–18. [Google Scholar] [CrossRef]
- Viera, M.; Schumacher, M.V.; Trüby, P.; de Araújo, E.F. Biomassa e nutrientes em um povoamento de Eucalyptus urophylla x Eucalyptus globulus, em Eldorado do Sul-RS. Ecol. Nutr. Florest. 2013, 1, 1–13. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Suderland, MA, USA, 2005. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 857. [Google Scholar]
- Guimarães, C.; Momolli, D.; Schumacher, M.; Ludvichak, A.; Souza, H.; Malheiros, A. Silvicultural Implications in Hibrid of Eucalyptus urophylla S.T. Blake × Eucalyptus grandis Hill ex Maiden Stand. J. Agric. Sci. 2019, 11, 273–281. [Google Scholar] [CrossRef]
- Santos, K.F.; Queiroz, T.B.; Ludvichak, A.A.; Momolli, D.R.; Garlet, C.; Schumacher, M.V.; de Araújo, E.F. Nutrient-use efficiency of Eucalyptus genotypes grown in Luvisol. Rev. Ambiente Água 2021, 16, e2608. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Liu, X.; Xu, D. Above-Ground Biomass and Nutrient Accumulation in Ten Eucalyptus Clones in Leizhou Peninsula, Southern China. Forests 2022, 13, 530. [Google Scholar] [CrossRef]
| Horizon | A | AC | CR | |
|---|---|---|---|---|
| Depth (cm) | 0–13 | 13–28/36 | 28/36–60+ | |
| Attributes | ||||
| Granulometry (%) | C.S. | 9.9 | 29.3 | - |
| F.S. | 3.4 | 3.4 | - | |
| M.S. | 5.0 | 5.8 | - | |
| Silt | 38.5 | 20.8 | - | |
| Clay | 43.3 | 40.8 | - | |
| pH | Water | 5.1 | 5.4 | - |
| (cmolc/dm3) | Ca2+ | 0.01 | 0.01 | - |
| Mg2+ | 6.2 | 8.5 | - | |
| K+ | 0.6 | 0.4 | - | |
| Na+ | 0.3 | 0.3 | - | |
| S | 7.1 | 9.2 | - | |
| Al3+ | 3.3 | 4.6 | - | |
| H+ | 10.9 | 10.9 | - | |
| t | 10.4 | 13.9 | - | |
| T | 21.3 | 24.8 | - | |
| O.M | % | 3.6 | 2.2 | - |
| Ta | clay | 41.6 | 49.5 | - |
| V | % | 39.6 | 45.8 | - |
| m | % | 31.3 | 33.3 | - |
| P | mg/dm3 | 2.9 | 1.5 | - |
| Group | Period of Time | Treatment |
|---|---|---|
| (1) Coexistence | 378 days | Ce 378 |
| 140 days | Ce 140 | |
| (2) Control | 28 days | Ct 28 |
| 168 days | Ct 168 | |
| 378 days | Ct 378 |
| Trat. | N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||||
| Leaves | |||||||||||
| Ce 378 | 20.23 a | 1.84 a | * 9.23 a | 7.94 a | 2.02 a | 1.33 a | 30.72 a | 9.46 a | 96.75 a | 702.86 a | 11.86 a |
| Ce 140 | 18.62 a | 1.66 a | 7.60 b | 7.37 a | 2.26 a | 1.07 a | 31.12 a | 8.88 a | 90.83 a | 843.52 a | 11.19 a |
| Ct 28 | 19.41 a | 1.62 a | 8.37 ab | 6.97 a | 2.05 a | 1.31 a | 26.64 a | 8.82 a | 93.23 a | 576.07 a | 11.48 a |
| Ct 168 | 18.73 a | 1.60 a | 8.17 ab | 7.04 a | 2.09 a | 1.23 a | 27.68 a | 10.12 a | 92.63 a | 696.45 a | 10.90 a |
| Ct 378 | 17.70 a | 1.70 a | 8.13 ab | 7.51 a | 2.26 a | 1.22 a | 26.56 a | 8.61 a | 88.23 a | 703.63 a | 10.71 a |
| Average | 18.94 A | 1.68 B | 8.30 B | 7.37 B C | 2.14 B | 1.23 A | 28.54 B | 9.18 A | 92.33 B | 704.51 B | 11.23 B |
| CV (%) | 4.99 | 5.66 | 7.14 | 5.32 | 5.43 | 8.33 | 7.77 | 6.69 | 3.40 | 13.45 | 4.08 |
| Branches | |||||||||||
| Ce 378 | 4.45 a | 1.07 a | 5.07 a | 10.01 a | 1.09 a | 0.30 a | 5.76 a | 7.38 a | 30.98 a | 383.08 a | 10.16 a |
| Ce 140 | 3.80 a | 0.84 a | 4.37 a | 9.83 a | 1.25 a | 0.20 a | 6.56 a | 7.80 a | 28.19 a | 522.92 a | 10.61 a |
| Ct 28 | 4.87 a | 1.07 a | 5.23 a | 9.13 a | 1.27 a | 0.37 a | 8.08 a | 7.55 a | 30.53 a | 369.80 a | 10.78 a |
| Ct 168 | 3.29 a | 1.23 a | 5.53 a | 8.79 a | 0.66 a | 0.25 a | 5.52 a | 7.67 a | 28.22 a | 354.25 a | 8.83 a |
| Ct 378 | 3.45 a | 0.82 a | 4.93 a | 10.35 a | 1.00 a | 0.21 a | 7.52 a | 6.89 a | 25.11 a | 429.27 a | 9.47 a |
| Average | 3.97 C | 1.01 C | 5.03 C | 9.62 B | 1.05 C | 0.27 C | 6.69 C | 7.46 B | 28.61 B | 411.86 B | 9.97 B |
| CV (%) | 16.90 | 17.25 | 8.54 | 6.69 | 23.46 | 26.40 | 16.50 | 4.74 | 8.18 | 16.54 | 8.17 |
| Stembark | |||||||||||
| Ce 378 | 9.89 a | 3.36 a | 13.30 a | 88.34 a | 13.00 a | 0.92 a | * 53.04 a | 10.80 a | 402.46 a | 2129.06 a | 28.29 a |
| Ce 140 | 9.08 a | 2.76 a | 13.10 a | 78.28 a | 12.87 a | 0.90 a | 37.92 a b | 9.61 a | 125.62 a | 2827.73 a | 25.09 a |
| Ct 28 | 9.40 a | 3.81 a | 13.10 a | 70.74 a | 14.54 a | 0.53 a | 46.56 a b | 9.81 a | 160.72 a | 2181.49 a | 26.66 a |
| Ct 168 | 9.12 a | 3.81 a | 13.40 a | 88.80 a | 14.32 a | 1.01 a | 48.48 a b | 9.24 a | 141.34 a | 2700.74 a | 28.01 a |
| Ct 378 | 8.43 a | 2.71 a | 13.00 a | 71.43 a | 12.09 a | 0.68 a | 34.08 b | 11.61 a | 212.81 a | 2340.72 a | 26.42 a |
| Average | 9.18 B | 3.29 A | 13.18 A | 79.52 A | 13.36 A | 0.81 B | 44.02 A | 10.21 A | 208.59 A | 2435.95 A | 26.89 A |
| CV (%) | 5.78 | 16.39 | 1.25 | 11.03 | 7.76 | 24.40 | 17.73 | 9.50 | 54.29 | 12.84 | 4.82 |
| Stemwood | |||||||||||
| Ce 378 | 1.04 a | 0.41 ab | 1.47 a | 0.98 a | 0.29 ab | 0.04 a | 2.80 a | 2.21 a | 45.97 a | 30.30 a | * 4.04 a |
| Ce 140 | 0.89 a | 0.31 b | 1.14 a | 1.00 a | 0.25 b | 0.02 a | 3.12 a | 1.96 a | 12.37 a | 35.61 a | 2.64 b |
| Ct 28 | 1.01 a | * 0.49 a | 1.61 a | 1.14 a | * 0.34 a | 0.02 a | 3.44 a | 1.89 a | 11.36 a | 32.20 a | * 4.16 a |
| Ct 168 | 0.99 a | 0.37 ab | 1.43 a | 0.92 a | 0.28 ab | 0.05 a | 3.20 a | 1.82 a | 22.31 a | 30.35 a | 3.13 ab |
| Ct 378 | 1.07 a | 0.29 b | 1.37 a | 1.03 a | 0.25 ab | 0.04 a | 3.36 a | 3.02 a | 18.81 a | 38.01 a | 3.30 ab |
| Average | 1.00 D | 0.37 D | 1.40 D | 1.01 C | 0.28 C | 0.03 D | 3.18 C | 2.18 C | 22.16 B | 33.29 C | 3.45 C |
| CV (%) | 6.86 | 21.52 | 12.25 | 8.00 | 13.13 | 39.46 | 7.83 | 22.57 | 63.43 | 10.23 | 18.50 |
| Trat. | N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg of biomass ha−1/kg of nutrient | |||||||||||
| Leaf | |||||||||||
| Ce 378 | 50 | 545 | 109 | 128 | 484 | 759 | 32,751 | 10,3986 | 10,395 | 1388 | 84,986 |
| Ce 140 | 54 | 590 | 131 | 131 | 443 | 939 | 32,141 | 111,712 | 11,062 | 1167 | 90,247 |
| Ct 28 | 52 | 619 | 120 | 143 | 490 | 761 | 37,493 | 113,452 | 10,806 | 1712 | 87,173 |
| Ct 168 | 54 | 627 | 124 | 143 | 481 | 810 | 36,551 | 98,009 | 10,922 | 1463 | 91,822 |
| Ct 378 | 57 | 583 | 123 | 133 | 446 | 833 | 37,574 | 116,861 | 11,489 | 1424 | 94,340 |
| Average | 53 c | 593 b | 121 c | 135 b | 468 c | 815 b | 35,274 c | 108,630 c | 10,937 c | 1417 b | 89,684 b |
| Branches | |||||||||||
| Ce 378 | 231 | 958 | 200 | 99 | 932 | 3450 | 173,804 | 134,766 | 32,228 | 2653 | 100,877 |
| Ce 140 | 252 | 1186 | 228 | 106 | 785 | 4636 | 147,399 | 129,442 | 35,003 | 1890 | 91,727 |
| Ct 28 | 205 | 921 | 191 | 108 | 778 | 2692 | 122,164 | 132,075 | 33,097 | 2665 | 92,961 |
| Ct 168 | 301 | 821 | 180 | 113 | 1500 | 4059 | 176,471 | 128,492 | 35,312 | 2829 | 111,470 |
| Ct 378 | 289 | 1190 | 201 | 92 | 1015 | 4600 | 133,981 | 141,684 | 38,961 | 2313 | 104,387 |
| Average | 250 b | 985 b | 197 bc | 103 bc | 956 b | 3685 b | 147,615 b | 133,333c | 34,753 bc | 2458 b | 100,214 b |
| Stembark | |||||||||||
| Ce 378 | 317 | 890 | 229 | 34 | 211 | 3421 | 68,638 | 251,938 | 8802 | 1405 | 105,863 |
| Ce 140 | 331 | 1097 | 230 | 39 | 234 | 3351 | 78,780 | 322,078 | 24,096 | 1063 | 118,888 |
| Ct 28 | 318 | 797 | 228 | 43 | 206 | 6000 | 64,353 | 310,976 | 18,326 | 1387 | 112,335 |
| Ct 168 | 304 | 677 | 207 | 35 | 181 | 2625 | 59,238 | 371,681 | 24,041 | 993 | 104,478 |
| Ct 378 | 351 | 1098 | 230 | 41 | 245 | 4870 | 87,843 | 245,077 | 15,577 | 1263 | 112,337 |
| Average | 328 b | 931 b | 227 b | 39 c | 220 c | 3973 b | 73,071 c | 288,774 b | 16,461 c | 1211 b | 112,260 b |
| Stemwood | |||||||||||
| Ce 378 | 975 | 2569 | 706 | 1055 | 3604 | 25,571 | 396,310 | 434,115 | 24,774 | 33,558 | 254,502 |
| Ce 140 | 1127 | 3204 | 877 | 993 | 3954 | 43,250 | 322,611 | 523,054 | 80,606 | 27,974 | 378,556 |
| Ct 28 | 986 | 2076 | 623 | 886 | 2939 | 44,929 | 294,476 | 528,571 | 86,591 | 30,976 | 244,082 |
| Ct 168 | 1021 | 2634 | 715 | 1070 | 3555 | 21,421 | 298,825 | 589,855 | 39,855 | 32,481 | 328,358 |
| Ct 378 | 945 | 3488 | 743 | 977 | 3936 | 23,000 | 292,063 | 336,073 | 50,380 | 26,205 | 303,630 |
| Average | 1009 a | 2731 a | 727 a | 994 a | 3572 a | 28,165 a | 313,235 a | 465,574 a | 46,969 a | 29,844 a | 298,476 a |
| Total biomass | |||||||||||
| Ce 378 | 323 | 1575 | 377 | 204 | 1116 | 5259 | 161,333 | 282,895 | 19,771 | 5559 | 177,686 |
| Ce 140 | 399 | 1948 | 459 | 191 | 1020 | 7146 | 154,988 | 349,642 | 44,678 | 4193 | 225,814 |
| Ct 28 | 328 | 1405 | 362 | 206 | 924 | 5846 | 141,373 | 326,829 | 40,657 | 5410 | 172,783 |
| Ct 168 | 420 | 1776 | 441 | 327 | 1545 | 6593 | 177,399 | 368,617 | 32,983 | 7298 | 235,645 |
| Ct 378 | 385 | 2008 | 413 | 208 | 1133 | 6403 | 159,728 | 264,131 | 33,240 | 5038 | 204,395 |
| Average | 370 | 1727 | 409 | 221 | 1121 | 6241 | 158,472 | 314,621 | 32,455 | 5331 | 202,153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momolli, D.R.; Schumacher, M.V.; Ludvichak, A.A.; Caldeira, M.V.W.; Faria, J.C.T.; Pereira, M.G.; Santos, K.F.d.; Souza, H.P.d.; Guimarães, C.d.C.; Delgado, R.C. Weed Coexistence in Eucalyptus Hybrid Stands Decreases Biomass and Nutritional Efficiency Mid-Rotation. Forests 2023, 14, 1816. https://doi.org/10.3390/f14091816
Momolli DR, Schumacher MV, Ludvichak AA, Caldeira MVW, Faria JCT, Pereira MG, Santos KFd, Souza HPd, Guimarães CdC, Delgado RC. Weed Coexistence in Eucalyptus Hybrid Stands Decreases Biomass and Nutritional Efficiency Mid-Rotation. Forests. 2023; 14(9):1816. https://doi.org/10.3390/f14091816
Chicago/Turabian StyleMomolli, Dione Richer, Mauro Valdir Schumacher, Aline Aparecida Ludvichak, Marcos Vinicius Winckler Caldeira, Júlio Cézar Tannure Faria, Marcos Gervasio Pereira, Kristiana Fiorentin dos Santos, Huan Pablo de Souza, Claudiney do Couto Guimarães, and Rafael Coll Delgado. 2023. "Weed Coexistence in Eucalyptus Hybrid Stands Decreases Biomass and Nutritional Efficiency Mid-Rotation" Forests 14, no. 9: 1816. https://doi.org/10.3390/f14091816
APA StyleMomolli, D. R., Schumacher, M. V., Ludvichak, A. A., Caldeira, M. V. W., Faria, J. C. T., Pereira, M. G., Santos, K. F. d., Souza, H. P. d., Guimarães, C. d. C., & Delgado, R. C. (2023). Weed Coexistence in Eucalyptus Hybrid Stands Decreases Biomass and Nutritional Efficiency Mid-Rotation. Forests, 14(9), 1816. https://doi.org/10.3390/f14091816









