Abstract
In this study, the growth and physiological performance of four newly bred black locust (Robinia pseudoacacia L.) clones (‘NK1’, ‘NK2’, ‘PL040’, ‘PL251’) together with one registered in Hungary (‘Üllői’) were monitored and compared in a field experiment located in the dry temperate climatic zone of Eastern Central Europe. Tree height and diameter at breast height were measured monthly during May–August 2022, an extremely dry period. Ecophysiological parameters such as leaf temperature, vapor pressure deficit, intercellular carbon dioxide level, transpiration and assimilation rates, and stomatal conductance to water and CO2 were measured in situ. There was a high clonal effect on survival rate and growth of the trees and on the physiological parameters. ‘NK1’ performed best regarding height (1.88 m), while ‘PL040’ (23.76 mm) had the highest diameter increment (n = 16–26). The highest carboxylation efficiency was found in ‘NK2’ (0.077 µmol m−2 s−1), while the lowest was in ‘NK1’ (0.035 µmol m−2 s−1), not showing a significant difference from the ‘Üllői’. Water-use efficiency values were found to be the highest in ‘NK2’ and ‘Üllői’ (4.92 and 4.78 kg m−3, respectively). Ci was found to be maximum in ‘NK1’ and ‘PL040’ (286.15 and 287.37 µmol mol−1, respectively), while it was minimum in ‘Üllői’ (248.30 µmol mol−1). Physiological parameters were found to be significantly different in the clones due to their genetic differences. A strong positive correlation was found between the transpiration and the assimilation rates (r = 0.843–0.994). Within the growing period, the loss of leaves due to abiotic stress was 0 for ‘NK1’ and negligible for the others. ‘NK2’ stood out among the other clones in most of the parameters tested (height, thickness, assimilation, WUE). In addition to its high photosynthetic intensity, its water-use efficiency was also high.
1. Introduction
Among the short rotation trees, black locust, poplar, and willow are the most important in the temperate climate zone. Under unstressful environmental conditions, poplar and willow perform better regarding their survivability and yield [1]. However, black locust is one of the drought-resistant species, with relatively low sensitivity to the high variation in weather conditions, successfully grown in the regions classified as warm temperate dry (WTED) climate zones by the Intergovernmental Panel on Climate Change, covering approximately one-third of the area of Hungary [2].
Widening the species palette in forestation, black locust (Robinia pseudoacacia L.) was introduced in the 18–19th centuries in Central Europe. For example, in Hungary, it was found efficient to control blown sand that is typical in one-fifth of the country’s area. Economic reasons had priority, leading to its widespread introduction. Furthermore, it is used for many purposes, such as timber production, honey production, and land reclamation. Its timber has extraordinary quality with multipurpose utilization [3,4], Hungarian black locust honey is on the National Treasures list, and black locust plays a considerable role in agroforestry and in the afforestation of eroded and marginal lands [3,5,6,7,8]. Additionally, as a carbon pool, the species contributes to the fight against global warming. Black locust is characterized by a high potential for acclimatization to Eastern European climatic conditions, suggesting high invasive potential [9]. However, proper choice of the planting sites and cultivation technologies ensure keeping its invasive potential under control [10,11].
Predicted climate change is expected to lower the profitability of beech, hornbeam, sessile oak, and Turkey oak forests, e.g., in Hungary, resulting from the drift in forest climate classes toward drier and warmer categories, and an increase in summer mean temperature, in particular [12]. As with all plants, black locust is sensitive to drought. For example, the lack of rain in areas with high atmospheric evaporative demand results in decreased sap flow density, stand transpiration, leaf area index, and stem diameter [13]. In the Chinese Loess Plateau, the annual percentage loss of whole-plant hydraulic conductance shows strong variation due to climatic variability, which was found positively correlating with the annual potential evapotranspiration and the aridity index [14]. Despite comparable bioclimatic conditions, the climate sensitivity of black locust stands was found to be highly variable, both temporally and spatially in Eastern Europe, suggesting a site-specific microclimate with winter and spring temperatures as key climatic drivers in the growth pattern. The growth response to the previous winter and current summer precipitation was different in the stands [9]. Differences in air temperature and precipitation, even within the same region, such as near or within a city acting as an urban heat island, influence significantly the annual ring widths [15]. The ecophysiological and morphological adaptation of black locust to prolonged drought—by reducing water loss through both reduced transpiration and leaf size—was demonstrated in the drier regions of Europe [16]. The trees have different transpiration levels by age; tree and stand respiration together with stomatal and hydraulic conductance were found to be higher in the older trees [17]. Drought memory affects plant growth performance; trees resprouting under drought conditions were found to be more drought tolerant than the well-watered ones. Black locust tolerates high variation in soil water availability without altering its water-use efficiency [18]. In one study, planting density (i.e., short rotation forestry (1000–2000 trees ha−1) vs. short rotation coppice (6000–15,000 trees ha−1)) was not found to significantly affect growth dynamics and water-use efficiency [19].
In most studies, the performance of a given clone has been tested under different environmental conditions to characterize its response to stressors such as drought, heat stress, and plantation density, as detailed above. Tree species used for wood biomass production have also been compared under the same environmental conditions to demonstrate one as an alternative to another, e.g., black locust vs. poplars (Populus spp.) [19] and/or willows (Salix spp.) [20,21] or European linden (Tilia × europaea) [22]. Bhusal et al. [23] studied and compared the growth responses of six gymnosperm species under long-term excessive irrigation. They also investigated the morphological, physiological, and biochemical traits of eleven tree species to assess their drought resistance. Highly resistant species have a large leaf mass per area and a high photosynthetic rate, according to this study [24]. Few studies focused on the differences in the performance of black locust clones, especially in dry regions, with the aim of clonal selection [25] or comparative analysis of selected clones [26,27,28,29]. In Hungary, forestry research to increase the yield of black locust, improve its stem quality, and enhance its drought tolerance has been ongoing for decades. This research effort resulted in several state-approved cultivars [5,28,30,31]. Suitability for the establishment of black locust plantations in marginal sites with extreme weather conditions, especially drought, can be expected to differ by clones, as proved by our preliminary investigation [32]. It must be noted that utilization determines which parameter has priority.
The objective of this report is to describe the in-depth results of a juvenile growth and plant physiology study characterizing newly bred, vegetatively propagated, 3-year-old black locust clones. Hypothesis 1 assumes that there are significant differences between the growth (height, diameter at the breast, and increments of these) and the plant physiology traits (rate of assimilation and transpiration, stomatal conductance, intercellular CO2 concentration, leaf temperature, carboxylation, and water-use efficiency) of the investigated clones in an extreme dry year. Hypothesis 2 assumes that there are correlations among the physiological parameters. Lastly, hypothesis 3 states that the clones can be distinguished by their growth and physiological parameters.
2. Materials and Methods
2.1. Study Site and Clone Trial
The experimental tree plantation was established near the village of Napkor (UTM 563,210 E; 5,307,770 N), in the region of Nyírség [32], the most considerable area of black locust forests in Hungary. The soil type is slightly acidic sandy soil with low humus content, classified as arenosol according to the USDA soil taxonomy.
Four black locust clones—’Laposi’ (breeding sign: NK1), ’Napkori’ (breeding sign: NK2), ’Farkasszigeti’ (breeding sign: PL040),’Püspökladányi’ (breeding sign: PL251)—along with a state-approved cultivar, ’Üllői’, were compared, focusing on their growth dynamics and selected physiological parameters. Vegetatively propagated 1-year-old seedlings were planted in spring 2020 with planting spacings of 2.5 × 2.5 m, 3.0 × 3.0 m, and 4.0 × 4.0 m (Figure 1). The original number of trees planted at setup in 2020 was 728 for PL251 and NK1, 598 for PL040, and 478 for Üllői. In 2021, according to the new regulation, 3.0 × 3.0 m, and 4.0 × 4.0 were modified to 3.0 × 1.0 m and 4.0 × 1.0 m, respectively. Preparation, planting, and maintenance were carried out in accordance with the national forest management practice.
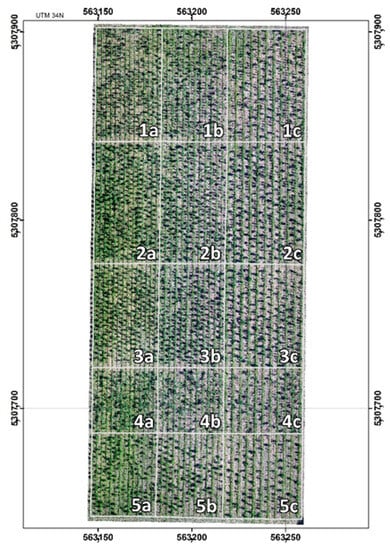
Figure 1.
Orthophoto of the experimental plantation. Numbers indicate the clones (1: PL251; 2: NK1; 3: PL040; 4: NK2; 5: Üllői), and the letters specify the planting spacings (a: 2.5 × 2.5 m; b: 3.0 × 3.0 m; c: 4.0 × 4.0 m) (taken by Szabó, G., Napkor, 16 June 2022).
2.2. Meteorological Data and Indicators
Based on the meteorological data provided by the National Weather Station located in Nyíregyháza–Napkor, the annual mean temperature was 10.6 °C, and the annual mean precipitation was 537 mm within the period 1991–2020. The year 2022 was the driest and hottest in the last century in Hungary, with 120 mm less precipitation and a 1.1 °C higher mean temperature in Napkor (Supplemental Figure S1).
To describe the weather extremes during 2022 compared to the years between 2002 and 2022, the simplified forestry aridity index (FAI) (Equation (1)) according to Führer et al. [33] and the temperature–precipitation factor (TPF) (Equation (2)) according to Koltay [34] were calculated.
The number of hot days (NHD) was defined as days when the daily maximum temperature Tmax ≥ 29.5 °C, which is 90% of daily Tmax over the 20-year reference period 2002–2021. A hot year was categorized as the year that had 30 or more hot days [35].
In the case of the FAI, temperature (T) and precipitation (P) data for May–August and July–August, the most intensive growing periods and the highest sensitivity with respect to vitality, are considered. Regarding the TPF, data for the period March–August were considered.
Based on the FAI, years were classified by four climate categories, as suggested by Járó [36], namely the beech forest climate with the lowest mean temperature and highest precipitation, the hornbeam–oak forest climate, the turkey oak–sessile oak forest climate, and the forest–steppe with the hottest and driest climate.
2.3. Measurement Methods and Calculations of Derived Data
In a preliminary study, the photosynthetic parameters, such as net assimilation rate (A), transpiration (Tr), water-use efficiency (WUE), normalized difference vegetation index (NDVI), and growth parameters (e.g., height and diameter at the base), were recorded in the same experiment in 2021 [32]. In this study, sampling strategy and parametrization were optimized, and the effect of the extreme weather conditions, drought in particular, on the growth and ecophysiological variables was evaluated the considering the data gathered in 2022.
In April, the ratio of trees that survived in the plantation was recorded, and the height (H) of each was measured with a straight edge. Thirty individuals were selected by clones, and the tree diameter (DBH) at breast height (1.3 m) was measured with a vernier in May and August, the most intensive growing period. Growth parameters were measured only in the parcels with the 2.5 × 2.5 m planting spacing. Growth in height and diameter within the three months was calculated.
In June, the assimilation parameters together with net assimilation rate, transpiration, stomatal conductance, intercellular CO2 concentration, and other physiological parameters were measured in situ using an LI-6800 (LI-COR, Lincoln, NE, USA) portable photosynthesis system [37]. The light was controlled in the sample chamber; we used 1500 µmol photons m−2 s−1 PAR, with 90% red (625 nm) and 10% blue (475 nm) light. A multiphase flash fluorometer head (Li-6800-01A) was used as a light source, the aperture was 2 cm2. The CO2 concentration was controlled in the chamber at 400 µmol mol−1 using an injector and carbon dioxide patrons. We selected three trees per plot and placed a newly fully developed, light-adapted leaf from each tree in the measuring chamber. Six measurements were recorded on each leaf at 6 s intervals, resulting in 18 measurements per plot. The values were recorded when the measured parameters had stabilized (CV < 1%), but after at least 120 s. Photosynthesis measurements were reported in a previous article [35]. In this paper, some derived parameters are presented, such as the difference between leaf and air temperature, vapor pressure deficit (VPD), carboxylation efficiency (CE), and water-use efficiency (WUE). WUE was calculated according to Tanner and Sinclair [38] (Equation (3)). Carboxylation efficiency (CE) was calculated according to Equation (4).
where WUE is the water-use efficiency (kg m−3), Tr is the transpirated H2O (mmol m−2 s−1), and A is the assimilated CO2 (μmol m−2 s−1). We used molar mass for CO2 44.0 g mol−1 and for H2O 18.0 g mol−1.
where CE is the carboxylation efficiency (μmol m2 s−1), A is the net assimilation rate (μmol m−2 s−1), and Ci is the intercellular CO2 concentration (μmol mol−1).
In September, a tree health condition survey was carried out with the consideration of 30 individuals by clones, where their leaf loss and damages in the trunk and the boughs were estimated. Clone NK2, where many individuals suffered from bark disease or had dieback on twigs, was not included.
2.4. Statistical Analysis
The distribution of the data was tested by means of the Shapiro–Wilk test, which revealed a normal distribution of the growth variables; the dataset was found to be parametric (p > 0.05). An analysis of variance (ANOVA) (Tukey’s HSD, p < 0.05) was used to test for differences among the clones. Where height, diameter, and physiological variables were nonparametric, differences were analyzed using the Kruskal–Wallis and the Games–Howell tests. The strength and direction of association between two ranked variables were measured by Spearman’s correlation coefficient.
3. Results
3.1. FAI, TPF, and NHD in the Period 2002–2022
In Hungary, change in the climate categories is predicted, with a decrease in the area of the beech forest climate and an increase in the area of the forest–steppe, in particular. Utilization of the latter, including forestry, has considerable limitations. FAI showed relatively low variation between 2002 and 2022 (8.37 ± 5.41). The ratio of the years above the FAI value of 7.25 representing the class of forest–steppe climate was 52%, and occurred in 2002, 2003, 2007, 2009, 2011, 2012, 2013, 2015, 2018, 2021, and 2022, with an average difference of 4.07 from the mean. There was no trend within the last 21 years. In 2022, the difference of FAI from the mean of the 2002–2022 period was 19.95, higher at 338% (Supplemental Figure S2).
TPF showed a comparable pattern in variation between 2002 and 2022 with 6.15 ± 2.73, similarly not showing any trend. Years above the mean occurred in 2002, 2003, 2007, 2009, 2011, 2012, 2015, 2018, and 2022 with the average difference of 2.26 from the mean. In 2022, the difference of TPF from the mean of the 21 (2002–2022) years was 8.53, higher at 239% (Supplemental Figure S3).
Despite the extremes in FAI and TPF, NHD in 2022 was not an outlier compared to the data between 2002 and 2021 (Supplemental Figure S4). NHD above 50 was measured in 2003, 2012, 2015, and 2018.
In general, plants exhibit different morphological, physiological, biochemical, and molecular alterations, resulting in stress tolerance under drought or heat stress, or both. In 2022, plantations were exposed to serious drought, primarily. In the study area, the extraordinary stress tolerance of black locust had considerable role in survival and relatively high performance in biomass production. Considering the A1B emission scenario published by the Intergovernmental Panel on Climate Change in 2020, approximately 10% of the forest area in Hungary is predicted to belong to the forest–steppe class in 2050, and 60% in 2070 [39], which makes keeping forests sustainable even more challenging. Such trends in climatic conditions motivate the use of alternative domesticated species, varieties, and clones. Technologies, e.g., black locust cultivation, are currently under development [32].
3.2. Survival and Height of 3-Year-Old Trees
The numbers of samplings in 2020 were 728 in the cases of PL251 and NK1 and 598 in the case of PL040. In 2022, the survival rates of the clones were 64%, 70%, and 72% for PL251, NK1, and PL040, respectively. In the case of NK2, 84% of the 356 saplings survived until April; however, it declined to 67% until September as a result of bark disease (Phomopsis oncostoma Thüm.). As a reference, ‘Üllői’ performed the worst; 248 trees from the 478 saplings survived (Figure 2).
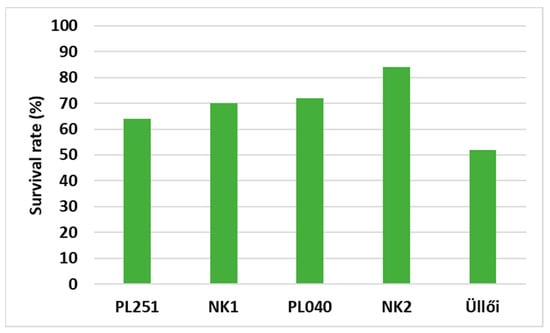
Figure 2.
Survival rate (%) of the clones (2022 April, Napkor, Hungary).
Tree height is a fundamental measure in forestry. It is used to assess the productivity of a site. Furthermore, it is related to aboveground biomass and canopy vertical structure. It can be a good proxy for forest status and useful in forecasting stand development and succession. The height of the clones in the case of different planting spacings is shown in Figure 3. NK2 reached the highest mean height (4.56 m) when planted in a 2.5 × 2.5 m space, with a significant difference from the others. PL251 performed second best (4.24 m), while NK1 and PL040 were not significantly different in their height (3.39 and 3.41 m, respectively). Tendency in a 3 × 3 m space was similar; NK2 and PL251 were the highest but with no significant difference (4.63 and 4.66 m), while NK1 was the shortest among all (3.52 m). There was no considerable difference in the clones’ order in the case of 4 × 4 m spacing. ’Üllői’, the only commercially available clone, showed the worst performance in all spacing plantations (2.8, 3.09, and 3.01 m in the case of 2.5 × 2.5 m, 3 × 3 m, and 4 × 4 m spacing, respectively).
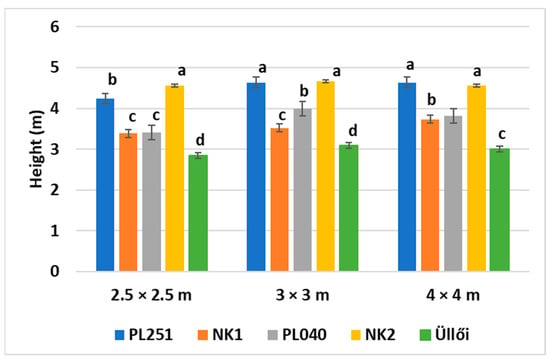
Figure 3.
Height of the clones in different planting spacings (2022 April, Napkor), ±standard error; small letters indicate the significant differences among the clones at p = 5% level.
3.3. Height and DBH in June
In June, in the case of 2.5 × 2.5 m spacing, NK2 and PL251 were the highest (5.17 and 4.99 m, respectively), while ’Üllői’ was the lowest (3.58 m), similar to the results found in April (Figure 4a). Despite its medium height, NK1 had a DBH comparable to that of NK2 and PL251 (55.23, 55.19, and 56.30 mm, respectively) (Figure 4b).
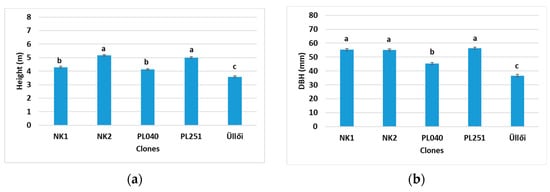
Figure 4.
Comparison of the clones in 2.5 × 2.5 m planting spacing by height (m) (a) and diameter at breast height (DBH) (mm) (b), based on measurement of 30 sample trees (15 June 2022), ±standard error; small letters indicate the significant differences among the clones at p = 5% level.
3.4. Growth in Height and DBH within the Period May–August
Comparing the growth in both height and DBH (Table 1), PL251 did not show a significant difference from ‘Üllői’. NK1 had the highest rate of height increase (1.81 m) but was second in diameter change (21.35 mm), after PL040 (23.76 mm).

Table 1.
Height and diameter increments in the main growing period (from 2022 May to 2022 August) in 2.5 × 2.5 m planting spacing.
In the main growing period of May–August, trends in height and DBH increments by clones are shown in Figure 5a,b. A linear trend in height growth rate was found in the cases of NK1 and PL040, while growth rate decreased after July in PL251 and ‘Üllői’. The DBH increase rate was lower after July in the case of all four clones. Close to one-third of the change in height and more than half of the DBH increase occurred between May and June. Regarding DBH, PL251 showed extraordinary performance, with a 66% increase in this period.
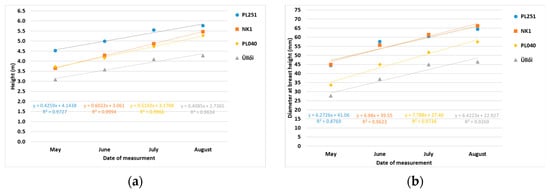
Figure 5.
Monthly height (a) and diameter at breast height (DBH) (b) increases in the black locust clones in the period May–August 2022.
3.5. Physiological Parameters
Vapor pressure deficit (VPD) has a major impact on plant assimilation and transpiration processes, such as stomatal opening and CO2 uptake. High VPD means dry air for the plant, and it can easily become too dry and stressed. The VPD values were relatively high at the time of measurement (3.0–3.8 kPa), suggesting that the very dry air around the plants induced high water stress. The VPD values of clones NK2 and PL040 were significantly lower than those of the other clones, indicating better stress tolerance (Figure 6).
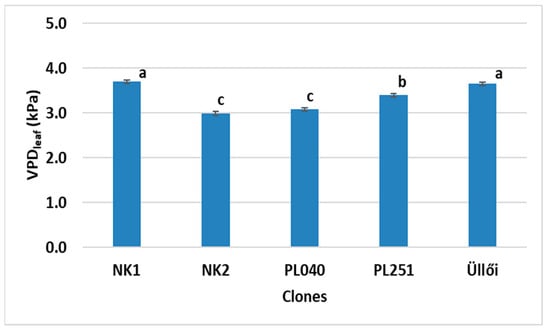
Figure 6.
VPDleaf values of the clones, ±standard error; small letters indicate significant differences at p = 5% level.
The total stomatal conductance (gtc) measurement showed significant differences between the black locust clones. The highest values were in NK2 (0.204 mol m−2 s−1) and PL040 (0.206 mol m−2 s−1), while the lowest were in NK1 and ‘Üllői’ clones with values of 0.116 and 0.096 mol m−2 s−1, respectively. There was no significant difference between the former two and the latter two (Figure 7). The total conductance values suggest that the stomata of clones NK2 and PL040 were more open, which may be related to the lower VPD values.
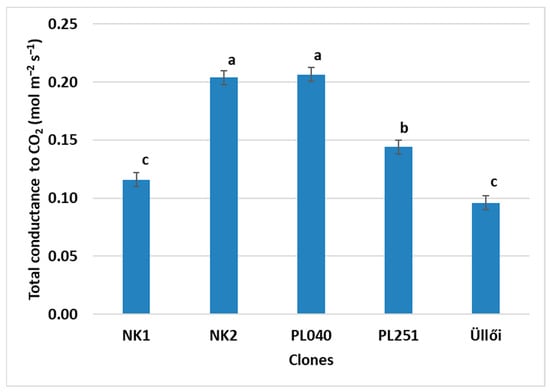
Figure 7.
Comparison of the clones by total conductance to CO2, ±standard error; small letters indicate the significant differences at p = 5% level.
Significant differences in the intercellular CO2 (Ci) concentration of the clones’ leaves were found among the clones at a p value of 5%. The highest Ci values were observed in clones NK1 (286.15 µmol mol−1), PL040 (287.37 µmol mol−1), and PL251 (277.20 µmol mol−1). ‘Üllői’ had the lowest Ci value with 248.30 µmol mol−1 (Figure 8). Many factors have effects on the Ci. It cannot be taken as a clear indicator for assessing the stress status of the plant or the functioning of the photosynthetic system.
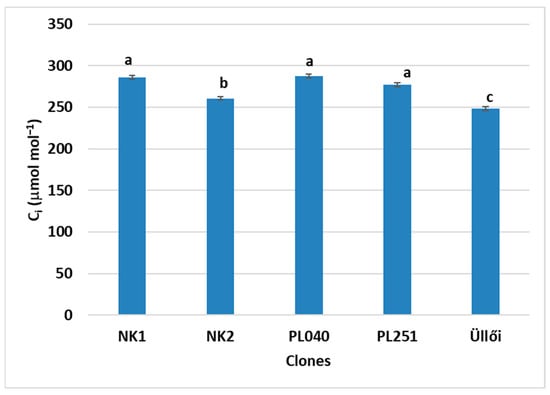
Figure 8.
Comparison of the clones by intercellular CO2 concentration (Ci), ±standard error; small letters indicate the significant differences at p = 5% level.
We calculated the CE from the A and Ci values. NK2 was significantly the best in this parameter with a value of 0.078 µmol m−2 s−1. Clone NK1 and ‘Üllői’ have the lowest CE values, 0.036 and 0.045 µmol m−2 s−1, respectively. We observed no significant difference at p = 5% level between these two clones. We did not find significant differences, neither between clone PL251 (0.048 µmol m−2 s−1) and ‘Üllői’, nor between clones PL251 and PL040 (0.052 µmol m−2 s−1) (Table 2).

Table 2.
Comparison of the clones’ statistics for carboxylation efficiency (CE) (µmol m−2 s−1); small letters indicate the significant differences at p = 5% level, n = 18.
As Figure 9 shows, there were differences in air and leaf temperatures among black locust clones as a function of transpiration. Kutasy et al. [40] also demonstrated that air and leaf temperature differences provide information on the water status of plants, and a higher difference means a better cooling effect from transpiration. Because the leaf’s stomata are closing under water stress, the cooling effect is reduced. On the date of measurement (29 June 2022), black locust clones had shown various water statuses: clones PL040 and NK2 were relatively good, they were able to cool their leaves, but the others (NK1, PL251, ‘Üllői’) were in water stress. In the case of transpiration, we found the same results. NK2 produced the highest transpiration (10.07 mmol m−2 s−1) and PL040 had the largest air–leaf temperature difference (1.03 °C). However, there was no significant difference between these two clones in these two parameters. The leaf cooling effect was very low in clones PL251, NK1, and ‘Üllői’, at −0.44, −0.29, and −0.06 °C, respectively. These three were not significantly different from each other.
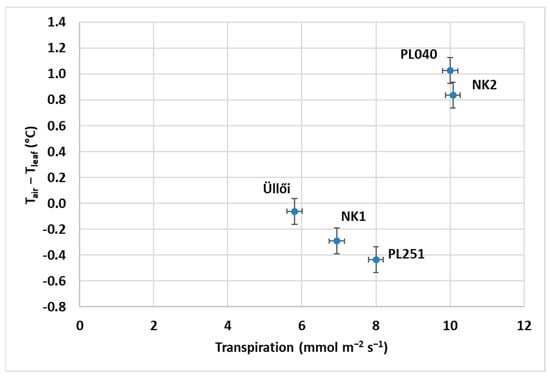
Figure 9.
The difference in air and leaf temperature of black locust clones as a function of transpiration (29 June 2022); means of 18 measurements per clone, ±standard error.
The clones underwent correlation analysis for the phytophysiological parameters. The results are summarized in Table 3, Table 4, Table 5, Table 6 and Table 7. Spearman correlation was found to be strong (p = 0.01) between the air minus the leaf temperature values and transpiration data in PL040 and NK2 (r = 0.716 and r = −0.758, respectively). In other clones, we did not find a correlation. Positive correlation was found between gsw and Tr for clones PL251 (r = 0.622; p = 0.01), NK1 (r = 0.476; p = 0.05), PL040 (r = 0.876; p = 0.01), NK2 (r = 0.695; p = 0.01), and ‘Üllői’ (r = 0.703; p = 0.01). A strong correlation (p = 0.01) was found between gsw and A in PL251 (r = 0.710), PL040 (r = 0.843), NK2 (r = 0.859), and ‘Üllői’ (r = 0.731). These parameters were found not to correlate for the NK1 clone. Regarding A and Tr, a strong correlation p = 0.01 was found, as follows: PL251 (r = 0.843), NK1 (r = 0.992), PL040 (r = 0.994), NK2 (r = 0.899), and ‘Üllői’ (r = 0.890). Negative correlation (p = 0.01) was found between gsw and VPDleaf for PL251 (r = −0.816), NK1 (r = −0.924), PL040 (r = −0.918), and ‘Üllői’ (r = −0.647). In this case, NK2 did not show a correlation. Strong positive correlation (p = 0.01) was found between gsw and Tair-Tleaf in the cases of PL251 (r = 0.864), NK1 (r = 0.758), and PL040 (r = 0.890) clones, while weak correlation (p = 0.05) was shown by ‘Üllői’ (r = 0.474). NK2 did not show a correlation. Regarding the relationship between gsw and WUE, a negative correlation (p = 0.05) was found for PL040 (r = −0.498) and ‘Üllői’ (r = −0.560), while a strong positive correlation (p = 0.01) was shown by PL251 (r = 0.709). Tair-Tleaf and WUE were positively correlated (p = 0.01) in the case of two clones, PL251 with the highest height and NK2 with the highest DBH (r = 0.777 and 0.796, respectively). These clones showed a negative correlation (p = 0.01) between VPDleaf and WUE (r = −0.593 and −0.661, respectively). Between Ci and WUE, a negative correlation was found for PL251 (r = −0.554 at p = 0.05) and NK1 (r = −0.950 at p = 0.01). Considering the relationship between A and Ci, PL251 (r = −0.690) and NK1 (r = −0.975) showed strong negative correlations, while PL040 (r = 0.769) showed a positive correlation, significant at p = 0.01. The Tr Ci correlation was found to be strongly significant (p = 0.01) for the same three clones: PL251 (r = −0.818), NK1 (r = −0.962), and PL040 (r = 0.810). The A and VPDleaf showed a negative correlation at the significance level of p = 0.01 only in the case of PL040 (r = −0.747). In the case of this clone, VPDleaf showed a strong negative correlation with most of the studied ecophysiological parameters, with the exception of Tair − Tleaf, where a strong negative correlation was found (r = −0.981), and WUE, with no correlation.

Table 3.
Correlations among the ecophysiological parameters of the black locust clone PL251.

Table 4.
Correlations among the ecophysiological parameters of the black locust clone NK1.

Table 5.
Correlations among the ecophysiological parameters of the black locust clone PL040.

Table 6.
Correlations among the ecophysiological parameters of the black locust clone NK2.

Table 7.
Correlations among the ecophysiological parameters of the black locust variety ‘Üllői’.
By analyzing the water-use efficiency (WUE) of the black locust clones, it can be concluded that NK2 stood out from the others. With a high CE, its WUE was also excellent (Figure 10). The second highest WUE value was measured in clone ‘Üllői’. There were no significant differences between these two. The other clones had lower WUE values. No significant differences were found between them.
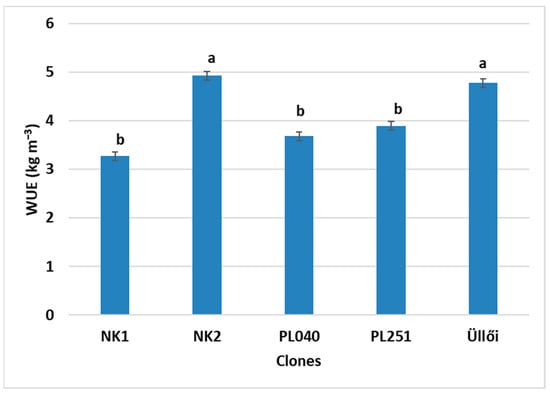
Figure 10.
Comparison of the clones by water-use efficiency (WUE), ±standard error; small letters indicates the significant differences at the p = 5% level.
3.6. Effects of Abiotic Stresses
Drought and wind were found to be the main environmental stressors leading to defoliation until September 2022. However, all the investigated clones were found to show low vulnerability despite the extreme drought in the study area during the investigation period. Among all, NK1 showed the highest resistance regarding defoliation, 0% in the case of row distances of 2.5 × 2.5 m and 3 × 3 m, and 10% in the case of 4 × 4 m. Nevertheless, the contribution of other factors at the production site cannot be ignored. The drought resistance of the different clones can be expected to be different under similar weather conditions but with different planting spacings, for example (Figure 11).
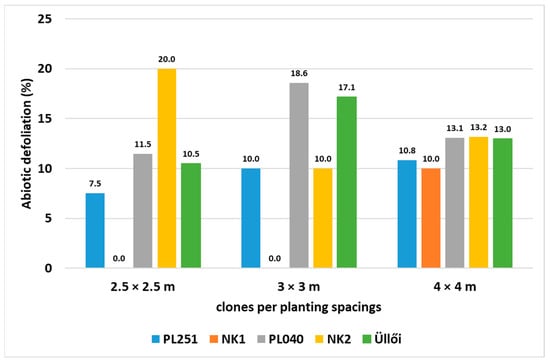
Figure 11.
Abiotic defoliation of the black locust clones in different planting spacings, measured in April 2022.
4. Discussion
In 2022, we published the partial results from our experiment. In 2021, we studied the height, the diameter at the base, NDVI, assimilation, transpiration, and water-use efficiency of these clones only in the 3 × 3 m planting spacing. We found clone NK2 to be the best in almost all of the studied parameters [35].
In this more recent article, we studied the survival rate and the height of the clones in all planting spacings, but we focused on the planting spacing of 2.5 × 2.5 m for the other parameters. We measured and evaluated the height and diameter increments of the clones in the main growing period (from May to August) of the sample trees and the physiological parameters. Here, we include not only the parameters we studied in 2021, but also add in the stomatal conductance, intercellular CO2 concentration, carboxylation efficiency, and the temperatures of the leaves. The correlation between the measured parameters was also analyzed according to clones.
Worldwide, tree plantations cover about 280 million hectares. Their importance is unquestionable, as approximately 80% of all industrial wood needs are met by such intensively cultivated plantations. Black locust is a fast-growing tree species with its most intensive growth in height and thickness in the first 10–15 years, depending on the site. It can grow up to 10 m in height by the age of 5, slowing after the age of 15. The diameter at breast height at 10-years-old can reach 10.8 cm [3]. The better the site potential is, the earlier the peak of height growth. This slows after 15 years [41], although growth remains relatively rapid in older trees; black locust stands in Europe can reach mean or dominant heights of over 20 m at 20 years and up to 25–30 m at 30 years.
The aim of our research work is to develop selected black locust varieties to improve yield and stem quality, and to achieve a uniform stand and better volume compared to common black locust at marginal sites.
The study of tree plantations is of great practical importance. In the future, a massive shortage of timber is forecast for the Central European region, especially in Hungary. Since black locust can tolerate even less favorable site conditions, the lack of timber can be reduced in the future by establishing black locust tree plantations [32]. The economic impact of this tree species is unquestionable, so it plays a key role in forest management. However, we should mention again that the technology for growing black locust should strive to achieve a sustainable coexistence of this tree species with people and nature. It means the strict eradication of black locust at valuable sites and tolerance in some areas [11]. Nevertheless, black locust has some positive effects on biodiversity. This finding is supported by the following facts: The black locust provides habitat for some rare and endangered plant species. Furthermore, in species-poor agricultural landscapes, black locust stands can increase diversity and play a significant role as migration corridors for forest animals [42]. Thus, it can be concluded that black locust can also play an important role in a given habitat from a nature conservation point of view.
To be able to produce good quality industrial wood on the plantations, it is essential to study the ecophysiological properties of the trees (biomass production, photosynthetic activity, transpiration, carbon sequestration, and others) in addition to the traditional stand full inventories [23,24,43].
It is well known that plants produce organic matter by using light energy. Photosynthesis is the basis for plant life. Its intensity is affected by numerous environmental factors such as CO2 concentration, temperature, illumination, water, and nutrient availability. During assimilation, CO2 is reduced, carbohydrates are incorporated, and oxygen is released by splitting water using the energy of incoming photons. Under stress, whether abiotic, biotic, or anthropogenic, the rate of photosynthesis decreases, with a concomitant decrease in the amount of assimilates produced and metabolic disturbances, so-called metabolic inhibition [44,45,46,47]. Nowadays, the most common stress factors are high temperatures and drought. Under water stress, stomata close and stomatal conductance becomes low, resulting in a decrease in intercellular CO2 concentration depending on the intensity of photosynthesis [46,48,49,50]. And thus, these parameters affect tree growth [51]. In the present study, we confirmed these effects and also found that different black locust clones respond differently to environmental stresses.
The extremely arid 2022 growing season allowed us to study black locust clones in a special situation. During the growth study, the height and diameter at breast height of sample trees or clones (plots) were measured monthly from May to August 2022. According to the measurement results, clone NK1 showed the best growth in height (1.88 m) and PL040 in diameter (23.76 mm) during the studied period. The NK2 clone was not measured because of its bark disease.
Transpiration is an important part of the hydrological cycle, where water in the soil is transferred through the plant to the air. As with photosynthesis (assimilation), transpiration in plants can be affected by several factors (environmental and anthropogenic). Jiao et al. [17,52] studied transpiration in black locust in a marginal (semiarid) site, the Loess Plateau, in northern China, southeast of the Gobi Desert. They found that as soil moisture content increases, transpiration increases, and with it, stomatal conductance. Their results also showed that older black locust trees are physiologically more active than younger ones. Our results show that the difference in transpiration between the clones is significant, and the difference in transpiration cooling effect on leaves can be clearly identified between the clones.
Significant differences were found between the clones for all the parameters tested. The Tleaf results were subtracted from the measured air temperature (Tair) values. This allowed us to compare the clones on the basis of their water stress resistance. In this respect, PL040, with the highest diameter growth, proved to be the best. For CE, NK2 performed the best (0.077 µmol m−2 s−1) and NK1 the weakest (0.035 µmol m−2 s−1). However, the latter did not differ significantly from the ‘Üllői’ control. In the case of WUE, NK2 and ‘Üllői’ produced the best results with values of 4.92 and 4.78 kg m−3, respectively, with no significant difference between them. For gtc, the clone PL040 (0.2065 mol m−2 s−1) was the best and ‘Üllői’ was the weakest (0.0961 mol m−2 s−1). For Ci, the highest values were measured for clones NK1 (286.15 µmol mol−1) and PL040 (287.37 µmol mol−1), and the lowest for ‘Üllői’ (248.30 µmol mol−1). Meng et al. [47] measured the Ci of 2-year-old diploid and tetraploid black locust, among others, which was 420 µmol mol−1 for the diploid and 500 µmol mol−1 for the tetraploid.
Zheng et al. [53] measured in situ the photosynthetic gas exchange (A, Tr, gtc and gsw, Ci) in an 18-year-old black locust stand growing on the Loess Plateau, comparing the environmental factors that affect it. Their results show that photosynthetically active radiation, air temperature, and humidity are the most important factors affecting photosynthesis in the black locust. Later, the effects of thinning on net assimilation and carbon sequestration capacity were also studied, and it was found that an increase in these two parameters was observed with decreasing stand density [54]. In black locust stands, similar studies using in situ phytophysiological measurements, have been carried out by several researchers on several occasions [47,55,56,57], but no such measurements have been made in a clone trial.
A correlation analysis was performed per clone to study the relationship between the ecophysiological traits. It was found that the correlation between physiological parameters varied between clones, which could be attributed to genetic differences between them. However, it should be emphasized that there was a strong positive correlation between A and Tr values for all clones (r = 0.843–0.994). For the relationship between A and gsw, we also found a correlation (r = 0.710–0.859) for all clones, but it was very weak for NK1 (r = 0.449). According to Wang et al. [51], A and gsw are linearly correlated with either growth or WUE, so these parameters are useful predictors of tree growth.
Abiotic defoliation of 30 randomly selected sample trees/clones was also investigated at the end of the main growing period (May–August) in the month of September. The test results showed low percentages for all clones, with clone NK1 highlighted at 0%.
We can improve the potential and resistance of this tree species to cope with extreme climates in a generative way, such as through human-assisted seedlot transfer from preadapted stands already in drier and warmer locations (e.g., southeastern Europe, e.g., Bulgaria and Turkey) or to establish a seed orchard from genotypes from these climatic conditions by grafting or using rooted cuttings and to exploit this seed stock; or by vegetative methods, for example, mass propagation of more drought-tolerant genotypes by vegetative means (tissue culture or micropropagation, establishment of a stool bed, production of rooting cuttings) based on ecophysiological studies.
5. Conclusions
The growth and physiological performance of different black locust (Robinia pseudoacacia L.) clones showed notable differences in response to the 2022 extremely dry season.
Of the clones previously considered the most promising, NK2 proved to be the best in terms of physiological parameters (CE, WUE, gtc, leaf temperature).
PL251, considered the second-best, performed poorly in physiological and growth parameters.
The clone PL040 seems promising under water stress conditions, as it showed the best thickness growth from May to August and performed well in drought tolerance parameters. Clone NK1 also showed good performance in height increment and abiotic stress tolerance.
The correlation between physiological parameters varied between clones, which could be attributed to genetic differences between clones. However, it should be pointed out that there was a strong positive correlation between A and Tr values for all clones.
We can conclude that it is possible to produce added value on marginal sites with selected black locust cultivars. Technology in growing and improving black locust is of increasing international importance, particularly in the face of the negative effects of global and local climate change. The additional cost of selective planting material can be compensated by the value-added tree plantations at a given age, or planting seed orchards of black locust clones could be a solution to reduce these costs.
Our findings can be adapted to any region potentially exposed to extreme climatic conditions, and clones of different species are expected to respond differently to abiotic stress, especially drought.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14091802/s1, Figure S1: Monthly sums of precipitation and averages of temperature (Napkor, 2022). Numbers in the bars: sum precipitation of the month. Figure S2: Representation of the extremity of FAI in the year 2022. Red line shows the mean FAI for the period 2002–2022, yellow line shows the value between the turkey oak-sessile oak forest and the forest-steppe climate classes. Figure S3: TPF value between 2002 and 2022. Red line shows the mean value for the period 2002–2022. Figure S4: Yearly NHD between 2002 and 2022. Hot days were defined as those when daily maximum air temperature, Tmax ≥ 29.5 °C.
Author Contributions
Conceptualization, T.Á. and J.C.; methodology, T.Á., Z.K., J.C. and K.R.; software, J.C.; validation, Z.K., A.B. and T.Á.; formal analysis, T.Á., J.C. and E.K.; investigation, T.Á., Z.K, J.C., A.K. and K.R.; resources, A.B. and J.C.; data curation, T.Á., Z.K., E.K. and J.C.; writing—original draft preparation, T.Á.; writing—review and editing, T.Á., Z.K., E.K., J.C. and K.R.; visualization, T.Á.; supervision, Z.K., J.C. and K.R.; project administration, J.C.; funding acquisition, T.Á., A.B. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made in the project frame TKP2021-NKTA-43, which was implemented with support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development, and Innovation Fund, financed under the TKP2021-NKTA funding scheme. Project no. 1021122 was implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development, and Innovation Fund, financed under the KDP-2020 funding scheme.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Thanks are due to Gergely Szabó for taking the UAV photos and to Miklós Támba, Napkori Erdőgazdák Kft., for his support in establishing and maintaining the experimental plantation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stolarski, M.J.; Stachowicz, P. Black locust, poplar or willow? Yield and energy value in three consecutive four-year harvest rotations. Ind. Crops Prod. 2023, 193, 116197. [Google Scholar] [CrossRef]
- Hungarian Meteorological Service, National Inventory Report for 1985–2014, Hungary. Available online: https://www.academia.edu/29067562/National_Inventory_Report_for_1985_2014_Hungary_Compiled_by_the_Hungarian_Meteorological_Service?email_work_card=view-paper (accessed on 12 May 2023).
- Nicolescu, V.N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Ciuvăț, A.L.; Abrudan, I.V.; Ciuvăț, C.G.; Marcu, C.; Lorenț, A.; Dincă, L.; Bartha, S. Black Locust (Robinia pseudoacacia L.) in Romanian Forestry. Diversity 2022, 14, 780. [Google Scholar] [CrossRef]
- Keresztesi, B. The Black Locust; Akadémiai Kiadó: Budapest, Hungary, 1988. [Google Scholar]
- Rédei, K.; Csiha, I.; Rásó, J.; Keserű, Z. Selection of promising black locust (Robinia pseudoacacia L.) cultivars in Hungary. J. For. Sci. 2017, 63, 339–343. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Hernea, C.; Bakti, B.; Keserű, Z.; Antal, B.; Rédei, K. Black locust (Robinia pseudoacacia L.) as a multi–purpose tree species in Hungary and Romania: A review. J. For. Res. 2018, 29, 1449–1463. [Google Scholar] [CrossRef]
- Honfy, V.; Pödör, Z.; Keserű, Z.; Rásó, J.; Ábri, T.; Borovics, A. The effect of tree spacing on yields of alley cropping systems–a case study from Hungary. Plants 2023, 12, 595. [Google Scholar] [CrossRef]
- Klisz, M.; Puchałka, R.; Netsvetov, M.; Prokopuk, Y.; Vítková, M.; Sádlo, J.; Matisons, R.; Mionskowksi, M.; Chakraborty, D.; Olszewski, P.; et al. Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate. For. Ecol. Manag. 2021, 492, 119194. [Google Scholar] [CrossRef]
- Rédei, K. Management of black Locust (Robinia pseudoacacia L.) stands in Hungary. J. For. Res. 2002, 13, 260–264. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Führer, E.; Jagodics, A.; Juhász, I.; Marosi, G.; Horváth, L. Ecological and economical impacts of climate change on Hungarian forestry practice. Időjárás—Q. J. Hung. Meteorol. Serv. 2013, 117, 159–174. [Google Scholar]
- Zhang, Q.; Jia, X.; Shao, M.; Zhang, C.; Li, X.; Ma, C. Sap flow of black locust in response to short-term drought in southern Loess Plateau of China. Sci. Rep. 2018, 8, 6222. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, M.; Yang, Y.; Zhao, X. Evaluating drought-induced mortality risk for Robinia pseudoacacia plantations along the precipitation gradient on the Chinese Loess Plateau. Agric. For. Meteorol. 2020, 284, 107897. [Google Scholar] [CrossRef]
- Kalbarczyk, R.; Ziemiańska, M.; Machowksa, A. Effect of climatic conditions on tree-ring widths in black locust (Robinia pseudoacacia L.) in the city of Wrocław. Drvna Industrija 2016, 67, 33–41. [Google Scholar] [CrossRef][Green Version]
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. N. Z. J. For. Sci. 2014, 44, 29. Available online: https://www.nzjforestryscience.com/content/44/1/29 (accessed on 12 May 2023). [CrossRef]
- Jiao, L.; Lu, N.; Fu, B.; Gao, G.; Wang, S.; Jin, T.; Zhang, L.; Liu, J.; Zhang, D. Comparison of transpiration between different aged black locust (Robinia pseudoacacia) trees on the semi-arid Loess Plateau, China. J. Arid Land 2016, 8, 604–617. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Freese, D. Effects of drought frequency on growth performance and transpiration of young black locust (Robinia pseudoacacia L.). Int. J. For. Res. 2014, 2014, 821891. [Google Scholar] [CrossRef]
- Toillon, J.; Priault, P.; Dallé, E.; Bodineau, G.; Bastien, J.-C.; Marron, F.B.N. Early effects of two planting densities on growth dynamics and water-use efficiency in Robinia pseudoacacia (L.) and Populus deltoides (Bartr. ex Marsh.) × P. nigra (L.) short rotation plantations. Ann. For. Sci. 2021, 78, 73. [Google Scholar] [CrossRef]
- Gruenewald, H.; Brandt, B.K.; Schneider, B.U.; Bens, O.; Kendzia, G.; Hüttl, R.F. Agroforestry systems for the production of woody biomass for energy transformation purposes. Ecol. Eng. 2007, 29, 319–328. [Google Scholar] [CrossRef]
- Gianni, F.; Sara, B.; Laura, R.; Minotta, G. Comparison between two and five years rotation models in poplar, willow and black locust Short Rotation Coppices (SRC) in North West Italy. Ann. Silvic. Res. 2020, 45, 12–20. [Google Scholar] [CrossRef]
- Rötzer, T.; Moser-Reischl, A.; Rahman, M.A.; Hartmann, C.; Paeth, H.; Pauleit, S.; Pretzsch, H. Urban tree growth and ecosystem services under extreme drought. Agric. For. Meteorol. 2021, 308–309, 108532. [Google Scholar] [CrossRef]
- Bhusal, N.; Adhikari, A.; Lee, M.; Han, A.; Han, A.R.; Kim, H.S. Evaluation of growth responses of six gymnosperm species under long-term excessive irrigation and traits determining species resistance to waterlogging. Agric. For. Meteorol. 2022, 323, 109071. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Dini-Papanastasi, O. Effects of clonal selection on biomass production and quality in Robinia pseudoacacia var. monophylla Carr. For. Ecol. Manag. 2008, 256, 849–854. [Google Scholar] [CrossRef]
- Rédei, K.; Keserű, Z.; Antal, B. Improved clonal approaches to growing black locust (Robinia pseudoacacia L.) in Hungary: A case study. Int. J. Hortic. Sci. 2015, 21, 53–56. [Google Scholar] [CrossRef]
- Rédei, K.; Keserű, Z.; Csiha, I.; Rásó, J.; Kamandiné Végh, Á.; Antal, B. Juvenile Growth and Morphological Traits of Micropropagated Black Locust (Robinia pseudoacacia L.) Clones under Arid Site Conditions. Acta Silv. Et Lignaria Hung. 2013, 9, 35–42. [Google Scholar] [CrossRef][Green Version]
- Keserű, Z.; Borovics, A.; Ábri, T.; Rédei, K.M.; Lee, I.H.; Lim, H. Growing of Black Locust (Robinia pseudoacacia L.) Candidate Cultivars on Arid Sandy Site. Acta Silv. Et Lignaria Hung. 2021, 17, 51–61. [Google Scholar] [CrossRef]
- Rédei, K.; Osváth-Bujtás, Z.; Keserű, Z. Clonal selection of black locust (Robinia pseudoacacia L.) in Hungary: A review. Int. J. Hortic. Sci. 2007, 13, 153–156. [Google Scholar] [CrossRef]
- Ábri, T.; Keserű, Z.; Rásó, J.; Rédei, K. Stand structure and growth of Robinia pseudoacacia ‘Jászkiséri’—‘Jászkiséri’black locust. J. For. Sci. 2021, 67, 489–497. [Google Scholar] [CrossRef]
- Rédei, K.; Ábri, T. Szelektált, államilag elismert akácfajták és fajtajelöltek. In Szelektált Akácfajták Termesztési Technológiája; Rédei, K., Ed.; Forest Research Institute, University of Sopron: Sopron, Hungary, 2023; pp. 13–18. (In Hungarian) [Google Scholar]
- Ábri, T.; Keserű, Z.; Borovics, A.; Rédei, K.; Csajbók, J. Comparison of juvenile, drought tolerant black locust (Robinia pseudoacacia L.) clones with regard to plant physiology and growth characteristics in Eastern Hungary: Early evaluation. Forests 2022, 13, 292. [Google Scholar] [CrossRef]
- Führer, E.; Horváth, L.; Jagodics, A.; Machon, A.; Szabados, I. Application of a new aridity index in Hungarian forestry practice. Időjárás—Q. J. Hung. Meteorol. Serv. 2011, 115, 205–216. [Google Scholar]
- Koltay, A. Report on the Results of Tree Health Condition Surveys Completed in the International Monitoring Areas (Forest Protection Network II); Forestry Research Institute: Mátrafüred, Hungary, 2017; pp. 1–37. Available online: http://klima.erti.hu/files/FAEG/2018/EVH_II_E%C3%9C_%C3%A1llapot_2017_Koltay.pdf (accessed on 12 May 2023). (In Hungarian)
- Arain, M.A.; Xu, B.; Brodeur, J.J.; Khomik, M.; Peichl, M.; Beamesderfer, E.; Restrepo-Couple, N.; Thorne, R. Heat and drought impact on carbon exchange in an age-sequence of temperate pine forests. Ecol. Process. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Járó, Z. Az erdészeti termőhelyértékelés rendszere. In Erdőművelés; Danszky, I., Ed.; Mezőgazdasági Kiadó: Budapest, Hungary, 1972; pp. 47–256. (In Hungarian) [Google Scholar]
- LI–COR, Inc. Li–6800 Portable Photosynthesis System, Software Version 1.2; LI–COR, Inc.: Lincoln, NE, USA, 2017. [Google Scholar]
- Tanner, C.B.; Sinclair, T.R. Efficient water use in crop production: Research or Re-search? In Limitations to Efficiency Water Use in Crop Production Limitations to Efficient Water Use in Crop Production; Taylor, H.M., Jordan, W.R., Sinclair, T.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1983; pp. 1–27. [Google Scholar]
- Gálos, B.; Führer, E. A klíma erdészeti célú előrevetítése. EK 2018, 8, 43–55. (In Hungarian) [Google Scholar] [CrossRef]
- Kutasy, E.; Buday-Bódi, E.; Virág, I.C.; Forgács, F.; Melash, A.A.; Zsombik, L.; Nagy, A.; Csajbók, J. Mitigating the negative effect of drought stress in oat (Avena sativa L.) with silicon and sulphur foliar fertilization. Plants 2022, 11, 30. [Google Scholar] [CrossRef]
- Haralamb, A.T. Culture of Tree Species; Editura Agrosilvică: Bucharest, Romania, 1967. (In Romanian) [Google Scholar]
- Vítková, M.; Pergl, J.; Sádlo, J. Black locust: From global ecology to local management—A case study from the Czech Republic. In Introduced Tree Species in European Forests: Opportunities and Challenges; Krumm, F., Vítková, L., Eds.; European Forest Institute: Freiburg, Germany, 2016; pp. 306–318. [Google Scholar]
- Doupis, G.; Kavroulakis, N.; Psarras, G.; Papadakis, I.E. Growth, photosynthetic performance and antioxidative response of ‘Hass’ and ‘Fuerte’avocado (Persea americana Mill.) plants grown under high soil moisture. Photosynthetica 2017, 55, 655–663. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, Ü.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Meng, F.; Peng, M.; Pang, H.; Huang, F. Comparison of photosynthesis and leaf ultrastructure on two black locust (Robinia pseudoacacia L.). Biochem. Syst. Ecol. 2014, 55, 170–175. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Wang, D.; LeBauer, D.; Kling, G.; Voigt, T.; Dietze, M.C. Ecophysiological screening of tree species for biomass production: Trade-off between production and water use. Ecosphere 2013, 4, 1–22. [Google Scholar] [CrossRef]
- Jiao, L.; Lu, N.; Fang, W.; Li, Z.; Wang, J.; Jin, Z. Determining the independent impact of soil water on forest transpiration: A case study of a black locust plantation in the Loess Plateau, China. J. Hydrol. 2019, 572, 671–681. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.; Zhou, J.; Zhou, H. Relationship between photosynthetic gas exchange of black locust and environmental factors. J. Northwest A F Univ.-Nat. Sci. Ed. 2011, 39, 8188. [Google Scholar]
- Zheng, Y.; Zhou, J.; Zhou, H.; Zhao, Z. Photosynthetic carbon fixation capacity of black locust in rapid response to plantation thinning on the semiarid Loess plateau in China. Pak. J. Bot. 2019, 51, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Meng, F.; Luo, Q.; Wang, Q.; Zhang, X.; Qi, Z.; Xu, F.; Lei, X.; Cao, Y.; Chow, W.S.; Sun, G. Physiological and proteomic responses to salt stress in chloroplasts of diploid and tetraploid black locust (Robinia pseudoacacia L.). Sci. Rep. 2016, 6, srep23098. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xi, W.; Rahmlow, A.; Kong, H.; Zhang, Z.; Shangguan, Z. Effects of forest plantation types on leaf traits of Ulmus pumila and Robinia pseudoacacia on the Loess Plateau, China. Ecol. Eng. 2016, 97, 416–425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).