Transcriptional Regulations and Hormonal Signaling during Somatic Embryogenesis in the Coconut Tree: An Insight
Abstract
1. Introduction
2. Somatic Embryogenesis System in Coconut
| Coconut Variety | Explant Type | Medium Composition | Plant Growth Regulators | Reference |
|---|---|---|---|---|
| Malayan Red Dwarf × Tagnanan | Rachilla | Y3 medium, gelrite (3 g/L), AC (2.5 g/L) | 2,4-D, BAP (0.3 mM) and GA3 (0.0046 mM) | [37] |
| Jamaican Malayan Dwarf | Rachilla and stem | 3 basal medium and sucrose (6.8%), agar (0.39%) | 2,4-D (0.1 μM), BAP (5 μM), and GA3 (10 μM) | [56] |
| West Coast Tall | Rachilla, stem, and foliage | Y3, AC (0.25%), sucrose (5%), agar (0.6%) | 2,4-D (452 μM), NAA (2.69 μM), BAP (8.88 μM), kinetin (4.65 μM) | [57] |
| Green Malayan Dwarf | Plumule | Y3, gelrite (3 g/L), AC (2.5 g/L), sucrose (50 g/L) | 2,4-D (6 μM) and (300 μM BAP) | [58] |
| MYD, Makapuno, XXD and PB121 | Plumule | Y3, agar (2.5 g/L), vitamins | 2,4-D and BAP | [59] |
| Green Malayan Dwarf | Plumule | Y3, gelrite (3 g/L), AC (2.5 g/L) | 2,4-D and BAP | [43,60] |
| Sri Lanka Tall | Plumule | BM72, sucrose (4% w/v), agar (0.8%) | 2,4-D | [61] |
| Malayan Dwarf | Plumule | Y3, gelrite (3 g/L), AC (2.5 g/L) | 2,4-D (1 μM) and BAP (50 μM) | [42] |
| Sri Lanka Tall | Immature embryo | BM72, AC (0.25%), sucrose (40 g/L), agar (0.8%) | 2,4-D (24 μM), ABA (2.5–7.5 μM), and cytokinin (2–10 μM) | [62] |
| Batu Layar Tall | Mature embryo slice | M2, AC (2.5 g/L), sucrose (0–100 g/L), agar (7.5 g/L) | 2,4-D and ABA | [47] |
| Typica | Embryo | AC (0.25%), sucrose (30 g/L), agar (0.8%) | 2,4-D (8 μM and 2 μM), BAP (10 μM) and kinetin (10 μM) | [63] |
| West Coast Tall | Young embryo | Gamborg’s B5 medium, agar (0.7%) | IAA, NAA, 2,4-D, BAP or kinetin (0.5 mg/L to 5 mg/L) | [64] |
| Malayan Yellow Dwarf (MYD) × West African Tall | Young foliage tissue | Sucrose (30 g/L), agar (0.8%), vitamins | 2,4-D, TCPP, and BAP | [41] |
| MYD × WAT, WAT × MYD and MYD | Immature inflorescence | Y3, AC (2 g/L), sucrose (116.8 mM), vitamins | 2,4-D and BAP (10−5 M) | [45] |
| PB 121 (MYD × WAT) | Immature inflorescence | Modified MS macronutrients, AC (3 g/L), agar (7.5 g/L) Nitsch micronutrients, vitamins, EDTA (26 mg), iron (24.9 mg), ascorbic acid (100 mg/L), malic acid (100 mg/L), adenine sulfate (30 mg/L) | 2,4-D and BAP | [44] |
| Malayan Yellow Dwarf | Immature inflorescence | Y3, AC (2.5 g/L), sucrose (30 g/L) | 2,4-D, spermine (0.01 µM), auxin (500 µ M), and water (10%) | [65] |
| Sri Lanka Tall | Inflorescence | CRI 72AC (0.1%), sucrose (40 g/L) | [66] | |
| Sri Lanka Tall | Unfertilized ovary | CRI 72, agar (2%) | 2,4-D and ABA (5 μM) | [67] |
| Dwarf Green | Leaf and inflorescence | Euewens medium, sucrose (60 g/L), TDZ (1.0 mg/L), 2-ip (1.0 mg/L) | 2,4-D (60 mg/L) and BAP (2 mg/L) | [36] |
2.1. Gene Regulatory Mechanism during the Development of SE
2.1.1. Cell Cycle
2.1.2. Genetic Component for Dedifferentiation and Totipotency
2.1.3. Release/Induction of Embryogenic Program
2.1.4. Formation of SE Meristem Maintenance and Regulation
2.2. Hormonal Regulatory Mechanisms Involved in SE
3. Epigenetic Regulations of Somatic Embryogenesis
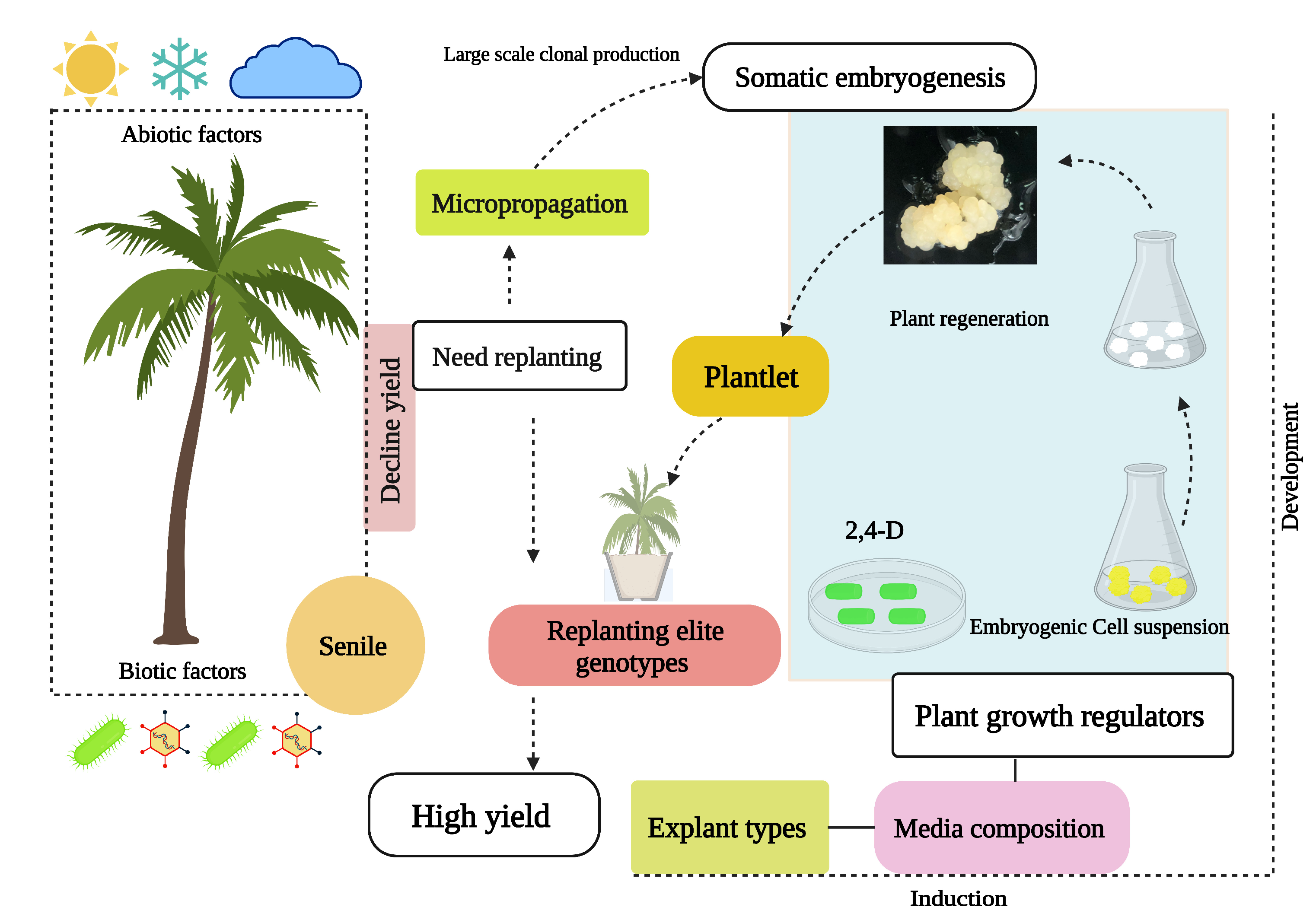
4. Prospects for Using Clonal Propagation to Meet Global Replanting Needs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SE | Somatic embryogenesis |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| CK | Cytokinin |
| BAP | 6-Benzylaminopurine |
| BRs | Brassinosteroids |
| TCPP | Tris(2-chloropropyl) phosphate |
| GA3 | Gibberellins |
| IAA | Indoleacetic acid |
| TDZ | Thidiazuron |
| 2-ip | 2-isopentenyl adenine |
References
- Zuraida, A.; Kumaran, G.; Ahmad, N.; Farhanah, M.; Nazreena, O. Callus induction from zygotic embryos of coconut MATAG F2. Asian Res. J. Agric. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Henrietta, H.M.; Kalaiyarasi, K.; Raj, A.S. Coconut Tree (Cocos nucifera) Products: A Review of Global Cultivation and its Benefits. J. Sustain. Environ. Manag. 2022, 1, 257–264. [Google Scholar] [CrossRef]
- Beegum, P.S.; Pandiselvam, R.; Ramesh, S.; Thube, S.H.; Pandian, T.P.; Khanashyam, A.C.; Manikantan, M.; Hebbar, K. A ritical appraisal on the antimicrobial, oral protective, and anti-diabetic functions of coconut and its derivatives. Qual. Assur. Safe Crops Foods 2022, 14, 86–100. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Nguyen, Q. Improving the value of the coconut with biotechnology. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 29–50. [Google Scholar]
- Zainol, F.A.; Arumugam, N.; Daud, W.N.W.; Suhaimi, N.A.M.; Ishola, B.D.; Ishak, A.Z.; Afthanorhan, A. Coconut Value Chain Analysis: A Systematic Review. Agriculture 2023, 13, 1379. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bourdeix, R.; Auroyé, A.; Pirmansah, A. Consumer interest in coconut products. In Coconut Risk Management and Mitigation Manual for the Pacific Region; Land Resources Division: Surrey, UK, 2021. [Google Scholar]
- Kairupan, A.N.; Kindangen, J.G.; Joseph, G.H.; Hutapea, R.T.; Malia, I.E.; Paat, P.C.; Polakitan, D.; Polakitan, A.; Rawung, J.B.M.; Lintang, M. Value Chain Implementation in Rural-Scale Integrated Coconut Farming System in North Sulawesi Province, Indonesia. In Agricultural Value Chains—Some Selected Issues; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Lédo, A.d.S.; Passos, E.E.M.; Fontes, H.R.; Ferreira, J.M.S.; Talamini, V.; Vendrame, W.A. Advances in Coconut palm propagation. Revi Bras. De. Fruticult 2019, 41, 2. [Google Scholar] [CrossRef]
- Gurr, G.M.; Johnson, A.C.; Ash, G.J.; Wilson, B.A.; Ero, M.M.; Pilotti, C.A.; Dewhurst, C.F.; You, M.S. Coconut lethal yellowing diseases: A phytoplasma threat to palms of global economic and social significance. Front. Plant Sci. 2016, 7, 1521. [Google Scholar] [CrossRef]
- Ganeshkumar, B.; GVT, G.K. Spatial assessment of climate variability effects on coconut crops in Tamil Nadu State—A case study. Theorl Appl. Clim. 2022, 148, 121–129. [Google Scholar] [CrossRef]
- Ramjegathesh, R.; Rajendran, L.; Karthikeyan, G.; Raguchander, T. Coconut (Cocos nucifera Linn.) Diseases And Management Strategies. In Diseases of Horticultural Crops; Apple Academic Press: Waretown, NJ, USA, 2021; pp. 73–96. [Google Scholar]
- Vadamalai, G.; Perera, A.; Hanold, D.; Rezaian, M.; Randles, J. Detection of Coconut cadang-cadang viroid sequences in oil and coconut palm by ribonuclease protection assay. Ann. Appl. Biol. 2009, 154, 117–125. [Google Scholar] [CrossRef]
- Sáenz-Carbonell, L.; Nguyen, Q.; López-Villalobos, A.; Oropeza-Salín, C. Coconut micropropagation for worldwide replanting needs. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 227–240. [Google Scholar]
- Karun, A.; Ramesh, S.; Rajesh, M.; Niral, V.; Sudha, R.; Muralikrishna, K. Conservation and Utilization of Genetic Diversity in Coconut (Cocos nucifera L.). In Cash Crops: Genetic Diversity, Erosion, Conservation Utilization; Springer: Berlin/Heidelberg, Germany, 2022; pp. 197–250. [Google Scholar]
- Khan, F.S.; Goher, F.; Zhang, D.; Shi, P.; Li, Z.; Htwe, Y.M.; Wang, Y. Is CRISPR/Cas9 a way forward to fast-track genetic improvement in commercial palms? Prospects and limits. Front. Plant Sci. 2022, 13, 1042828. [Google Scholar] [CrossRef]
- Bourdeix, R.; Prades, A. A Global Strategy for the Conservation and Use of Coconut Genetic Resources 2018–2028; Bioversity International: Montpellier, France, 2017. [Google Scholar]
- Yousefi, K.; Abdullah, S.N.A.; Hatta, M.A.M.; Ling, K.L. Genomics and Transcriptomics Reveal Genetic Contribution to Population Diversity and Specific Traits in Coconut. Plants 2023, 12, 1913. [Google Scholar] [CrossRef]
- Batugal, P.; Bourdeix, R.; Baudouin, L. Coconut breeding. In Breeding Plantation Tree Crops: Tropical Species; Springer: Berlin/Heidelberg, Germany, 2009; pp. 327–375. [Google Scholar]
- Perera, L.; Manimekalai, R.; Sudarsono, S. Advances in breeding of coconut. In Achieving Sustainable Cultivation of Tropical Fruits; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 323–344. [Google Scholar]
- Nair, K.P. The coconut palm (Cocos nucifera L.). In Tree Crops; Springer: Berlin/Heidelberg, Germany, 2021; pp. 79–128. [Google Scholar]
- Elhiti, M.; Stasolla, C. Somatic embryogenesis: The molecular network regulating embryo formation. In Somatic Embryogenesis in Ornamentals and Its Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 217–229. [Google Scholar]
- Smertenko, A.; Bozhkov, P.V. Somatic embryogenesis: Life and death processes during apical–basal patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Pathirana, R.; Vidhanaarchchi, V. Somatic embryogenesis in anther-derived fast-growing callus as a long-term source for doubled-haploid production of coconut (Cocos nucifera L.). J. Natl. Sci. Found. Sri Lanka 2021, 49, 39. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Veluru, A.; Mohammed, N.S.; Shil, S.S.S.; Prakash, K.; Kotian, K.; Muralikrishna, K.; Karun, A. Induction of somatic embryogenesis and plantlet regeneration in Areca concinna, an endangered palm species. Res. Squar 2022. [Google Scholar] [CrossRef]
- Ferreira, J.C.B.; de Araújo Silva-Cardoso, I.M.; de Oliveira Meira, R.; Scherwinski-Pereira, J.E. Somatic embryogenesis and plant regeneration from zygotic embryos of the palm tree Euterpe precatoria Mart. Plant Cell Tissue Organ. Cult. 2022, 148, 667–686. [Google Scholar] [CrossRef]
- Solangi, N.; Abul-Soad, A.A.; Jatoi, M.A.; Mirani, A.A.; Markhand, G.S. Micropropagation of elite date palm (Phoenix dactylifera L.) cultivars Samany and Bertamoda through immature inflorescence explants. J. Saudi Soc. Agric. Sci. 2023. [Google Scholar] [CrossRef]
- Fernando, S.; Weerakoon, L.; Gunathilake, T. Micropropagation of coconut through plumule culture. Cocos 2004, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Kong, E.Y.; Biddle, J.; Kalaipandian, S.; Adkins, S.W. Coconut Callus Initiation for Cell Suspension Culture. Plants 2023, 12, 968. [Google Scholar] [CrossRef]
- Tung, H.T.; Ngan, H.T.M.; Cuong, D.M.; Hien, V.T.; Huong, T.T.; Vinh, B.V.T.; Mo, V.T.; Anh, T.T.L.; Van Binh, N.; Diem, L.T. Somatic embryo as a tool for micropropagating of some plants. In Plant Tissue Culture: New Techniques and Application in Horticultural Species of Tropical Region; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–166. [Google Scholar]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic embryogenesis. An overview. In Somatic Embryogenesis: Fundamental Aspects Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–8. [Google Scholar]
- Aparna, V.; Neema, M.; Chandran, K.; Muralikrishna, K.; Karun, A. Enhancement of callogenesis from plumular explants of coconut (Cocos nucifera) via exogenous supplementation of amino acids and casein hydrolysate. Curr. Horti 2023, 11, 40–43. [Google Scholar] [CrossRef]
- Perera, P.I.; Hocher, V.; Verdeil, J.L.; Doulbeau, S.; Yakandawala, D.; Weerakoon, L.K. Unfertilized ovary: A novel explant for coconut (Cocos nucifera L.) somatic embryogenesis. Plant Cell Rep. 2007, 26, 21–28. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Mu, Z.; Kong, E.Y.Y.; Biddle, J.; Cave, R.; Bazrafshan, A.; Wijayabandara, K.; Beveridge, F.C.; Nguyen, Q.; Adkins, S.W. Cloning coconut via somatic embryogenesis: A review of the current status and future prospects. Plants 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Nwite, P.A.; Eke, C.; Osemwegie, Q.; Ikhajiagbe, B. Assessment of media modification and induction of calli on coconut (Cocos nucifera L.) palm explants. Res. J. Agric. 2023, 2320, 6063. [Google Scholar]
- Sandoval-Cancino, G.; Sáenz, L.; Chan, J.; Oropeza, C. Improved formation of embryogenic callus from coconut immature inflorescence explants. Vitr. Cell. Dev. Biol. Plant 2016, 52, 367–378. [Google Scholar] [CrossRef]
- Elhiti, M.; Stasolla, C. Transduction of signals during somatic embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef]
- Sáenz-Carbonell, L.; Montero-Cortés, M.; Pérez-Nuñez, T.; Azpeitia-Morales, A.; Andrade-Torres, A.; Córdova-Lara, I.; Chan-Rodríguez, J.L.; Sandoval-Cancino, G.; Rivera-Solis, G.; Oropeza-Salín, C. Somatic Embryogenesis in Cocos nucifera L. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 297–318. [Google Scholar]
- Rabechault, H.; Ahée, J.; Guenin, G. Colonies cellulaires et formes embryoides obtenues in vitro a partir de cultures d’embryons de Palmier a huile (Elaeis guineensis Jacq. var. dura Becc.). Acad. Sci. Compt Rend. Ser. 1970, 270, 3067–3070. [Google Scholar]
- Pannetier, C.; Buffard-Morel, J. Production of somatic embryos from leaf tissues of coconut, Cocos nucifera L. In Proceedings of the Plant Tissue Culture, Tokyo, Japan, 11–16 July 1982; pp. 755–756. [Google Scholar]
- Chan, J.; Sáenz, L.; Talavera, C.; Hornung, R.; Robert, M.; Oropeza, C. Regeneration of coconut (Cocos nucifera L.) from plumule explants through somatic embryogenesis. Plant Cell Rep. 1998, 17, 515–521. [Google Scholar] [CrossRef]
- Pérez-Núñez, M.; Chan, J.; Saenz, L.; González, T.; Verdeil, J.-L.; Oropeza, C. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. Vitr. Cell. Dev. Biol. Plant 2006, 42, 37–43. [Google Scholar] [CrossRef]
- Magnaval, C.; Noirot, M.; Verdeil, J.-L.; Blattes, A.; Huet, C.; Grosdemange, F.; Beulé, T.; Buffard-Morel, J. Specific nutritional requirements of coconut calli (Cocos nucifera L.) during somatic embryogenesis induction. J. Plant Physiol. 1997, 150, 719–728. [Google Scholar] [CrossRef]
- Verdeil, J.-L.; Buffard-Morel, J. Somatic embryogenesis in coconut (Cocos nucifera L.). In Somatic Embryogenesis and Synthetic Seed I; Springer: Berlin/Heidelberg, Germany, 1995; pp. 299–317. [Google Scholar]
- Samosir, Y.M.S. Optimisation of Somatic Embryogenesis in Coconut (Cocos nucifera L.). Ph.D. Thesis, School of Land, Crop and Food Sciences, The University of Queensland, Brisbane, Australia, 1999. [Google Scholar]
- Samosir, Y.; Godwin, I.; Adkins, S. The use of osmotically active agents and abscisic acid can optimise the maturation of coconut somatic embryos. In Current Advances in Coconut Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 341–354. [Google Scholar]
- Adkins, S.; Samosir, Y.; Godwin, I. Control of environmental conditions and the use of polyamines can optimise the conditions for the initiation and proliferation of coconut somatic embryos. In Current Advances in Coconut Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 321–340. [Google Scholar]
- Rajesh, M.; Radha, E.; Sajini, K.; Anitha, K. Polyamine-induced somatic embryogenesis and plantlet regeneration in vitro from plumular explants of dwarf cultivars of coconut (Cocos nucifera). Ind. J. Agric. Sci. 2014, 84, 527–530. [Google Scholar]
- Khan, F.; Goher, F.; Paulsmeyer, M.; Hu, C.G.; Zhang, J.Z. Calcium (Ca2+) sensors and MYC2 are crucial players during jasmonates-mediated abiotic stress tolerance in plants. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Biddle, J.; Nguyen, Q.; Mu, Z.H.; Foale, M.; Adkins, S. Germplasm reestablishment and seedling production: Embryo culture. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–225. [Google Scholar]
- Keshvari, T.; Najaphy, A.; Kahrizi, D.; Zebarjadi, A. Callus induction and somatic embryogenesis in Stevia rebaudiana Bertoni as a medicinal plant. Cell. Mol. Biol. 2018, 64, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.P.; Prinsen, E.; Ayaydin, F.; Miskolczi, P.; Potters, G.; Asard, H.; Van Onckelen, H.A.; Dudits, D.; Fehér, A. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002, 129, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Maulida, D.; Erfa, L.; Sesanti, R.; Hidayat, H. Induction of kopyor coconut embryogenic callus using 2.4-D and TDZ. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changchun, China, 21–23 August 2020; p. 012013. [Google Scholar]
- El Gioushy, S.F.; Rui, L.; Fan, H. Impact of various 2, 4-D concentrations and different vitamin mixtures on in vitro culture of coconut (Cocos nucifera L.) by utilizing seedlings shoot tip. Acta Sci. Pol. Hort. Cultus 2020, 19, 135–145. [Google Scholar] [CrossRef]
- Eeuwens, C.; Blake, J. Culture of coconut and date palm tissue with a view to vegetative propagation. In Symposium on Tissue Culture for Horticultural Purposes 78; ISHS: Leuven, Belgium, 1977; pp. 277–286. [Google Scholar]
- Gupta, P.; Kendurkar, S.; Kulkarni, V.; Shirgurkar, M.; Mascarenhas, A. Somatic embryogenesis and plants from zygotic embryos of coconut (Cocos nucifera L.) in vitro. Plant Cell Rep. 1984, 3, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, L.; Chan, J.L.; Narvaez, M.; Oropeza, C. Protocol for the micropropagation of coconut from plumule explants. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 161–170. [Google Scholar]
- Nguyen, T.Q. Clonal Propagation of Coconut (Cocos nucifera L.) for Elite Seedling Production and Germplasm Exchange. Ph.D. Thesis, School of Agriculture and Food Sciences, The University of Queensland, Brisbane, Australia, 2018. [Google Scholar]
- Saenz, L.; Azpeitia, A.; Chuc-Armendariz, B.; Chan, J.; Verdeil, J.-L.; Hocher, V.; Oropeza, C. Morphological and histological changes during somatic embryo formation from coconut plumule explants. Vitr. Cell. Dev. Biol. -Plant 2006, 42, 19–25. [Google Scholar] [CrossRef]
- Fernando, S.; Verdeil, J.-L.; Hocher, V.; Weerakoon, L.K.; Hirimburegama, K. Histological analysis of plant regeneration from plumule explants of Cocos nucifera. Plant Cell Tissue Organ. Cult. 2003, 72, 281–283. [Google Scholar] [CrossRef]
- Fernando, S.; Gamage, C. Abscisic acid induced somatic embryogenesis in immature embryo explants of coconut (Cocos nucifera L.). Plant Sci. 2000, 151, 193–198. [Google Scholar] [CrossRef]
- Karunaratne, S.; Periyapperuma, K. Culture of immature embryos of coconut, Cocos nucifera L: Callus proliferation and somatic embryogenesis. Plant Sci. 1989, 62, 247–253. [Google Scholar] [CrossRef]
- Bhalla-Sarin, N.; Bagga, S.; Sopory, S.K.; Guha-Mukherjee, S. Induction and differentiation of callus from embryos of Cocos nucifera L. by IAA-conjugates. Plant Cell Rep. 1986, 5, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Antonova, I. Somatic Embryogenesis for Micropropagation of Coconut (Cocos nucifera L.). Ph.D. Thesis, School of Land, Crop and Food Sciences, The University of Queensland, Brisbane, Australia, 2009. [Google Scholar]
- Perera, P.I.; Yakandawala, D.; Hocher, V.; Verdeil, J.-L.; Weerakoon, L.K. Effect of growth regulators on microspore embryogenesis in coconut anthers. Plant Cell Tissue Organ. Cult. 2009, 96, 171–180. [Google Scholar] [CrossRef]
- Perera, P.; Yakandawala, D.; Verdeil, J.; Hocher, V.; Weerakoon, L. Somatic embryogenesis and plant regeneration from unfertilised ovary explants of coconut (Cocos nucifera L.). Trop. Agric. Res. 2008, 20, 226–233. [Google Scholar]
- Sahara, A.; Roberdi, R.; Wiendi, N.M.A.; Liwang, T. Transcriptome profiling of high and low somatic embryogenesis rate of oil palm (Elaeis guineensis Jacq. var. Tenera). Front. Plant Sci. 2023, 14, 1142868. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Fayas, T.; Naganeeswaran, S.; Rachana, K.; Bhavyashree, U.; Sajini, K.; Karun, A. De novo assembly and characterization of global transcriptome of coconut palm (Cocos nucifera L.) embryogenic calli using Illumina paired-end sequencing. Protoplasma 2016, 253, 913–928. [Google Scholar] [CrossRef]
- Pérez-Núñez, M.; Souza, R.; Sáenz, L.; Chan, J.; Zuniga-Aguilar, J.; Oropeza, C. Detection of a SERK-like gene in coconut and analysis of its expression during the formation of embryogenic callus and somatic embryos. Plant Cell Rep. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Montero-Cortés, M.; Sáenz, L.; Cordova, I.; Quiroz, A.; Verdeil, J.-L.; Oropeza, C. GA3 stimulates the formation and germination of somatic embryos and the expression of a KNOTTED-like homeobox gene of Cocos nucifera (L.). Plant Cell Rep. 2010, 29, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Montero-Cortés, M.; Rodríguez-Paredes, F.; Burgeff, C.; Pérez-Nunez, T.; Córdova, I.; Oropeza, C.; Verdeil, J.-L.; Sáenz, L. Characterisation of a cyclin-dependent kinase (CDKA) gene expressed during somatic embryogenesis of coconut palm. Plant Cell Tissue Organ. Cult. 2010, 102, 251–258. [Google Scholar] [CrossRef]
- Sabana, A.; Antony, G.; Rahul, C.; Rajesh, M. In silico identification of microRNAs and their targets associated with coconut embryogenic calli. Agri Gene 2018, 7, 59–65. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Bandupriya, H.; López-Villalobos, A.; Sisunandar, S.; Foale, M.; Adkins, S.W. Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): A review. Planta 2015, 242, 1059–1076. [Google Scholar] [CrossRef]
- Osorio-Montalvo, P.; De-la-Peña, C.; Oropeza, C.; Nic-Can, G.; Córdova-Lara, I.; Castillo-Castro, E.; Sáenz-Carbonell, L. A peak in global DNA methylation is a key step to initiate the somatic embryogenesis of coconut palm (Cocos nucifera L). Plant Cell Rep. 2020, 39, 1345–1357. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Angulo-Bejarano, P.I.; Bandyopadhyay, A.; Sharma, A.; Paul, S. Regulatory roles of noncoding RNAs in callus induction and plant cell dedifferentiation. Plant Cell Rep. 2023, 42, 689–705. [Google Scholar] [CrossRef]
- Sandoval, A.; Hocher, V.; Verdeil, J.-L. Flow cytometric analysis of the cell cycle in different coconut palm (Cocos nucifera L.) tissues cultured in vitro. Plant Cell Rep. 2003, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Shafique Khan, F.; Zeng, R.-F.; Gan, Z.-M.; Zhang, J.-Z.; Hu, C.-G. Genome-wide identification and expression profiling of the WOX gene family in Citrus sinensis and functional analysis of a CsWUS member. Int. J. Mol. Sci. 2021, 22, 4919. [Google Scholar] [CrossRef]
- Carneiro, A.K.; Montessoro, P.d.F.; Fusaro, A.F.; Araújo, B.G.; Hemerly, A.S.J.P. Plant CDKs—Driving the cell cycle through climate change. Plants 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.J.; Takatsuka, H.; Hirota, J.; Mineta, K.; Nomoto, Y.; Ito, M. Members of SIAMESE-RELATED Class Inhibitor Proteins of Cyclin-Dependent Kinase Retard G2 Progression and Increase Cell Size in Arabidopsis thaliana. Life 2022, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, A.; Aki, S.S.; Takahashi, N.; Umeda, M. Regulation of the plant cell cycle in response to hormones and the environment. Annu. Rev. Plant Biol. 2021, 72, 273–296. [Google Scholar] [CrossRef]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2021, 63, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Herrera, A.; Ku Gonzalez, A.; Canche Moo, R.; Quiroz-Figueroa, F.R.; Loyola-Vargas, V.; Rodriguez-Zapata, L.; Burgeff, D.; Hondt, C.; Suárez-Solís, V.; Castaño, E. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ. Cult. 2008, 94, 171–180. [Google Scholar] [CrossRef]
- Orłowska, A.; Kępczyńska, E. Identification of Polycomb Repressive Complex1, Trithorax group genes and their simultaneous expression with WUSCHEL, WUSCHEL-related Homeobox5 and SHOOT MERISTEMLESS during the induction phase of somatic embryogenesis in Medicago truncatula Gaertn. Plant Cell Tissue Organ. Cult. 2018, 134, 345–356. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V.J.P.c.r. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Grenier De March, G.; Guerineau, F.; Cosson, V.; Ratet, P. WUSCHEL Overexpression promotes callogenesis and somatic embryogenesis in Medicago truncatula Gaertn. Plants 2021, 10, 715. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Jaskóła, K.; Gąsiorek, P.; Meus, M.; Nowak, K.; Gaj, M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 2013, 238, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.; Frigerio, L.; Cui, Y.; Menassa, R. Following vegetative to embryonic cellular changes in leaves of Arabidopsis overexpressing LEAFY COTYLEDON2. Plant Physiol. 2013, 162, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Morończyk, J.; Grzyb, M.; Szczygieł-Sommer, A.; Gaj, M.D. miR172 Regulates WUS during Somatic Embryogenesis in Arabidopsis via AP2. Cells 2022, 11, 718. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; López-Torres, A.; Barredo-Pool, F.; Wrobel, K.; Loyola-Vargas, V.M.; Rojas-Herrera, R.; De-la-Pena, C. New insights into somatic embryogenesis: LEAFY COTYLEDON1, BABY BOOM1 and WUSCHEL-RELATED HOMEOBOX4 are epigenetically regulated in Coffea canephora. PLoS ONE 2013, 8, e72160. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Maas, L.; Figueiredo, D.; Zhong, Y.; Reis, R.; Li, M.; Horstman, A.; Riksen, T.; Weemen, M.; Liu, H. BABY BOOM regulates early embryo and endosperm development. Proc. Natl. Acad. Sci. USA 2022, 119, e2201761119. [Google Scholar] [CrossRef]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2022, 188, 1095–1110. [Google Scholar] [CrossRef]
- Hecht, V.; Vielle-Calzada, J.-P.; Hartog, M.V.; Schmidt, E.D.; Boutilier, K.; Grossniklaus, U.; de Vries, S.C. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001, 127, 803–816. [Google Scholar] [CrossRef]
- Nolan, K.E.; Irwanto, R.R.; Rose, R.J. Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol. 2003, 133, 218–230. [Google Scholar] [CrossRef]
- Kang, H.-I.; Lee, C.-B.; Kwon, S.-H.; Park, J.-M.; Kang, K.-S.; Shim, D. Comparative transcriptome analysis during developmental stages of direct somatic embryogenesis in Tilia amurensis Rupr. Sci. Rep. 2021, 11, 6359. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.I.; Vidhanaarachchi, V.; Gunathilake, T.; Yakandawala, D.; Hocher, V.; Verdeil, J.-L.; Weerakoon, L.K. Effect of plant growth regulators on ovary culture of coconut (Cocos nucifera L.). Plant Cell Tissue Organ. Cult. 2009, 99, 73–81. [Google Scholar] [CrossRef]
- Iwakawa, H.; Shinmyo, A.; Sekine, M. Arabidopsis CDKA; 1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2006, 45, 819–831. [Google Scholar] [CrossRef]
- Bandupriya, H.; Dunwell, J. Overexpression of CnANT, Coconut BABYBOOM Homologue Alters Plant Growth and Morphology in Transgenic Arabidopsis Plants. Trop. Agric. Res. 2012, 23, 249. [Google Scholar] [CrossRef]
- Bandupriya, H.; Gibbings, J.G.; Dunwell, J.M. Isolation and characterization of an AINTEGUMENTA-like gene in different coconut (Cocos nucifera L.) varieties from Sri Lanka. Tree Genet. Genom. 2013, 9, 813–827. [Google Scholar] [CrossRef]
- Bueno, N.; Cuesta, C.; Centeno, M.L.; Ordás, R.J.; Alvarez, J.M. In Vitro Plant Regeneration in Conifers: The Role of WOX and KNOX Gene Families. Genes 2021, 12, 438. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Tahir, M.; Schroeder, D.; Stasolla, C. Overexpression of HBK3, a class I KNOX homeobox gene, improves the development of Norway spruce (Picea abies) somatic embryos. J. Exp. Bot. 2007, 58, 2851–2861. [Google Scholar] [CrossRef][Green Version]
- Cole, M.; Nolte, C.; Werr, W. Nuclear import of the transcription factor SHOOT MERISTEMLESS depends on heterodimerization with BLH proteins expressed in discrete sub-domains of the shoot apical meristem of Arabidopsis thaliana. Nucleic Acids Res. 2006, 34, 1281–1292. [Google Scholar] [CrossRef]
- Lenhard, M.; Jürgens, G.; Laux, T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 2002, 129, 3195–3206. [Google Scholar] [CrossRef]
- Lopes, F.L.; Galvan-Ampudia, C.; Landrein, B. WUSCHEL in the shoot apical meristem: Old player, new tricks. J. Exp. Bot. 2021, 72, 1527–1535. [Google Scholar] [CrossRef]
- Dao, T.Q.; Weksler, N.; Liu, H.M.-H.; Leiboff, S.; Fletcher, J.C. Interactive CLV3, CLE16, and CLE17 signaling mediates stem cell homeostasis in the Arabidopsis shoot apical meristem. Development 2022, 149, 200787. [Google Scholar] [CrossRef]
- Thibaud-Nissen, F.; Shealy, R.T.; Khanna, A.; Vodkin, L.O. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol. 2003, 132, 118–136. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, Y.; Perry, S.E. AGAMOUS-Like15 promotes somatic embryogenesis in Arabidopsis and soybean in part by the control of ethylene biosynthesis and response. Plant Physiol. 2013, 161, 2113–2127. [Google Scholar] [CrossRef]
- Radoeva, T.; Weijers, D. A roadmap to embryo identity in plants. Trends Plant Sci. 2014, 19, 709–716. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Zhao, S. Functional characterization of a WRKY family gene involved in somatic embryogenesis in Panax ginseng. Protoplasma 2020, 257, 449–458. [Google Scholar] [CrossRef]
- Khan, F.S.; Gan, Z.-M.; Li, E.-Q.; Ren, M.-K.; Hu, C.-G.; Zhang, J.-Z. Transcriptomic and physiological analysis reveals interplay between salicylic acid and drought stress in citrus tree floral initiation. Planta 2022, 255, 1–22. [Google Scholar] [CrossRef]
- Chen, B.; Fiers, M.; Dekkers, B.J.; Maas, L.; van Esse, G.W.; Angenent, G.C.; Zhao, Y.; Boutilier, K. ABA signalling promotes cell totipotency in the shoot apex of germinating embryos. J. Exp. Bot. 2021, 72, 6418–6436. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, N.; Wang, H.; Stromberg, A.J.; Perry, S.E. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell 2009, 21, 2563–2577. [Google Scholar] [CrossRef]
- Kim, H.U.; Jung, S.-J.; Lee, K.-R.; Kim, E.H.; Lee, S.-M.; Roh, K.H.; Kim, J.-B. Ectopic overexpression of castor bean LEAFY COTYLEDON2 (LEC2) in Arabidopsis triggers the expression of genes that encode regulators of seed maturation and oil body proteins in vegetative tissues. FEBS Open Bio 2014, 4, 25–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Clemens, A.; Maximova, S.N.; Guiltinan, M.J. The Theobroma cacao B3 domain transcription factor TcLEC2plays a duel role in control of embryo development and maturation. BMC Plant Biol. 2014, 14, 106. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Tsuchiya, Y.; Lumba, S.; Okamoto, M.; McCourt, P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev. Cell 2004, 7, 373–385. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Luo, Y.; Koop, H.-U. Somatic embryogenesis in cultured immature zygotic embryos and leaf protoplasts of Arabidopsis thaliana ecotypes. Planta 1997, 202, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Khurana, P. Ectopic expression of Triticum aestivum SERK genes (TaSERKs) control plant growth and development in Arabidopsis. Sci. Rep. 2017, 7, 12368. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Gunnerås, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hall, T.C.; Holmes-Davis, R. Plant chromatin: Development and gene control. Bioessays 2002, 24, 234–243. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018, 23, 235–247. [Google Scholar] [CrossRef]
- Ree, J.F.; Polesi, L.G.; Back, F.; Bertolazi, A.A.; Silveira, V.; Guerra, M.P. Aging peach palm (Bactris gasipaes Kunth) cultures lose embryogenic potential and metabolic cellular function due to continuous culture in hypoxic environments. Plant Cell Tissue Organ. Cult. 2020, 140, 49–67. [Google Scholar] [CrossRef]
- de Araújo Silva-Cardoso, I.M.; Gomes, A.C.M.M.; Scherwinski-Pereira, J.E. Cellular responses of oil palm genotypes during somatic embryogenesis involve participation of procambial cells, DNA demethylation, and auxin accumulation. Plant Cell Rep. 2022, 41, 1875–1893. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.; Galaz-Ávalos, R.M.; Peña-Cabrera, E.; Loyola-Vargas, V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal Behav. 2013, 8, e26998. [Google Scholar] [CrossRef]
- Pila Quinga, L.A.; Pacheco de Freitas Fraga, H.; do Nascimento Vieira, L.; Guerra, M.P. Epigenetics of long-term somatic embryogenesis in Theobroma cacao L.: DNA methylation and recovery of embryogenic potential. Plant Cell Tissue Organ. Cult. 2017, 131, 295–305. [Google Scholar] [CrossRef]
- Duarte-Aké, F.; De-La-Peña, C. Epigenetic Advances in Somatic Embryogenesis in Sequenced Genome Crops. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Cham, Switzerland, 2016; pp. 81–102. [Google Scholar]
- Jackson, J.P.; Lindroth, A.M.; Cao, X.; Jacobsen, S.E. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 2002, 416, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Molitor, A.M.; Bu, Z.; Yu, Y.; Shen, W.-H. Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLoS Genet. 2014, 10, e1004091. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, X.; Huang, H.; Xu, L. Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Arabidopsis Tissues. PLoS Genet. 2012, 8, e1002911. [Google Scholar] [CrossRef]
- Bratzel, F.; López-Torrejón, G.; Koch, M.; Del Pozo, J.C.; Calonje, M. Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr. Biol. 2010, 20, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.R.; Mehalick, A.J.; Packer, R.L.; Jenik, P.D. MicroRNAs regulate the timing of embryo maturation in Arabidopsis. Plant Physiol. 2011, 155, 1871–1884. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Cheng, Z.J.; Su, Y.H.; Han, H.N.; Zhang, Y.; Zhang, X.S. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 2011, 7, e1002243. [Google Scholar] [CrossRef]
- Jaligot, E.; Rival, A.; Beulé, T.; Dussert, S.; Verdeil, J.-L. Somaclonal variation in oil palm (Elaeis guineensis Jacq.): The DNA methylation hypothesis. Plant Cell Rep. 2000, 19, 684–690. [Google Scholar] [CrossRef]
- Wei, Q.; Shi, P.; Khan, F.S.; Htwe, Y.M.; Zhang, D.; Li, Z.; Wei, X.; Yu, Q.; Zhou, K.; Wang, Y. Cryopreservation and Cryotolerance Mechanism in Zygotic Embryo and Embryogenic Callus of Oil Palm. Forests 2023, 14, 966. [Google Scholar] [CrossRef]
- Weckx, S.; Inzé, D.; Maene, L. Tissue culture of oil palm: Finding the balance between mass propagation and somaclonal variation. Front. Plant Sci. 2019, 10, 722. [Google Scholar] [CrossRef]
- Punchihewa, P. Current status of the coconut industry. In Current Advances in Coconut Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 3–17. [Google Scholar]
- Bandupriya, H.; Iroshini, W.; Perera, S.; Vidhanaarachchi, V.; Fernando, S.; Santha, E.; Gunathilake, T. Genetic fidelity testing using SSR marker assay confirms trueness to type of micropropagated coconut (Cocos nucifera L.) plantlets derived from unfertilized ovaries. Open Plant Sci. J. 2017, 10, 46–54. [Google Scholar] [CrossRef][Green Version]
- Xiao, Y.; Xu, P.; Fan, H.; Baudouin, L.; Xia, W.; Bocs, S.; Xu, J.; Li, Q.; Guo, A.; Zhou, L. The genome draft of coconut (Cocos nucifera). Gigascience 2017, 6, gix095. [Google Scholar] [CrossRef] [PubMed]
- Lantican, D.V.; Strickler, S.R.; Canama, A.O.; Gardoce, R.R.; Mueller, L.A.; Galvez, H.F. De novo genome sequence assembly of dwarf coconut (Cocos nucifera L.‘Catigan Green Dwarf’) provides insights into genomic variation between coconut types and related palm species. G3 Genes Genom. Genet. 2019, 9, 2377–2393. [Google Scholar] [CrossRef] [PubMed]
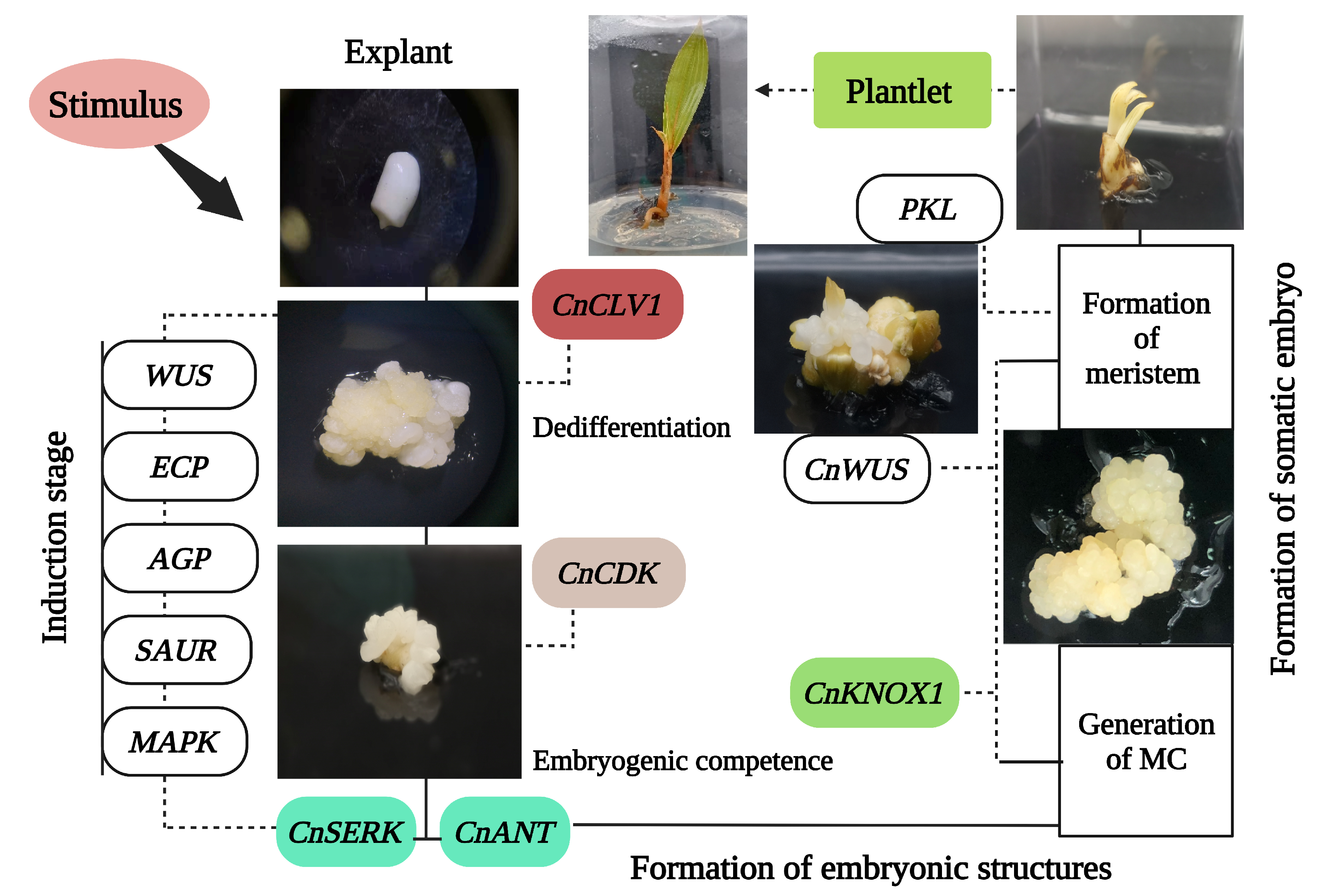
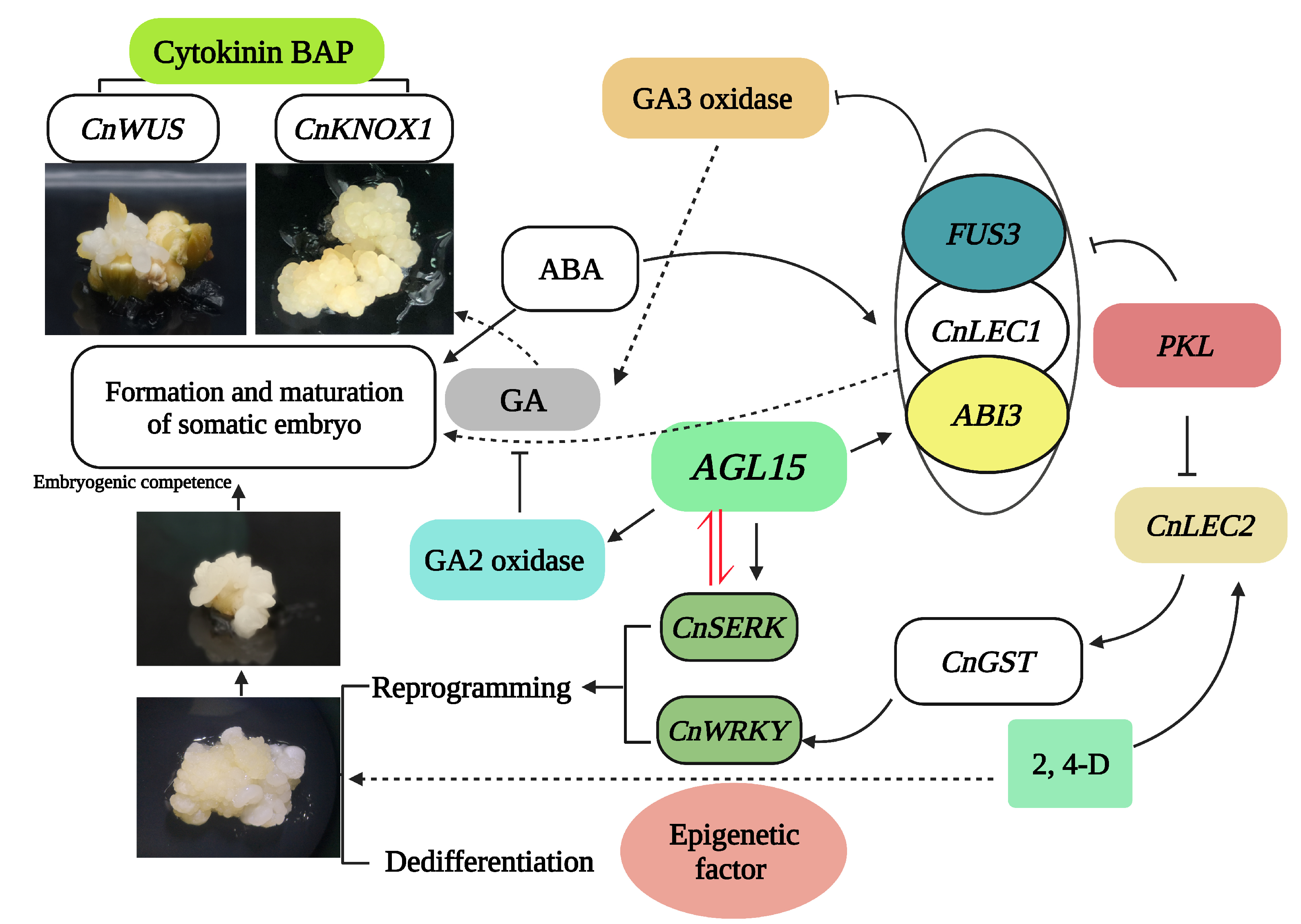

| Expression Pattern | Gene | Abbreviation | Reference |
|---|---|---|---|
| SE developmental stage | WUSCHEL | CnWUS | [69] |
| Callus tissues at the initiation stage of SE | SOMATIC EMBRYOGENESIS RECEPTOR-like KINASE | CnSERK | [70] |
| Early stages of callus formation | CLAVATA | CLV | [69] |
| Embryogenic calli | AINTEGUMENTA-like | ANT | [69] |
| Globular and coleoptilar SE growth | KNOTTED-like homeobox | CnKNOX | [71] |
| SE developmental stage | GLUTATHIONE STRANSFERASE | GST | [69] |
| Embryogenic callus and germinated | Cyclin-Dependent Kinases | CnCDK | [72] |
| embryogenic calli | MITOGEN-ACTIVATED PROTEIN KINASE | MAPK | [69] |
| APETALA2/ETHYLENE RESPONSIVE FACTOR | AP2/ERF | [69] | |
| SAUR Family Protein | SAUR | [69] | |
| EMBRYOGENIC CELL PROTEIN | ECP | [69] | |
| LATE EMBRYOGENESIS ABUNDANT PROTEIN | LEA | [69] | |
| ARABINOGALACTAN PROTEIN | AGP | [69] | |
| SE developmental stage | WRKY transcription factor | WRKY | [69] |
| GERMIN-LIKE PROTEIN | GLP | [69] | |
| Embryogenic and non-embryogenic calli | MicroRNAs | miRNAs | [73] |
| SE developmental stage | PICKLE | PKL | [69] |
| SE Development Stage | Gene | Coconut Dwarf Accession | Gene Accession | Molecular and Biological Function |
|---|---|---|---|---|
| Cell cycle | CDK | AZ04G0076960 AZ13G0236040 | AT1G15570 AT1G18040 AT1G20930 AT1G76540 | G2/M transition of the mitotic cell cycle, protein binding, regulation of cell cycle, regulation of G2/M transition of the mitotic cell cycle |
| Dedifferentiation | WUS | AZ11G0210850 | AT2G17950 | Stem cell population maintenance, DNA-binding transcription factor activity, protein binding |
| CLV3 | AT2G27250 | Cell differentiation, cell–cell signaling involved in cell fate commitment, protein binding | ||
| WOX5 | AZ03G0055410 | AT4G32980 AT3G11260 | Positive regulation of stem cell population maintenance, response to auxin, DNA-binding transcription factor activity | |
| AIL | AZ01G0008180 | AT1G72570 AT3G20840 | DNA binding, regulation of transcription factor activity | |
| Quiescent center (QC) specification and stem cell activity, DNA binding | ||||
| BBM | AZ07G0145330 | AT5G17430 | Cell population proliferation, DNA-binding transcription factor activity | |
| CDK | AZ04G0076960 AZ13G0236040 | AT1G73690 | Involved in cell cycle regulation and cell differentiation, protein binding, | |
| Totipotent potential acquisition | WUS | AZ11G0210850 | AT2G17950 | Stem cell population maintenance, DNA-binding transcription factor activity, protein binding |
| LEC1 | AZ07G0152850 AZ05G0112880 | AT1G21970 | Somatic embryogenesis, DNA binding | |
| Meristem maintenance | KNOX | AZ10G0201430 AZ02G0037220 | AT1G62990 AT1G14760 | Leaf proximal/distal pattern formation, DNA binding |
| STM | AZ07G0160530 | AT1G75410 AT2G23760 AT2G35940 AT3G54220 | DNA binding, regulation of timing of the transition from vegetative to reproductive phase | |
| AS2 | AZ14G0257890 | AT1G65620 | Protein binding, proximal/distal pattern formation | |
| WUS | AZ11G0210850 | AT2G17950 | Stem cell population maintenance, DNA-binding transcription factor activity, protein binding | |
| Somatic embryo | SERK | AZ15G0264500 | AT1G71830 | Protein phosphorylation and protein kinase binding, hormonal signaling pathway, brassinosteroid homeostasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.S.; Li, Z.; Shi, P.; Zhang, D.; Htwe, Y.M.; Yu, Q.; Wang, Y. Transcriptional Regulations and Hormonal Signaling during Somatic Embryogenesis in the Coconut Tree: An Insight. Forests 2023, 14, 1800. https://doi.org/10.3390/f14091800
Khan FS, Li Z, Shi P, Zhang D, Htwe YM, Yu Q, Wang Y. Transcriptional Regulations and Hormonal Signaling during Somatic Embryogenesis in the Coconut Tree: An Insight. Forests. 2023; 14(9):1800. https://doi.org/10.3390/f14091800
Chicago/Turabian StyleKhan, Faiza Shafique, Zhiying Li, Peng Shi, Dapeng Zhang, Yin Min Htwe, Qun Yu, and Yong Wang. 2023. "Transcriptional Regulations and Hormonal Signaling during Somatic Embryogenesis in the Coconut Tree: An Insight" Forests 14, no. 9: 1800. https://doi.org/10.3390/f14091800
APA StyleKhan, F. S., Li, Z., Shi, P., Zhang, D., Htwe, Y. M., Yu, Q., & Wang, Y. (2023). Transcriptional Regulations and Hormonal Signaling during Somatic Embryogenesis in the Coconut Tree: An Insight. Forests, 14(9), 1800. https://doi.org/10.3390/f14091800








