A Statistical Approach to Macrofungal Diversity in a Mediterranean Ecosystem of the Iberian Peninsula Dominated by the Holm Oak (Quercus ilex L. subsp. ballota (Desf.) Samp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Meteorological Data
2.4. Statistical Procedure
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolton, J. An History of Fungusses, Growing about Halifax; Author and sold in Halifax; Ulan Press: Halifax, UK, 1788; Volume 1. [Google Scholar]
- Bel, G.; Hagberg, A.; Meron, E. Gradual regime shifts in spatially extended ecosystems. Theor. Ecol. 2012, 5, 591–604. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Morin, P.J. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102. [Google Scholar] [PubMed]
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar]
- Opdam, P.; Wiens, J.A. Fragmentation, habitat loss and landscape management. In Conserving Bird Biodiversity: General Principles and Their Application; Norris, K., Pain, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 202–223. [Google Scholar]
- Beule, L.; Grüning, M.; Karlovsky, P.; lM-Arnold, A. Changes of Scots pine phyllosphere and soil fungal communities during outbreaks of defoliating insects. Forests 2017, 8, 316. [Google Scholar]
- Kauserud, H.; Stige, L.C.; Vik, J.O.; Økland, R.H.; Høiland, K.; Stenseth, N.C. Mushroom fruiting and climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 3811–3814. [Google Scholar] [CrossRef]
- Kauserud, H.; Heegaard, E.; Semenov, M.A.; Boddy, L.; Halvorsen, R.; Stige, L.C.; Stenseth, N.C. Climate change and spring-fruiting fungi. Proc. R. Soc. B. 2009, 277, 1169–1177. [Google Scholar] [CrossRef]
- Kauserud, H.; Heegaard, E.; Büntgen, U.; Halvorsen, R.; Egli, S.; Senn-Irlet, B.; Høiland, K. Warming-induced shift in European mushroom fruiting phenology. Proc. Natl. Acad. Sci. USA 2012, 109, 14488–14493. [Google Scholar]
- Jang, S.K.; Kim, S.W. Relationship between Ectoycorrhizal Fruiting Bodies and Climatic and Environmental Factors in Naejangsan National Park. Mycobiology 2015, 43, 122–134. [Google Scholar] [CrossRef]
- Barnes, C.J.; van der Gast, C.J.; McNamara, N.P.; Rowe, R.; Bending, G.D. Extreme rainfall affects assembly of the root-associated fungal community. New Phytol. 2018, 220, 1172–1184. [Google Scholar]
- Shi, L.L.; Mortimer, P.E.; Slik, J.F.; Zou, X.M.; Xu, J.; Feng, W.T.; Qiao, L. Variation in forest soil fungal diversity along a latitudinal gradient. Fungal Divers. 2014, 64, 305–315. [Google Scholar]
- Angelini, P.; Compagno, R.; Arcangeli, A.; Bistocchi, G.; Gargano, M.L.; Venanzoni, R.; Venturella, G. Macrofungal diversity and ecology in two Mediterranean forest ecosystems. Plant Biosyst. 2016, 150, 540–549. [Google Scholar] [CrossRef]
- Buée, M.; De Boer, W.; Martin, F.; Van Overbeek, L.; Jurkevitch, E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 2009, 321, 189–212. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Kjøller, R.; Nilsson, L.O.; Hansen, K.; Schmidt, I.K.; Vesterdal, L.; Gundersen, P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol. 2012, 194, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Vaquerizo, M.D.; Gallardo, A. Nitrógeno orgánico disuelto en comunidades vegetales del SO de la Península Ibérica. Cuad. Soc. Esp. Cienc. For. 2008, 25, 153–159. [Google Scholar]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlák, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Egli, S.; Peter, M.; Buser, C.; Stahel, W.; Ayer, F. Mushroom picking does not impair future harvests–results of a long-term study in Switzerland. Biol. Conserv. 2006, 129, 271–276. [Google Scholar] [CrossRef]
- García Jiménez, P.; Fernández Ruiz, A.; Sánchez Sánchez, J.; Rodríguez de la Cruz, D. Mycological Indicators in Evaluating Conservation Status: The Case of Quercus spp. Dehesas in the Middle-West of the Iberian Peninsula (Spain). Sustainability 2020, 12, 10442. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Yuan, H.; Lian, B. The effect of environmental contamination on the community structure and fructification of ectomycorrhizal fungi. Microbiol. Open 2017, 6, e00396. [Google Scholar] [CrossRef]
- Zelnik, Y.R.; Meron, E.; Bel, G. Localized states qualitatively change the response of ecosystems to varying conditions and local disturbances. Ecol. Complex. 2016, 25, 26–34. [Google Scholar] [CrossRef]
- Mehus, H. Fruit Body Production of Macrofungi in Some North Norwegian Forest Types. Nord. J. Bot. 1986, 6, 679–702. [Google Scholar] [CrossRef]
- Richard, F.; Roy, M.; Shahin, O.; Sthultz, C.; Duchemin, M.; Joffre, R.; Selosse, M.A. Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: Seasonal dynamics and response to drought in the surface organic horizon. Ann. For. Sci. 2011, 68, 57–68. [Google Scholar] [CrossRef]
- Perotto, S.; Angelini, P.; Bianciotto, V.; Bonfante, P.; Girlanda, M.; Kull, T. Interaction of fungi with other organisms. Plant Biosyst. 2013, 147, 208–218. [Google Scholar] [CrossRef][Green Version]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis; Elsevier: New York, NY, USA, 2008. [Google Scholar]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestial Ecosystem Ecology, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Markkola, A.M.; Saravesi, K.; Aikio, S.; Taulavuori, E.; Taulavuori, K. Light-driven host-symbiont interactions under hosts’ range shifts caused by global warming: A review. Environ. Exp. Bot. 2016, 121, 48–55. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Moreno, G.; Manjón, J.L.; Álvarez-Jiménez, J. Los hongos y el cambio climático. In Desert Truffle; Kagan-Zur, V., Sitrit, Y., Roth-Bejerano, N.A., Morte, Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2013; pp. 129–135. [Google Scholar]
- Capel Molina, J.J. Los Climas de España; Oikos-tau. S.A.: Barcelona, Spain, 1981. [Google Scholar]
- State Meteorological Agency. Monthly Weather Forecast; Ministry of Agriculture, Food and Environment. Territorial Delegation of Castile & Leon: Valladolid, Spain, 2012. [Google Scholar]
- Kirk, P.M.; Cannon, P.; Minter, D.; Stalpers, J. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Fernández, A.; Sánchez, S.; García, P.; Sánchez, J. Macrofungal diversity in an isolated and fragmented Mediterranean Forest ecosystem. Plant Biosyst. 2020, 154, 139–148. [Google Scholar] [CrossRef]
- Castile & Leon InfoRiego System Network. Available online: http://www.inforiego.org/opencms/opencms/estaciones/listado/index.html (accessed on 1 November 2015).
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2016. Available online: https://www.R-project.org/ (accessed on 5 February 2018).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. R Package Version 2.4-2. Vegan: Community Ecology Package. 2017. Available online: https://cran.r-project.org/ (accessed on 5 January 2018).
- Vicente-Villardón, J.L. MultBiplot(R): Multivariate Analysis Using Biplots. R Package Version 0.3.3. 2016. Available online: http://biplot.usal.es/classicalbiplot/multbiplot-in-r/ (accessed on 5 January 2018).
- Vicente-Villardón, J.L. MULTBIPLOT: A package for Multivariate Analysis using Biplots. Statistics Department. University of Salamanca: Salamanca, Spain. 2016. Available online: http://biplot.usal.es/ClassicalBiplot/index.html (accessed on 6 January 2018).
- Eyssartier, G.; Roux, P. Le Guide des Champignons: France et Europe, 1st ed.; Belin: Périgueux, France, 2011. [Google Scholar]
- Courtecuisse, R.; Duhem, B. Guide des Champignons de France et d’Europe: 1752 Espèces Décrites et Illustrées; Delachaux & Niéstlé: Paris, France, 1994. [Google Scholar]
- Hering, T.F. The terricolous higher fungi of four lake district woodlands. Trans. Br. Mycol. Soc. 1966, 49, 369–383. [Google Scholar] [CrossRef]
- Durall, D.M.; Gamiet, S.; Simard, S.W.; Kudrna, L.; Sakakibara, S.M. Effects of clearcut logging and tree species composition on the diversity and community composition of epigeous fruit bodies formed by ectomycorrhizal fungi. Botany 2006, 84, 966–980. [Google Scholar] [CrossRef]
- Gabel, A.C.; Gabel, M.L. Comparison of diversity of macrofungi and vascular plants at seven sites in the Black Hills of South Dakota. Am. Midl. Nat. 2007, 157, 258–296. [Google Scholar] [CrossRef]
- Halme, P.; Heilmann-Clausen, J.; Rämä, T.; Kosonen, T.; Kunttu, P. Monitoring fungal biodiversity—Towards an integrated approach. Fungal Ecol. 2012, 5, 750–758. [Google Scholar] [CrossRef]
- Martin-Vide, J.; Lopez-Bustins, J.A. The western Mediterranean oscillation and rainfall in the Iberian Peninsula. Int. J. Climatol. 2006, 26, 1455–1475. [Google Scholar] [CrossRef]
- Gange, A.C.; Gange, E.G.; Sparks, T.H.; Boddy, L. Rapid and recent changes in fungal fruiting patterns. Science 2007, 316, 71. [Google Scholar] [CrossRef] [PubMed]
- Tuno, N.; Akaishi, D.; Kimura, M.T. Abundance and phenology of macrofungal fruiting bodies in central and northern Japan. Mycoscience. 2020, 61, 331–336. [Google Scholar] [CrossRef]
- Sanz-Benito, I.; Stadler, T.; Mediavilla, O.; Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Dejene, T.; Geml, J.; Martín-Pinto, P. Into the void: ECM fungal communities involved in the succession from rockroses to oak stands. Sci Rep 2023, 13, 10085. [Google Scholar] [CrossRef]
- Sato, H.; Morimoto, S.; Hattori, T. A thirty-year survey reveals that ecosystem function of fungi predicts phenology of mushroom fruiting. PLoS ONE 2012, 7, e49777. [Google Scholar] [CrossRef]
- Sebastiana, M.; da Silva, A.B.; Matos, A.R.; Alcântara, A.; Silvestre, S.; Malhó, R. Ectomycorrhizal inoculation with Pisolithus tinctorius reduces stress induced by drought in cork oak. Mycorrhiza 2018, 28, 247–258. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Gonçalves, A.; Louro, R. Canopy cover influence on macrofungal richness and sporocarp production in montado ecosystems. Agroforest Syst. 2011, 82, 149–159. [Google Scholar] [CrossRef]
- Sanz, A.; Fernández Ruiz, A.; García Jiménez, P.; Sánchez Sánchez, J.; Rodríguez de la Cruz, D. Preliminary survey of macrofungal diversity in El Monte de Villoria (Salamanca, Spain). Bot. Complut. 2022, 46, e80421. [Google Scholar] [CrossRef]
- Boddy, L.; Heilmann-Clausen, J. Basidiomycete Community Development in Temperate Angiosperm Wood. Br. Mycol. Soc. Symp. Ser. 2008, 28, 211–237. [Google Scholar]
- Fischer, M.; González García, V. An annotated checklist of European basidiomycetes related to white rot grapevine (Vitis vinifera). Phytol. Mediterr. 2011, 54, 281–298. [Google Scholar]
- Sabaté, S.; Gracia, C.A.; Sánchez, A. Likely effects of climate change on growth of Quercus ilex, Pinus halepensis, Pinus pinaster, Pinus sylvestris and Fagus sylvatica forests in the Mediterranean region. For. Ecol. Manag. 2002, 162, 23–37. [Google Scholar] [CrossRef]
- Pickles, B.J.; Egger, K.N.; Massicotte, H.B.; Green, D.S. Ectomycorrhizas and climate change. Fungal Ecol. 2012, 5, 73–84. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Fang, C.; Smith, P.; Moncrieff, J.B.; Smith, J.U. Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 2005, 433, 57–59. [Google Scholar] [CrossRef]
- Limousin, J.M.; Rambal, S.; Ourcival, J.M.; Joffre, R. Modelling rainfall interception in a Mediterranean Quercus ilex ecosystem: Lesson from a throughfall exclusion experiment. J. Hydrol. 2008, 357, 57–66. [Google Scholar] [CrossRef]
- Misson, L.; Rocheteau, A.; Rambal, S.; Ourcival, J.M.; Limousin, J.M.; Rodriguez, R. Functional changes in the control of carbon fluxes after 3 years of increased drought in a Mediterranean holm forest. Glob. Chang. Biol. 2010, 16, 2461–2475. [Google Scholar]
- Sardans, J.; Penuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Barnard, R.; Le Roux, X.; Hungate, B.A.; Cleland, E.E.; Blankinship, J.C.; Barthes, L.; Leadley, P.W. Several components of global change alter nitrifying and denitrifying activities in an annual grassland. Funct. Ecol. 2006, 20, 557–564. [Google Scholar] [CrossRef]
- Salerni, E.; Laganà, A.; Perini, C.; Loppi, S.; Dominicis, V.D. Effects of temperature and rainfall on fruiting of macrofungi in oak forests of the Mediterranean area. Isr. J. Plant Sci. 2002, 50, 189–198. [Google Scholar] [CrossRef]
- Su, N.; Jarvie, S.; Yan, Y.; Gong, X.; Li, F.; Han, P.; Zhang, Q. Landscape context determines soil fungal diversity in a fragmented habitat. Catena 2022, 213, 106163. [Google Scholar] [CrossRef]
- Kirschbaum, M.U. Forest growth and species distribution in a changing climate. Tree Physiol. 2000, 20, 309–322. [Google Scholar] [CrossRef]
- Martin, F.; Cullen, D.; Hibbett, D.; Pisabarro, A.; Spatafora, J.W.; Baker, S.E.; Grigoriev, I.V. Sequencing the fungal tree of life. New Phytol. 2011, 190, 818–821. [Google Scholar] [CrossRef]
- Reverchon, F.; del Ortega-Larrocea, P.M.; Pérez-Moreno, J. Saprophytic fungal communities change in diversity and species composition across a volcanic soil chronosequence at Sierra del Chichinautzin, Mexico. Ann. Microbiol. 2010, 60, 217–226. [Google Scholar] [CrossRef]
- Birgander, J.; Rousk, J.; Olsson, P.A. Comparison of fertility and seasonal effects on grassland microbial communities. Soil Biol. Biochem. 2014, 76, 80–89. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Durling, M.B.; Clemmensen, K.E.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef]
- Adamo, I.; Dashevskaya, S.; Alday, J.G. Fungal perspective of pine and oak colonization in Mediterranean degraded ecosystems. Forests 2022, 13, 88. [Google Scholar] [CrossRef]
- Braga-Neto, R.; Luizão RC, C.; Magnusson, W.E.; Zuquim, G.; de Castilho, C.V. Leaf litter fungi in a Central Amazonian forest: The influence of rainfall, soil and topography on the distribution of fruiting bodies. Biodivers Conserv. 2008, 17, 2701–2712. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Rosa-Gruszecka, A.; Gawryś, R.; Horak, J. Effect of soil properties and vegetation characteristics in determining the frequency of Burgundy truffle fruiting bodies in Southern Poland. Écoscience 2019, 26, 113–122. [Google Scholar] [CrossRef]
- Ponce, Á.; Alday, J.G.; Martínez de Aragón, J.; Collado, E.; Morera, A.; Bonet, J.A.; de-Miguel, S. Environmental drivers shaping the macrofungal sporocarp community in Mediterranean Quercus ilex stands. For. Ecol. Manag. 2022, 524, 120523. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Friesen, J.; Gamiet, S.; Kroeger, P. Epigeous fruiting bodies of ectomycorrhizal fungi as indicators of soil fertility and associated nitrogen status of boreal forests. Mycorrhiza 2009, 19, 535–548. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Magarzo, A.; Sanz-Benito, I.; Mediavilla, O.; Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Dejene, T.; Martín-Pinto, P. Mosaic forest management at landscape scale to enhance fungal diversity and production, in a context of forest fire-prevention in Mediterranean ecosystems. Ecol. Indic. 2023, 150, 110272. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Gang, L.; Chen, H.Y. Advanced research tools for fungal diversity and its impact on forest ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 45044–45062. [Google Scholar] [CrossRef]
- Gonçalves, S.C.; Haelewaters, D.; Furci, G.; Mueller, G.M. Include all fungi in biodiversity goals. Science 2021, 373, 403. [Google Scholar] [CrossRef]
- Kuikka, K.; Härmä, E.; Markkola, A.; Rautio, P.; Roitto, M.; Saikkonen, K.; Ahonen-Jonnarth, U.; Finlay, R.; Tuomi, J. Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 2003, 84, 2051–2061. [Google Scholar] [CrossRef]
- Sarrionandia, E.; Salcedo, I. Macrofungal diversity of holm-oak forests at the northern limit of their distribution range in the Iberian Peninsula. Scand. J. For. Res. 2018, 33, 23–31. [Google Scholar] [CrossRef]
- Sun, W.; Yang, B.; Zhu, Y.; Wang, H.; Qin, G.; Yang, H. Ectomycorrhizal fungi enhance the tolerance of phytotoxicity and cadmium accumulation in oak (Quercus acutissima Carruth.) seedlings: Modulation of growth properties and the antioxidant defense responses. Environ. Sci. Pollut. Res. 2022, 29, 6526–6537. [Google Scholar] [CrossRef] [PubMed]


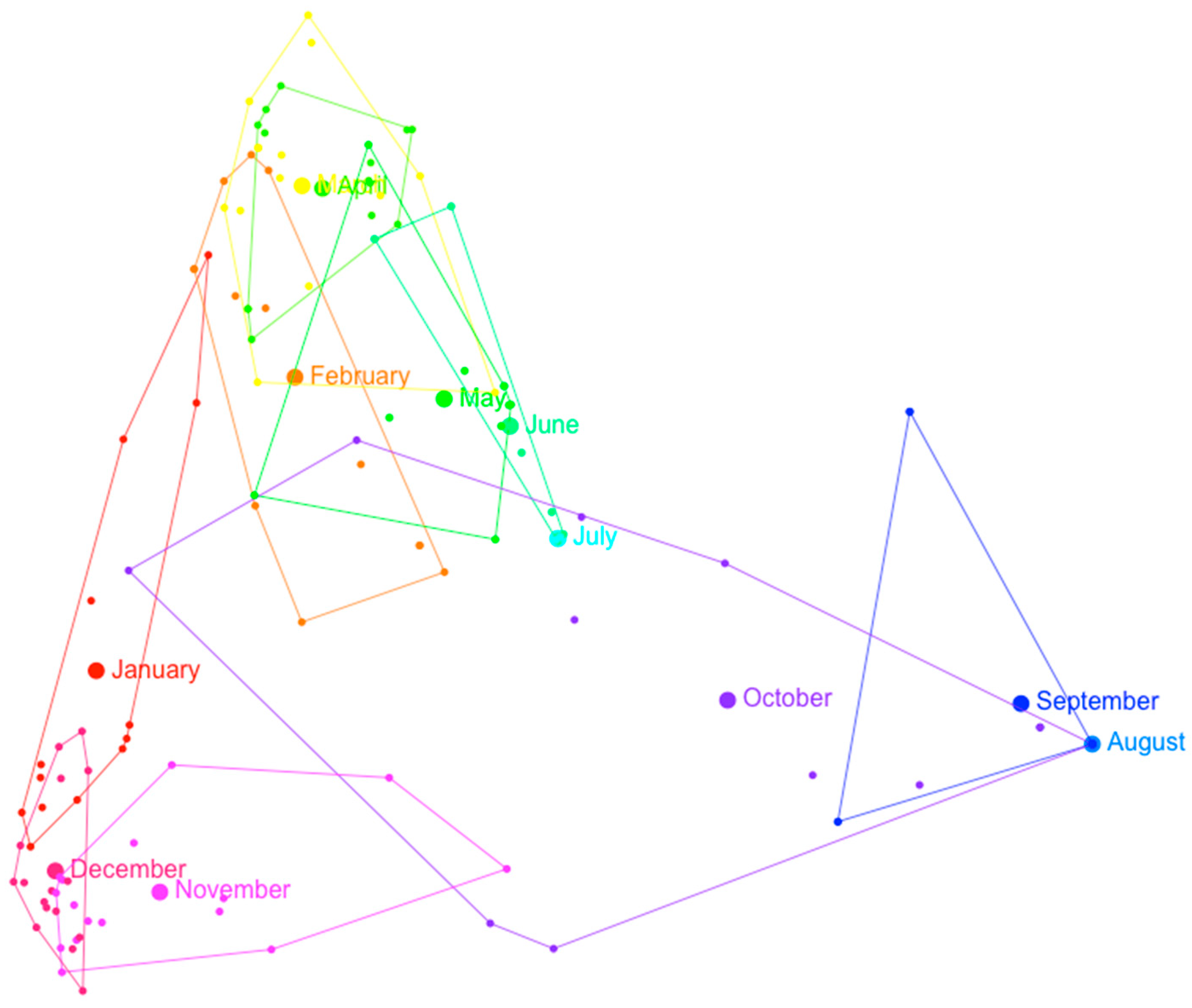



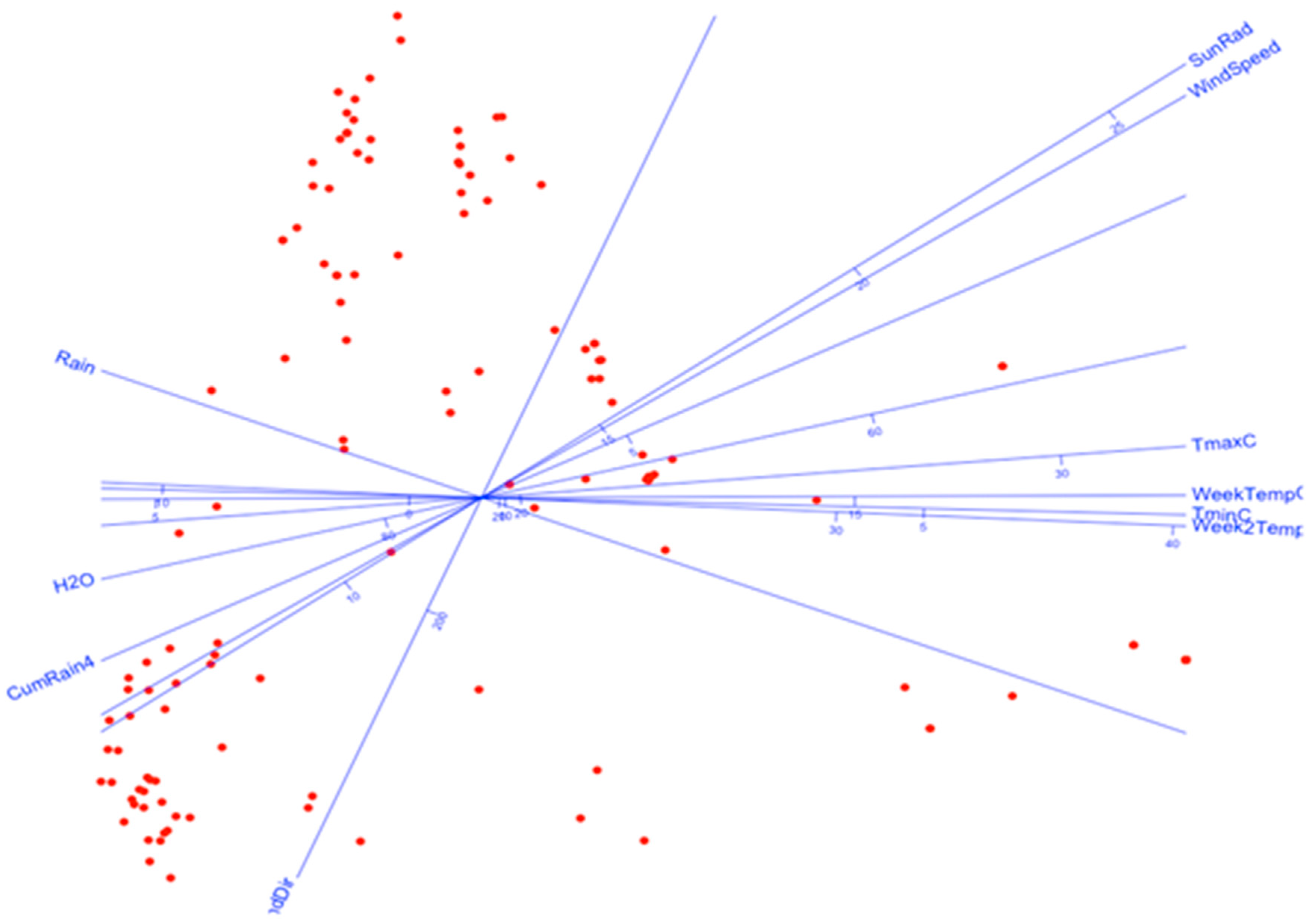


| Group (Subgroup) | Week | Date | N Taxa |
|---|---|---|---|
| A (Upper subgroup) | 154 | 6 December 2011 | 29 |
| A (Lower subgroup) | 204 | 19 November 2012 | 43 |
| B (Left subgroup) | 174 | 26 April 2012 | 10 |
| B (Right subgroup) | 167 | 6 March 2012 | 2 |
| C | 72 | 11 May 2010 | 3 |
| D | 38 | 15 September 2009 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ruiz, A.; Vicente-Villardón, J.L.; Sánchez-Sánchez, J.; García-Jiménez, P.; Sánchez-Durán, S.; Rodríguez-de la Cruz, D. A Statistical Approach to Macrofungal Diversity in a Mediterranean Ecosystem of the Iberian Peninsula Dominated by the Holm Oak (Quercus ilex L. subsp. ballota (Desf.) Samp.). Forests 2023, 14, 1662. https://doi.org/10.3390/f14081662
Fernández-Ruiz A, Vicente-Villardón JL, Sánchez-Sánchez J, García-Jiménez P, Sánchez-Durán S, Rodríguez-de la Cruz D. A Statistical Approach to Macrofungal Diversity in a Mediterranean Ecosystem of the Iberian Peninsula Dominated by the Holm Oak (Quercus ilex L. subsp. ballota (Desf.) Samp.). Forests. 2023; 14(8):1662. https://doi.org/10.3390/f14081662
Chicago/Turabian StyleFernández-Ruiz, Abel, José Luis Vicente-Villardón, José Sánchez-Sánchez, Prudencio García-Jiménez, Sergio Sánchez-Durán, and David Rodríguez-de la Cruz. 2023. "A Statistical Approach to Macrofungal Diversity in a Mediterranean Ecosystem of the Iberian Peninsula Dominated by the Holm Oak (Quercus ilex L. subsp. ballota (Desf.) Samp.)" Forests 14, no. 8: 1662. https://doi.org/10.3390/f14081662
APA StyleFernández-Ruiz, A., Vicente-Villardón, J. L., Sánchez-Sánchez, J., García-Jiménez, P., Sánchez-Durán, S., & Rodríguez-de la Cruz, D. (2023). A Statistical Approach to Macrofungal Diversity in a Mediterranean Ecosystem of the Iberian Peninsula Dominated by the Holm Oak (Quercus ilex L. subsp. ballota (Desf.) Samp.). Forests, 14(8), 1662. https://doi.org/10.3390/f14081662








