Optimized Ultrasound-Assisted Extraction of Psidium laruotteanum Roots: A Concentrated Source of Piceid from the Brazilian Savanna
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Chemicals
2.3. Ultrasound-Assisted Extraction (UAE) of Piceid
2.4. Determination of Piceid
2.5. Design of Experiments
2.6. Performance of the Method
2.7. Extraction Kinetics
3. Results
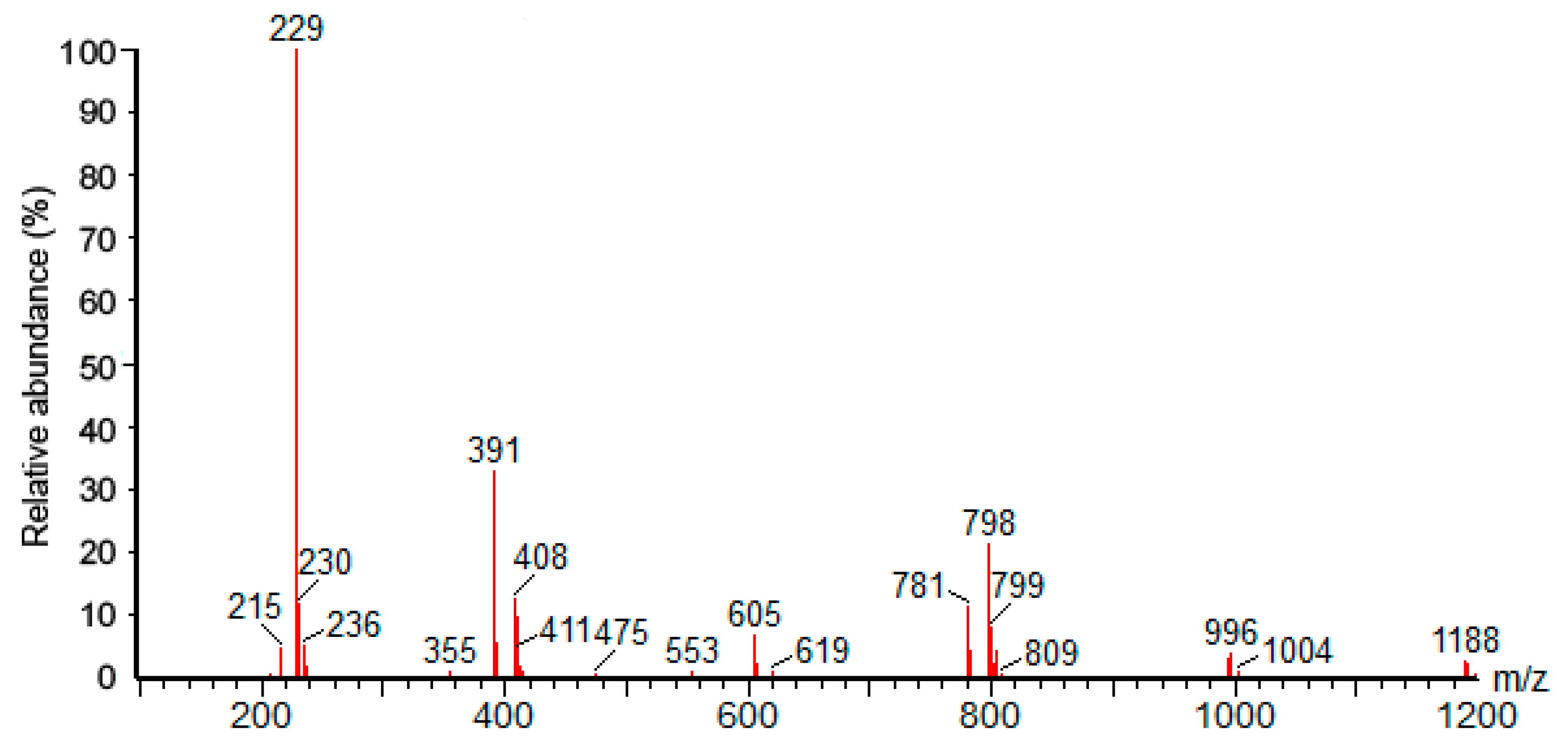
3.1. Identification of Piceid
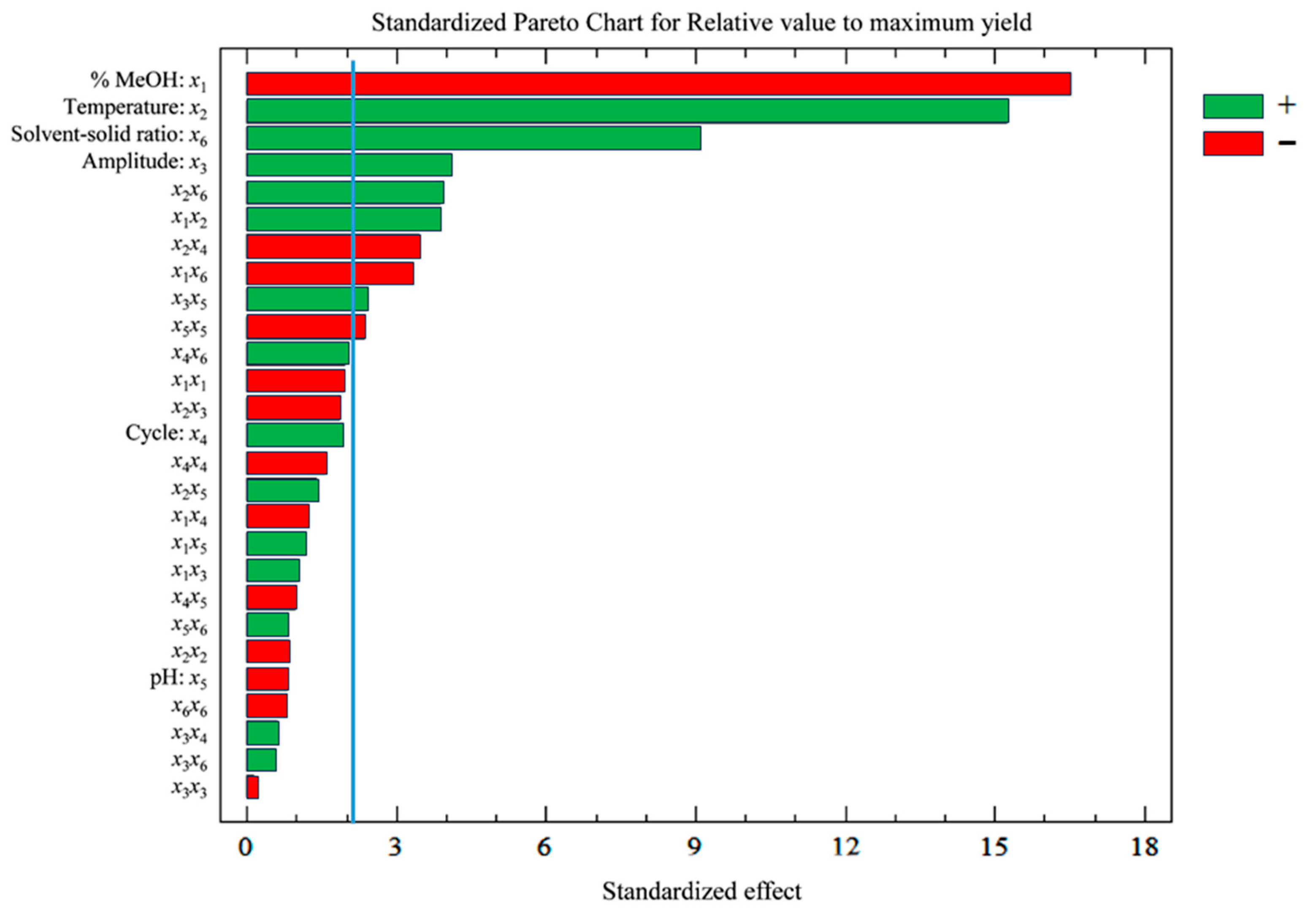
3.2. Development of the UAE Method
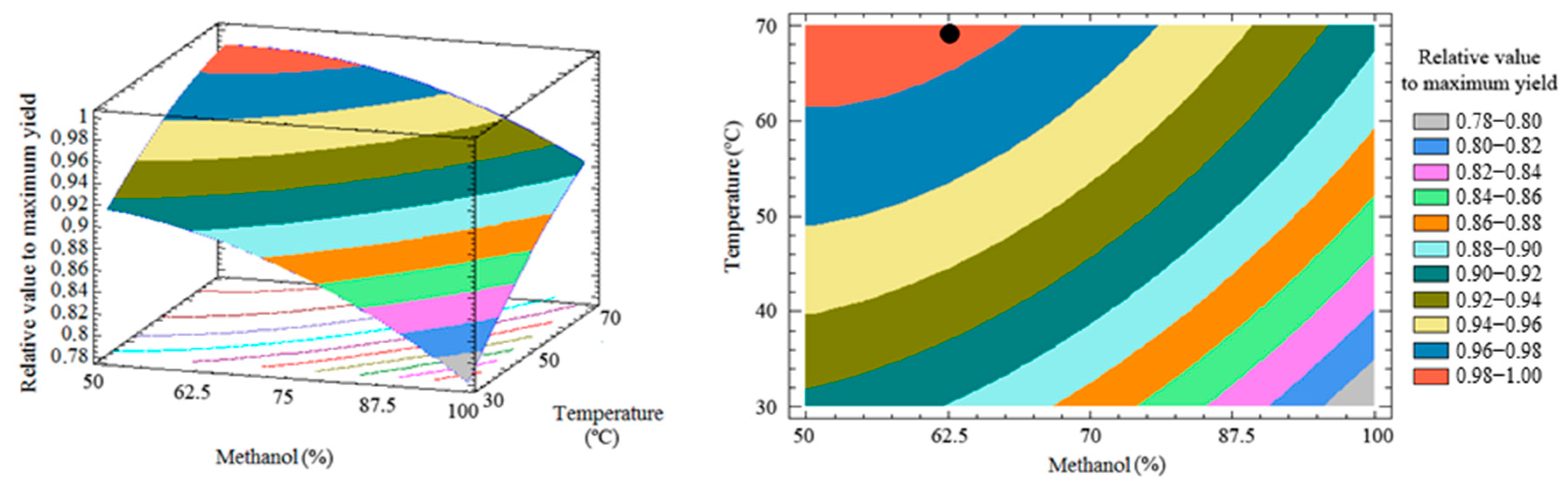
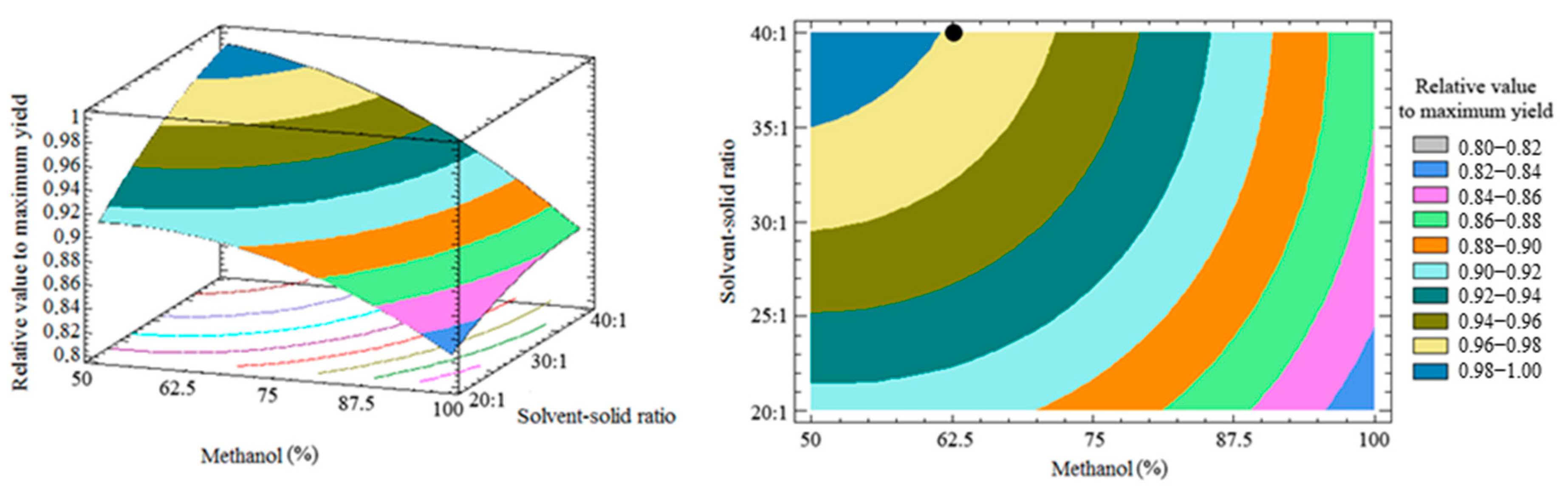
3.3. Response Surface and Optimization of the Method
3.4. Optimal Condition and Method Validation
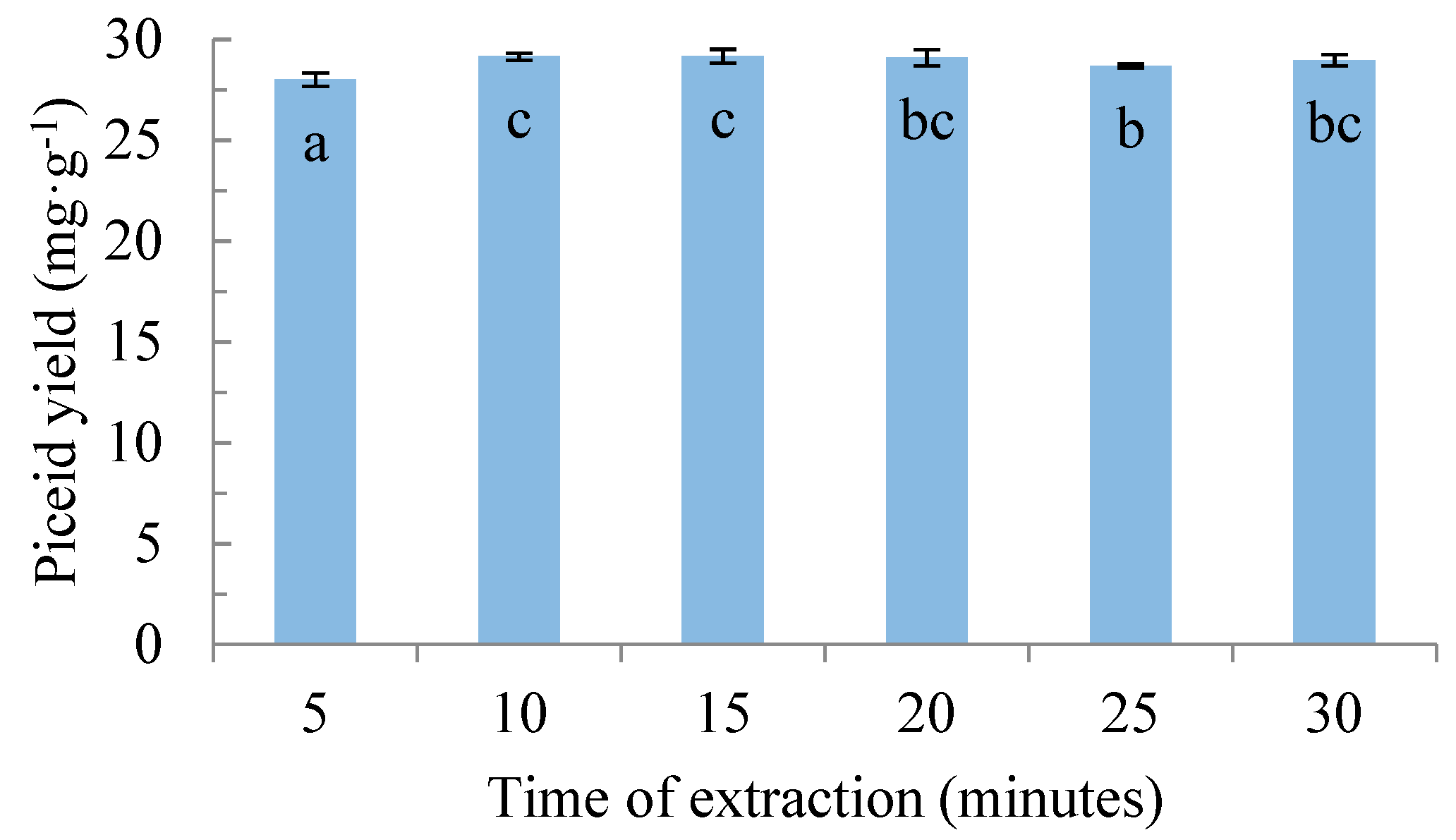
3.5. Extraction Kinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application—A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant Intervention to Improve Cognition in the Aging Brain: The Example of Hydroxytyrosol and Resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wang, W.; Tang, X.; Goh, R.M.; Thuya, W.L.; Ho, P.C.; Chen, L.; Wang, L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers 2023, 15, 2758. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, C.; Quincozes-Santos, A.; Basli, K.; Richard, T. Resveratrol and Neuroprotection. In Resveratrol: Sources, Production and Health Benefits; Delmas, D., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 399–417. [Google Scholar]
- Trindade, C.S.; Canas, S.; Inácio, M.L.; Pereira-Lorenzo, S.; Sousa, E.; Naves, P. Phenolic Compounds Regulating the Susceptibility of Adult Pine Species to Bursaphelenchus xylophilus . Forests 2022, 13, 500. [Google Scholar] [CrossRef]
- Shi, W.; He, W.; Zhang, Z.; Sun, J.; Zhu, C.; Liu, Z.; Xu, Y.; Zhao, B. Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis . Sustainability 2022, 14, 15193. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of Resveratrol Protects It from Enzymic Oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the Trans-Resveratrol and Trans-Piceid Content of Cocoa-Containing and Chocolate Products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef] [PubMed]
- Ibern-Gómez, M.; Roig-Pérez, S.; Lamuela-Raventós, R.M.; De La Torre-Boronat, M.C. Resveratrol and Piceid Levels in Natural and Blended Peanut Butters. J. Agric. Food Chem. 2000, 48, 6352–6354. [Google Scholar] [CrossRef]
- Ragab, A.S.; Van Fleet, J.; Jankowski, B.; Park, J.-H.; Bobzin, S.C. Detection and Quantitation of Resveratrol in Tomato Fruit (Lycopersicon esculentum Mill.). J. Agric. Food Chem. 2006, 54, 7175–7179. [Google Scholar] [CrossRef]
- Jerkovic, V.; Collin, S. Occurrence of Resveratrol and Piceid in American and European Hop Cones. J. Agric. Food Chem. 2007, 55, 8754–8758. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Andrés-Lacueva, C.; De La Carmen Torre-Boronat, M. Method for the Quantitative Extraction of Resveratrol and Piceid Isomers in Grape Berry Skins. Effect of Powdery Mildew on the Stilbene Content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Moreno-Labanda, J.F.; Mallavia, R.; Pérez-Fons, L.; Lizama, V.; Saura, D.; Micol, V. Determination of Piceid and Resveratrol in Spanish Wines Deriving from Monastrell (Vitis vinifera L.) Grape Variety. J. Agric. Food Chem. 2004, 52, 5396–5403. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, C.; Du, H.; Chen, Y.; Huang, X.; Gong, L.; You, P.; Deng, J.; Liu, Y.; Feng, H.; et al. Novel Functional Food from an Invasive Species Polygonum Cuspidatum: Safety Evaluation, Chemical Composition, and Hepatoprotective Effects. Food Qual. Saf. 2022, 6, fyac032. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Guerrero, R.F.; Fernández-Marin, M.I.; Cantos-Villar, E.; Palma, M. Ultrasound-Assisted Extraction of Stilbenoids from Grape Stems. J. Agric. Food Chem. 2013, 61, 12549–12556. [Google Scholar] [CrossRef] [PubMed]
- Francezon, N.; Meda, N.-S.-B.R.; Stevanovic, T. Optimization of Bioactive Polyphenols Extraction from Picea mariana Bark. Molecules 2017, 22, 2118. [Google Scholar] [CrossRef] [PubMed]
- Suprun, A.R.; Dubrovina, A.S.; Aleynova, O.A.; Kiselev, K.V. The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes. Metabolites 2021, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Atanasova, V.; Tardif, C.; Richard-Forget, F. Stilbenoids as Promising Natural Product-Based Solutions in a Race against Mycotoxigenic Fungi: A Comprehensive Review. J. Agric. Food Chem. 2023, 71, 5075–5092. [Google Scholar] [CrossRef]
- Psidium laruotteanum Cambess. Available online: http://servicos.jbrj.gov.br/flora/search/Psidium_laruotteanum (accessed on 10 June 2023).
- Imatomi, M.; Novaes, P.; Gualtieri, S.C.J. Interspecific Variation in the Allelopathic Potential of the Family Myrtaceae. Acta Bot. Bras. 2013, 27, 54–61. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Barbero, G.F.; Piñeiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Extraction of Natural Products: Principles and Fundamental Aspects; Royal Society of Chemistry: London, UK, 2013; ISBN 9781849736060. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Ultrasound-Assisted Extraction. In Natural Product Extraction. Principles and Applications; Rostagno, M., Prado, J., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 89–112. [Google Scholar]
- Liazid, A.; Schwarz, M.; Varela, R.M.; Palma, M.; Guillén, D.A.; Brigui, J.; Macías, F.A.; Barroso, C.G. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod. Process. 2010, 88, 247–252. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef]
- Ba, D.; Boyaci, I.H. Modeling and Optimization i: Usability of Response Surface Methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- ICH. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 2005; Volume 1994. [Google Scholar]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chang, C.-M.-J.; Deng, T.-S.; Chen, J.-H.; Shieh, C.-J. Optimized Ultrasound-Assisted Extraction of Phenolic Compounds from Polygonum Cuspidatum. Molecules 2014, 19, 67–77. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Beňová, B.; Adam, M.; Pavlíková, P.; Fischer, J. Supercritical Fluid Extraction of Piceid, Resveratrol and Emodin from Japanese Knotweed. J. Supercrit. Fluids 2010, 51, 325–330. [Google Scholar] [CrossRef]
- Viganó, J.; Sanches, V.L.; de Souza Mesquita, L.M.; de Souza, M.C.; da Silva, L.C.; Chaves, J.O.; Forster-Carneiro, T.; Rostagno, M.A. Comprehensive Analysis of Phenolic Compounds from Natural Products: Integrating Sample Preparation and Analysis. Anal. Chim. Acta 2021, 1178, 338845. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of Trans-Resveratrol in Grapes by Pressurised Liquid Extraction and Fast High-Performance Liquid Chromatography. J. Chromatogr. A 2006, 1110, 61–65. [Google Scholar] [CrossRef]
- Flores, I.R.; Vásquez-Murrieta, M.S.; Franco-Hernández, M.O.; Márquez-Herrera, C.E.; Ponce-Mendoza, A.; del Socorro López-Cortéz, M. Bioactive Compounds in Tomato (Solanum lycopersicum) Variety Saladette and Their Relationship with Soil Mineral Content. Food Chem. 2021, 344, 128608. [Google Scholar] [CrossRef] [PubMed]
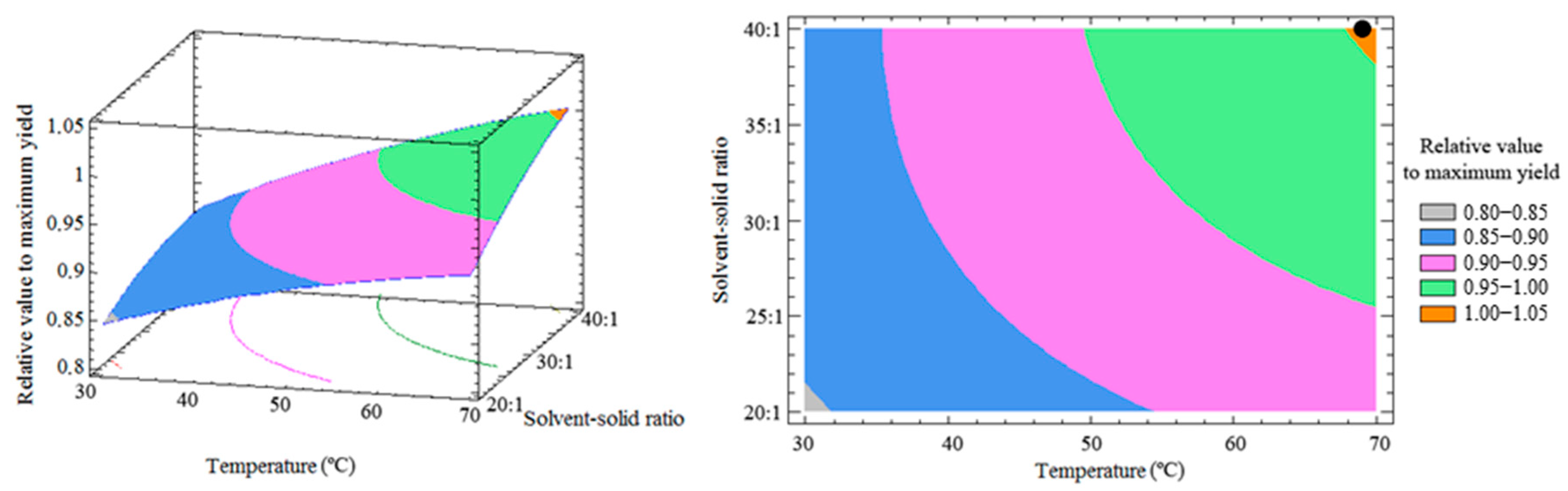
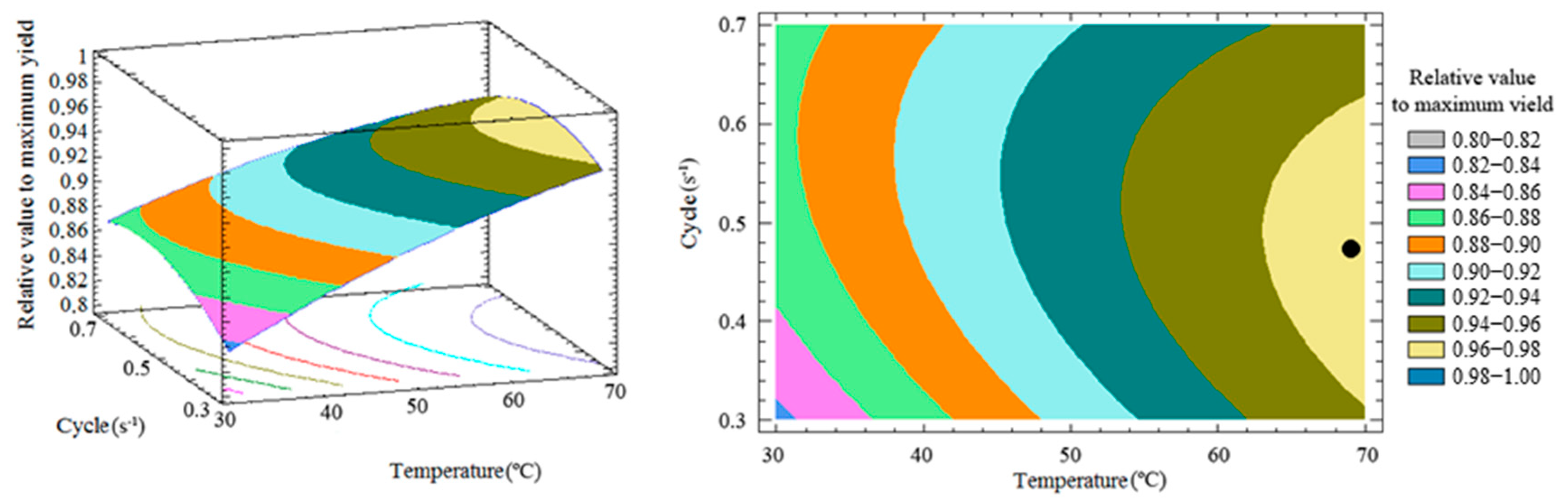
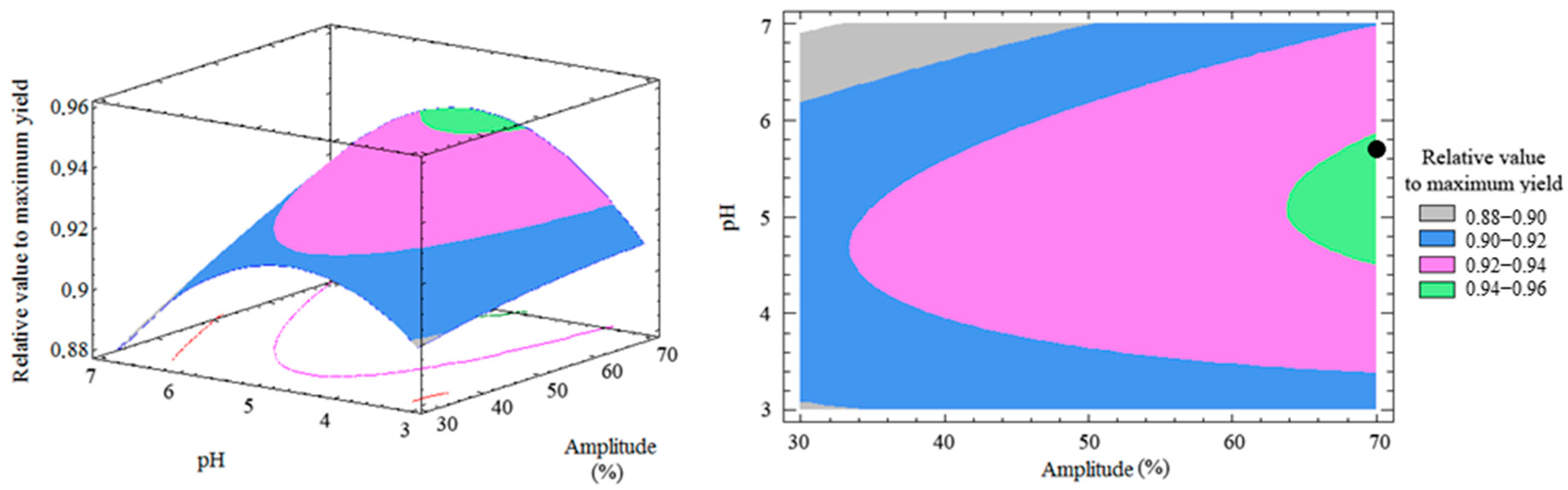
| DOE Runs | Extraction Factors | Relative Values to Maximum Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| x1: Solvent (MeOH %) | x2: Temperature (°C) | x3: Amplitude (%) | x4: Cycle (s–1) | x5: pH | x6: Solvent–Solid Ratio | ||
| 1 | 50 (−1) | 30 (−1) | 30 (−1) | 0.7 (+1) | 7 (+1) | 20 (−1) | 80.4 |
| 2 | 100 (+1) | 30 (−1) | 30 (−1) | 0.7 (+1) | 7 (+1) | 40 (+1) | 70.8 |
| 3 | 100 (+1) | 70 (+1) | 70 (+1) | 0.3 (−1) | 3 (−1) | 40 (+1) | 86.8 |
| 4 | 50 (−1) | 30 (−1) | 30 (−1) | 0.3 (−1) | 7 (+1) | 40 (+1) | 84.1 |
| 5 | 50 (−1) | 70 (+1) | 30 (−1) | 0.3 (−1) | 7 (+1) | 20 (−1) | 85.2 |
| 6 | 50 (−1) | 70 (+1) | 30 (−1) | 0.3 (−1) | 3 (−1) | 40 (+1) | 98.5 |
| 7 | 100 (+1) | 70 (+1) | 30 (−1) | 0.7 (+1) | 3 (−1) | 40 (+1) | 84.9 |
| 8 | 50 (−1) | 70 (+1) | 30 (−1) | 0.7 (+1) | 3 (−1) | 20 (−1) | 88.2 |
| 9 | 75 (0) | 50 (0) | 70 (+1) | 0.5 (0) | 5 (0) | 30 (0) | 93.0 |
| 10 | 100 (+1) | 30 (−1) | 70 (+1) | 0.7 (+1) | 7 (+1) | 20 (−1) | 73.7 |
| 11 | 75 (0) | 30 (−1) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 84.0 |
| 12 | 100 (+1) | 30 (−1) | 70 (+1) | 0.3 (−1) | 7 (+1) | 40 (+1) | 73.6 |
| 13 | 75 (0) | 50 (0) | 30 (−1) | 0.5 (0) | 5 (0) | 30 (0) | 92.7 |
| 14 | 75 (0) | 50 (0) | 50 (0) | 0.3 (−1) | 5 (0) | 30 (0) | 90.7 |
| 15 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 20 (−1) | 88.7 |
| 16 | 50 (−1) | 30 (−1) | 70 (+1) | 0.7 (+1) | 3 (−1) | 20 (−1) | 87.0 |
| 17 | 75 (0) | 70 (+1) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 100.0 |
| 18 | 50 (−1) | 70 (+1) | 70 (+1) | 0.3 (−1) | 7 (+1) | 40 (+1) | 98.5 |
| 19 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 3 (−1) | 30 (0) | 89.1 |
| 20 | 50 (−1) | 30 (−1) | 70 (+1) | 0.3 (−1) | 7 (+1) | 20 (−1) | 83.6 |
| 21 | 100 (+1) | 30 (−1) | 70 (+1) | 0.7 (+1) | 3 (−1) | 40 (+1) | 79.0 |
| 22 | 100 (+1) | 70 (+1) | 70 (+1) | 0.7 (+1) | 7 (+1) | 40 (+1) | 91.3 |
| 23 | 100 (+1) | 30 (−1) | 30 (−1) | 0.3 (−1) | 3 (−1) | 40 (+1) | 67.0 |
| 24 | 100 (+1) | 70 (+1) | 70 (+1) | 0.3 (−1) | 7 (+1) | 20 (−1) | 84.6 |
| 25 | 100 (+1) | 70 (+1) | 30 (−1) | 0.7 (+1) | 7 (+1) | 20 (−1) | 77.1 |
| 26 | 50 (−1) | 30 (−1) | 30 (−1) | 0.3 (−1) | 3 (−1) | 20 (−1) | 80.8 |
| 27 | 75 (0) | 50 (0) | 50 (0) | 0.7 (+1) | 5 (0) | 30 (0) | 91.5 |
| 28 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 93.7 |
| 29 | 100 (+1) | 30 (−1) | 70 (+1) | 0.3 (−1) | 3 (−1) | 20 (−1) | 72.5 |
| 30 | 50 (−1) | 30 (−1) | 70 (+1) | 0.7 (+1) | 7 (+1) | 40 (+1) | 90.8 |
| 31 | 50 (−1) | 30 (−1) | 70 (+1) | 0.3 (−1) | 3 (−1) | 40 (+1) | 87.5 |
| 32 | 50 (−1) | 70 (+1) | 30 (−1) | 0.7 (+1) | 7 (+1) | 40 (+1) | 96.7 |
| 33 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 40 (+1) | 95.4 |
| 34 | 100 (+1) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 86.2 |
| 35 | 50 (−1) | 70 (+1) | 70 (+1) | 0.7 (+1) | 3 (−1) | 40 (+1) | 98.9 |
| 36 | 100 (+1) | 30 (−1) | 30 (−1) | 0.7 (+1) | 3 (−1) | 20 (−1) | 72.5 |
| 37 | 50 (−1) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 95.1 |
| 38 | 50 (−1) | 70 (+1) | 70 (+1) | 0.3 (−1) | 3 (−1) | 20 (−1) | 87.5 |
| 39 | 50 (−1) | 70 (+1) | 70 (+1) | 0.7 (+1) | 7 (+1) | 20 (−1) | 88.8 |
| 40 | 50 (−1) | 30 (−1) | 30 (−1) | 0.7 (+1) | 3 (−1) | 40 (+1) | 91.5 |
| 41 | 100 (+1) | 30 (−1) | 30 (−1) | 0.3 (−1) | 7 (+1) | 20 (−1) | 67.8 |
| 42 | 100 (+1) | 70 (+1) | 30 (−1) | 0.3 (−1) | 7 (+1) | 40 (+1) | 87.3 |
| 43 | 100 (+1) | 70 (+1) | 70 (+1) | 0.7 (+1) | 3 (−1) | 20 (−1) | 78.4 |
| 44 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 5 (0) | 30 (0) | 93.8 |
| 45 | 75 (0) | 50 (0) | 50 (0) | 0.5 (0) | 7 (+1) | 30 (0) | 91.2 |
| 46 | 100 (+1) | 70 (+1) | 30 (−1) | 0.3 (−1) | 3 (−1) | 20 (−1) | 84.5 |
| MeOH in Water (%) | Temperature (°C) | Amplitude (%) | Cycle (s−1) | pH | Solvent–Solid Ratio | Extraction Yield of Piceid (mg·g−1) | |
|---|---|---|---|---|---|---|---|
| Optimal condition | 62.7 | 69.1 | 70 | 0.47 | 5.7 | 40:1 | 29.13 * |
| Used condition | 62.5 | 69.1 | 70 | 0.50 | 5.7 | 40:1 | 29.15 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takao, L.K.; Setyaningsih, W.; Gualtieri, S.C.J.; Ruíz-Rodríguez, A.; Varela, R.M.; Palma, M. Optimized Ultrasound-Assisted Extraction of Psidium laruotteanum Roots: A Concentrated Source of Piceid from the Brazilian Savanna. Forests 2023, 14, 1608. https://doi.org/10.3390/f14081608
Takao LK, Setyaningsih W, Gualtieri SCJ, Ruíz-Rodríguez A, Varela RM, Palma M. Optimized Ultrasound-Assisted Extraction of Psidium laruotteanum Roots: A Concentrated Source of Piceid from the Brazilian Savanna. Forests. 2023; 14(8):1608. https://doi.org/10.3390/f14081608
Chicago/Turabian StyleTakao, Leandro Kenji, Widiastuti Setyaningsih, Sonia C. J. Gualtieri, Ana Ruíz-Rodríguez, Rosa M. Varela, and Miguel Palma. 2023. "Optimized Ultrasound-Assisted Extraction of Psidium laruotteanum Roots: A Concentrated Source of Piceid from the Brazilian Savanna" Forests 14, no. 8: 1608. https://doi.org/10.3390/f14081608
APA StyleTakao, L. K., Setyaningsih, W., Gualtieri, S. C. J., Ruíz-Rodríguez, A., Varela, R. M., & Palma, M. (2023). Optimized Ultrasound-Assisted Extraction of Psidium laruotteanum Roots: A Concentrated Source of Piceid from the Brazilian Savanna. Forests, 14(8), 1608. https://doi.org/10.3390/f14081608







