Functional Role of Intestinal Symbiotic Microorganisms in Improving the Adaptability of Anoplophora glabripennis to Resistant Host Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Plants and Maintaining Condition of Anoplophora glabripennis
2.2. Plant Metabolomics Analysis
2.3. Preparation and Determination of Anoplophora glabripennis Enzyme Activity
2.4. DNA Extraction and PCR Amplification
2.5. Analysis of Metabonomics, Enzyme Activity, and Sequence Processing Data
2.6. Correlation Analysis of Differential Metabolites with Intestinal Enzyme Activity and Intestinal Microorganisms
3. Results
3.1. Principal Component Analysis and System Stability
3.2. Screening of Differential Metabolites of Insect Resistance
3.3. Changes in Digestive Enzyme and Detoxifying Enzyme Activity in Larval Gut
3.4. OTU Annotation and Venn Diagram Analysis
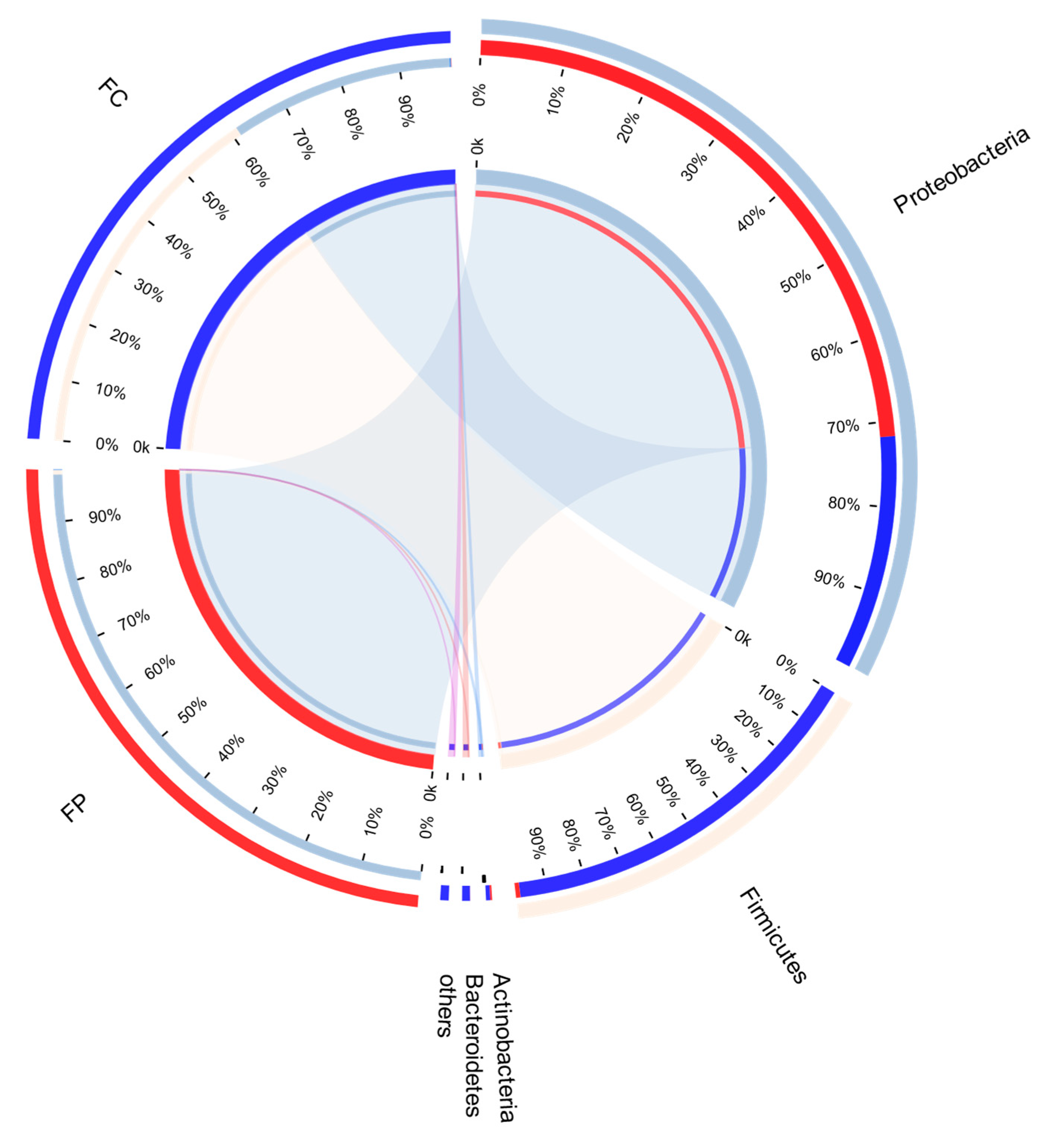
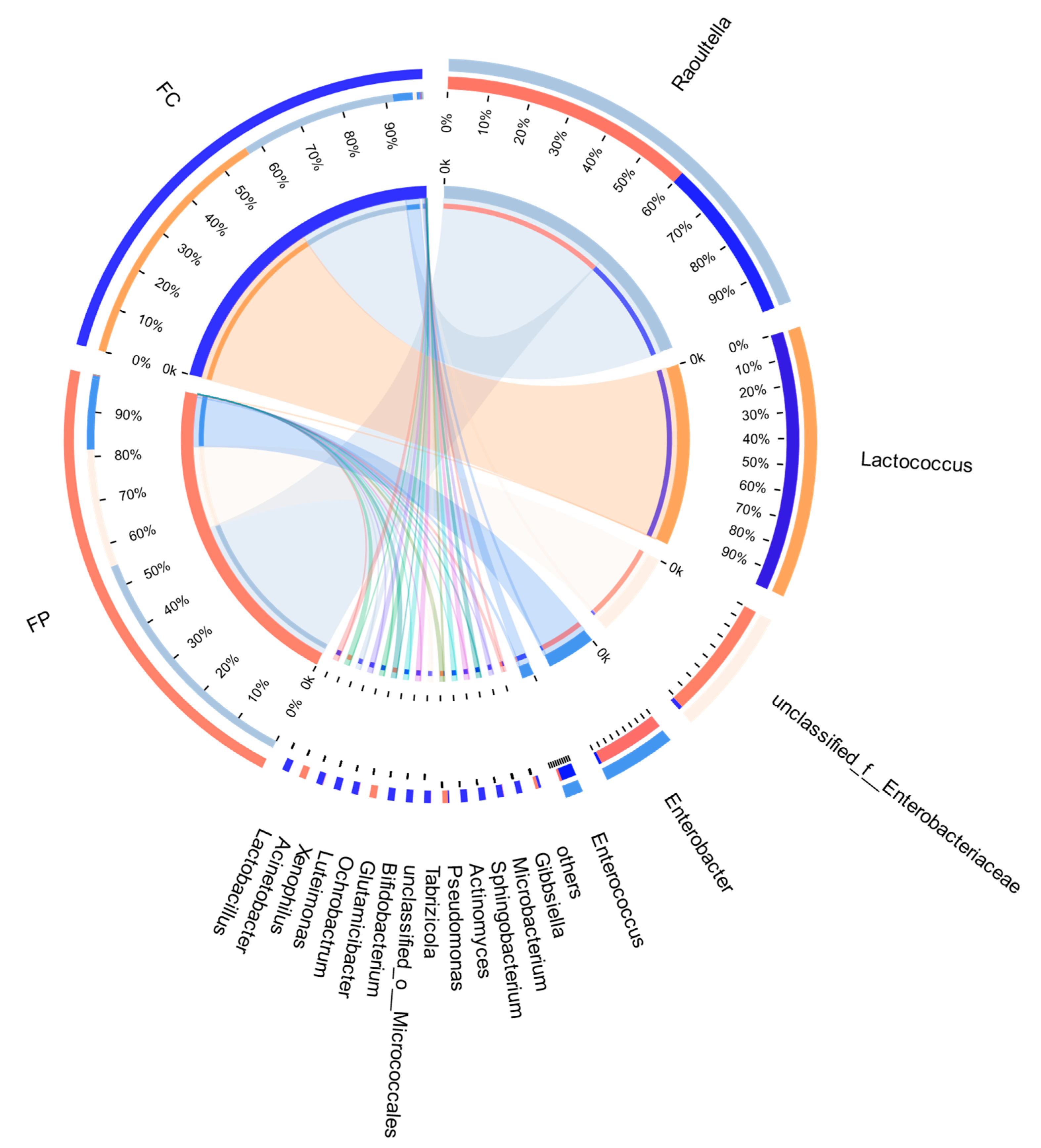
3.5. Analysis of Midgut Bacterial Community
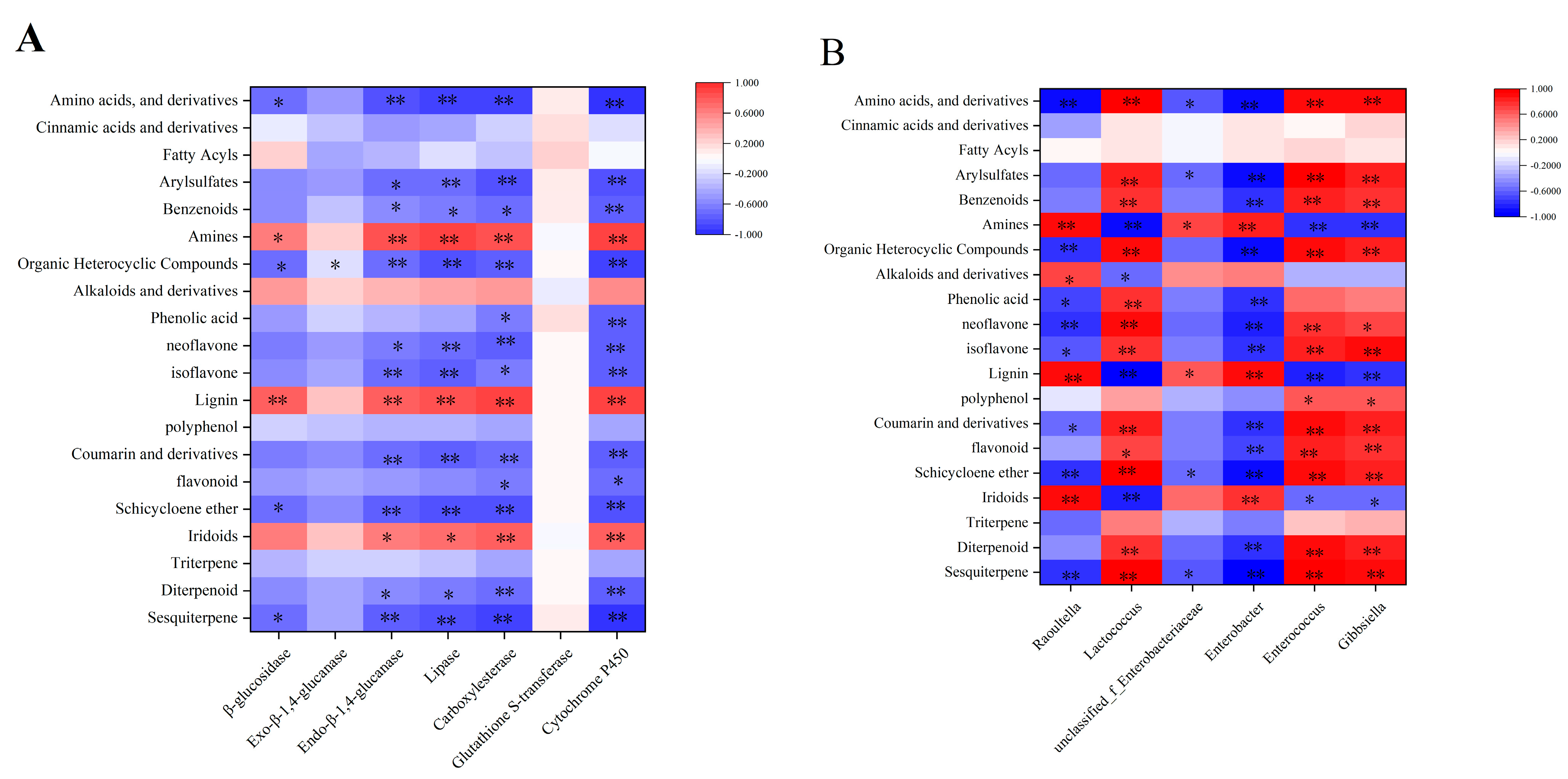
3.6. Correlation between Differential Metabolites of Insect Resistance and Intestinal Enzyme Activity
3.7. Correlation between Differential Metabolites of Insect Resistance and Intestinal Bacteria
4. Discussion
4.1. Host Metabolites Affect Intestinal Physiological and Biochemical Status of Anoplophora glabripennis Larvae
4.2. Effects of Host Metabolites on Symbiotic Community
4.3. Potential Role of Microbial Community Structure Changes in Host Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hölttä, T.; Kurppa, M.; Nikinmaa, E. Scaling of xylem and phloem transport capacity and resource usage with tree size. Front. Plant Sci. 2013, 4, 496. [Google Scholar] [CrossRef]
- Sugio, A.; Dubreuil, G.; Giron, D.; Simon, J.C. Plant-insect interactions under bacterial influence: Ecological implications and underlying mechanisms. J. Exp. Bot. 2015, 66, 467–478. [Google Scholar] [CrossRef]
- Gurung, K.; Wertheim, B.; Falcao, S.J. The microbiome of pest insects: It is not just bacteria. Entomol. Exp. Appl. 2019, 167, 156–170. [Google Scholar] [CrossRef]
- Ohgushi, T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 81–105. [Google Scholar] [CrossRef]
- Waleed, A.N.; Junaid, A.S.; Ye, L.Q.; Huang, X.L. Functional interactions among phytophagous insects, plants and microbial symbiont. Wuyi Sci. J. 2020, 36, 1–15. [Google Scholar]
- Clissold, F.J.; Sanson, G.D.; Read, J. Indigestibility of plant cell wall by the Australian plague locust, Chortoicetes terminifera. Entomol. Exp. Appl. 2004, 112, 159–168. [Google Scholar] [CrossRef]
- Schaller, F.; Schaller, A.; Stintzi, A. Biosynthesis and metabolism of jasmonates. J. Plant Growth Regul. 2004, 23, 179–199. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Genes. Enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 665–675. [Google Scholar] [CrossRef]
- Shao, Y.Y. Effects of Nutrients and Secondary Metabolites of Hippophae rhamnoides on the Transfer Damage of Holcocerus hippophaecolus Larvae. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Chen, H.; McCaig, B.C.; Melotto, M.; He, S.Y.; Howe, G.A. Regulation of plant arginase by wounding, jasmonate, and the phytotoxin coronatine. J. Biol. Chem. 2004, 279, 45998–46007. [Google Scholar] [CrossRef]
- Ma, J.P.; Mou, Z.M. Research Progress of Plant Protease Inhibitors. China Seric. 2006, 3, 4–8. [Google Scholar] [CrossRef]
- David, L. Function of Plant Secondary Metabolites and their Exploitation in Biotechnology. Annual Plant Reviews, Volume 3. Biochem. Syst. Ecol. 2002, 30, 279. [Google Scholar] [CrossRef]
- Lazarević, J.; Janković-Tomanić, M. Dietary and phylogenetic correlates of digestive trypsin activity in insect pests. Entomol. Exp. Appl. 2015, 157, 123–151. [Google Scholar] [CrossRef]
- Cris, O.; William, E.K.; Willisa, J.D.; Oppertc, B.; Jurat-Fuentes, J.L. Prospecting for cellulolytic activity in insect digestive fluids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 145–154. [Google Scholar] [CrossRef]
- Wang, J.M.; Chen, X.O.; Feng, Y.; Duan, Z.Y. Comparison of compounds and activity of digestive enzyme between two xylophagous insects. J. Forest. Res. 2007, 2, 170–175. [Google Scholar]
- Yang, S.; Cao, Q.; Peng, K.; Xie, J. Jasmonic Acid-Treated Cotton Plant Leaves Impair Larvae Growth Performance, Activities of Detoxification Enzymes, and Insect Humoral Immunity of Cotton Bollworm. Neotrop. Entomol. 2022, 51, 570–582. [Google Scholar] [CrossRef]
- Walker, C.H.; Mackness, M.I. Esterases: Problems of identification and classification. Biochem. Pharmacol. 1983, 32, 3265–3269. [Google Scholar] [CrossRef]
- Francis, F.; Haubruge, E.; Gaspar, C.; Dierickx, P.J. Glutathione S-transferases of Aulacorthum solani and Acyrthosiphon pisum: Partial purification and characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 165–171. [Google Scholar] [CrossRef]
- Daborn, P.J.; Lumb, C.; Boey, A.; Wong, W.; ffrench-Constant, R.H.; Batterham, P. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 2007, 37, 512–519. [Google Scholar] [CrossRef]
- Li, X.; Baudry, J.; Berenbaum, M.R.; Schuler, M.A. Structural and functional divergence of insect CYP6B proteins: From specialist to generalist cytochrome P450. Proc. Natl. Acad. Sci. USA 2004, 101, 2939–2944. [Google Scholar] [CrossRef]
- Zhou, F.; Pang, Z.C.; Yu, X.Q.; Wang, Z.Y. Insect Intestinal Microorganism: Progress and Application. J. Appl. Entomol. 2020, 57, 600–607. [Google Scholar]
- Geib, S.M.; Filley, T.R.; Hatcher, P.G.; Kelli, H.; Carlson, J.E.; Jimenez-Gasco, M.M.; Nakagawa-Izumi, A.; Sleighter, R.L.; Tien, M. Lignin degradation in wood-feeding insects. Proc. Natl. Acad. Sci. USA 2008, 105, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Geib, S.M.; Jimenez-Gasco, M.; Carlson, J.E.; Ming, T.; Hoover Jabbour, R.; Kelli, H. Microbial Community Profiling to Investigate Transmission of Bacteria Between Life Stages of the Wood-Boring Beetle, Anoplophora. glabripennis. Microb. Ecol. 2009, 58, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S.; Bohlmann, J.; Raffa, K.F. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.N.; Rodríguez, R.; Gutiérrez-Sánchez, G.; Augur, C.; Favela-Torres, E.; Prado-Barragan, L.A.; Ramírez-Coronel, A.; Contreras-Esquivel, J.C. Microbial tannases: Advances and perspectives. Appl. Microbiol. 2007, 76, 47–59. [Google Scholar] [CrossRef]
- Thiyonila, B.; Kannan, M.; Reneeta, N.P.; Ramya, T.; Krishnan, M. Influence of tannase from serratia marcescens strain imbl5 on enhancing antioxidant properties of green tea. Biocatal. Agric. Biotechnol. 2020, 27, 101675. [Google Scholar] [CrossRef]
- Aylward, F.O.; Mcdonald, B.R.; Adams, S.M.; Valenzuela, A.; Schmidt, R.A.; Goodwin, L.A.; Woyke, T.; Currie, C.R.; Suen, G.; Poulsen, M. Comparison of 26 Sphingomonad Genomes Reveals Diverse Environmental Adaptations and Biodegradative Capabilities. Appl. Environ. Microbiol. 2013, 79, 3724–3733. [Google Scholar] [CrossRef]
- Jian, X.; Jeffrey, I.G. Honor Thy Symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef]
- Carissimo, G.; Pondeville, E.; McFarlane, M.; Failloux, A.; Kohl, A.; Vernick, K.D. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, 176–185. [Google Scholar] [CrossRef]
- Fukatsu, T.; Hosokawa, T. Capsule-Transmitted Gut Symbiotic Bacterium of the Japanese Common Plataspid Stinkbug, Megacopta punctatissima. Appl. Environ. Microb. 2002, 68, 389–396. [Google Scholar] [CrossRef]
- Stephen, T.; Hardin, K.; Gries, G.; Ward, S.; Robb, B. Vibratory communication signal produced by male western conifer seed bugs (Hemiptera: Coreidae). Can. Entomol. 2007, 140, 174–183. [Google Scholar] [CrossRef]
- Li, H.P.; Lv, F.; Bi, Y.G.; Wang, Z.G. Reviews on the serious wood-boring pest Anoplophora glabripennis in forestry of China. For. Ecol. Manag. 2020, 1, 1–9. [Google Scholar]
- Liu, G.R. Advances in Landscape Studies of Fraxinus Plants in China. J. Hengshui Univ. 2013, 29, 73–74. [Google Scholar]
- Shi, W.; Ding, S.Y.; Yuan, J.S. Comparison of Insect Gut Cellulase and Xylanase Activity Across Different Insect Species with Distinct Food Sources. Bioenerg. Res. 2011, 4, 1–10. [Google Scholar] [CrossRef]
- Wang, J.M. Feeding Amount and Digestive Enzymes and Tree Decom Position of Three Species of XyloPhagous Insects. Ph.D. Thesis, China Academy of Forestry Sciences, Beijing, China, 2007. [Google Scholar]
- Wer, D.F. The Digestive Enzyme Activity and Bacterial Diversity in the Gut of Hyphantria cunea (Lepidoptera: Arctiidae) Larcae. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2017. [Google Scholar]
- Zhao, A.P. Study of Midgut Trypsin Activity and Expression in Oriental Fruit Moths during Larval Development. Ph.D. Thesis, Northwest Agricultural and Forestry University, Xianyang City, China, 2017. [Google Scholar]
- Kotkar, H.M.; Sarate, P.J.; Tamhane, V.A.; Gupta, V.S.; Giri, A.P. Responses of midgut amylases of Helicoverpa armigera to feeding on various host plants. J. Insect Physiol. 2009, 55, 663–670. [Google Scholar] [CrossRef]
- Jin, M.; Liao, C.; Fu, X.; Holdbrook, R.; Wu, K.; Xiao, Y. Adaptive regulation of detoxification enzymes in Helicoverpa armigera to different host plants. Insect Mol. Biol. 2019, 28, 628–636. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Wang, H.; Song, C.; Shangguan, X.; Ma, Y.; Zhu, L.; He, G. Functional study of cytochrome P450 enzymes from the brown planthopper (Nilaparvata lugens Stål) to analyze its adaptation to BPH-resistant rice. Front. Physiol. 2017, 8, 972. [Google Scholar] [CrossRef]
- Ren, S.Z.; Wen, Z.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea. J. Chem. Ecol. 2007, 33, 449–461. [Google Scholar] [CrossRef]
- An, Z.L.; Chu, D.; Guo, D.F.; Fan, Z.X.; Tao, Y.L.; Liu, G.X.; Zhang, Y.J. Effects of host plant on activities of some detoxification enzymes in Bemisia tabaci biotype B. Acta Geol. Sin. 2008, 4, 1536–1543. [Google Scholar]
- Karban, R.; Agrawal, A.A. Herbivore offense. Annu. Rev. Entomol. 2002, 33, 641–664. [Google Scholar] [CrossRef]
- Despre’s, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Li, X.C.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms to metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Rigsby, C.M.; Showalter, D.N.; Herms, D.A.; Koch, J.L.; Bonello, P.; Cipollini, D. Physiological responses of emerald ash borer larvae to feeding on different ash species reveal putative resistance mechanisms and insect counter-adaptations. J. Insect Physiol. 2015, 78, 47–54. [Google Scholar] [CrossRef]
- Mason, C.J. Complex relationships at the intersection of insect gut microbiomes and plant defenses. J. Chem. Ecol. 2020, 46, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Jurkevitch, E.; Yuval, B. Bringing back the fruit into fruit fly-bacteria interactions. Mol. Ecol. 2008, 17, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Kim, S.H.; Lee, W.J.; Bae, J.W. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; He, C.; Li, M.L. Analysis of Intestinal Bacterial Community Diversity of Adult Dastarcus helophoroides. J. Insect Sci. 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Ma, S.L.; Yang, Y.; Jack, C.J.; Diao, Q.Y.; Fu, Z.M.; Dai, P.L. Effects of Tropilaelaps mercedesae on midgut bacterial diversity of Apis mellifera. Exp. Appl. Acarol. 2019, 79, 169–186. [Google Scholar] [CrossRef]
- Jones, R.T.; Sanchez, L.G.; Fierer, N. A cross-taxon analysis of insect- associated bacterial diversity. PLoS ONE 2013, 8, e61218. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Shi, F.M.; Pei, J.H.; Hou, Z.-H.; Zong, S.-X.; Ren, L.-L. Gut Bacteria Associated with Monochamus saltuarius (Coleoptera: Cerambycidae) and Their Possible Roles in Host Plant Adaptations. Front. Microbiol. 2021, 12, 687211. [Google Scholar] [CrossRef]
- Martinson, V.G.; Douglas, A.E.; Jaenike, J. Community structure of the gut microbiota in sympatric species of wild Drosophila. Ecol. Lett. 2017, 20, 629–639. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, M.S.; Qu, D.; Li, C.L.; Cui, J.T.; Hu, Y.H.; Wang, Y.J. Identification and Characterization of a Newly Isolated Carbendazim-degrading Bacterial Strain MBC. J. Environ. Sci. 2012, 30, 563–569. [Google Scholar]
- Wang, Y. Quantitative and Localization of Several Groups in the Gut of Reticulitermes Chinensis Snyder and Primary Characterization of a Lactococcus. sp Strain. Ph.D. Thesis, Central China Normal University, Wuhan, China, 2014. [Google Scholar]
- Li, M.Y.; Xu, H.Q.; Yu, X.H.; Wang, Y.R. Studies on intestinal flora of silkworm. Jiangsu Seric. 2000, 2, 5–7. [Google Scholar]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh PJamey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Wang, Z.K.; Yang, Y.S.; Stefka, A.T.; Sun, G.; Peng, L.H. Review article: Fungal microbiota and digestive diseases. Aliment Pharm. Ther. 2014, 39, 751–766. [Google Scholar] [CrossRef]
- Butler, È.; Alsterfjord, M.; Olofsson, T.C.; Karlsson, C.; Malmström, J.; Vásquez, A. Proteins of novel lactic acid bacteria from Apis mellifera mellifera: An insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol. 2013, 13, 235. [Google Scholar] [CrossRef]
- Gilles, S.; Arnaud, D.; Berra, E.; Pascal, H.; Julien, R.; François, L. Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef]
- Manero, A.; Blanch, A.R. Identification of Enterococcus spp. with a biochemical key. Appl. Environ. Microbiol. 1999, 65, 4425–4430. [Google Scholar] [CrossRef]
- Chen, B.; The, B.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera littoralis. Sci. Rep. 2016, 66, 29505. [Google Scholar] [CrossRef]
- Gao, S.S. Diversity of Intestinal Bacterial in Three Lepidoptera Larvae Damaging Maize Ears. Ph.D. Thesis, Shandong Agricultural University, Tai’an City, China, 2018. [Google Scholar]
- Serra, A.; Macia, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Schneider, H.; Schwiertz, A.; Collins, M.D.; Blaut, M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch. Microbiol. 1999, 171, 81–91. [Google Scholar] [CrossRef]
- Mason, C.J.; Scully, E.D.; Geib, S.M.; Kelli, H. Contrasting diets reveal metabolic plasticity in the tree-killing beetle, Anoplophora glabripennis (Cerambycidae: Lamiinae). Sci. Rep. 2016, 6, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, R.L.; Zou, Y.X.; Liu, X.M. Recent Advances in Biotransformation of Natural Polyphenols by Gut Microflflora. Food Sci. 2019, 35, 319–325. [Google Scholar] [CrossRef]
- Gokulakrishnan, S.; Chandraraj, K.; Gummadi, S.N. A preliminary study of caffeine degradation by Pseudomonas sp. GSC 1182. Int. J. Food Microbiol. 2007, 113, 346–350. [Google Scholar] [CrossRef]
- Li, N.; Ji, B.P.; Li, B.; Wu, W. Screening and Identification of a Pseudomonas lutea Strain Capable of Degrading Caffeine. Food Sci. 2010, 31, 218–221. [Google Scholar]
- Mika, A.; Greenwood, B.N.; Chichlowski, M.; Borchert, D.; Hulen, K.A.; Berg, B.M.; Paton, M.; Fleshner, M. 155. Dietary prebiotics increase Bifidobacterium spp. and Lactobacillus spp. in the gut and promote stress resistance. Brain Behav. 2014, 40, e45. [Google Scholar] [CrossRef]
- Sun, Y.F. Effect of Salt Tolerance and Growth Promotion of Strain Glutamiciactor Soli 1-3-3 and Its. Application. Patent CN202010242767.0A, 17 July 2020. [Google Scholar]
- Wang, L.X.; Chen, W.; Xie, G.L.; Zhou, L. Isolation and identification of intestinal bacteria and screening of cellulolytic bacteria in Proisotoma ananevae (Collembola: Isotomidae). Acta Petrol. Sin. 2018, 61, 835–847. [Google Scholar]
- Li, S.Y.; Li, X.P.; Shpigelman, A.; Lorenzo, J.M.; Montesano, D.; Barba, F.J. Direct and indirect measurements of enhanced phenolic bioavailability from litchi pericarp procyanidins by Lactobacillus casei-01. Food Funct. 2017, 8, 2760–2770. [Google Scholar] [CrossRef]
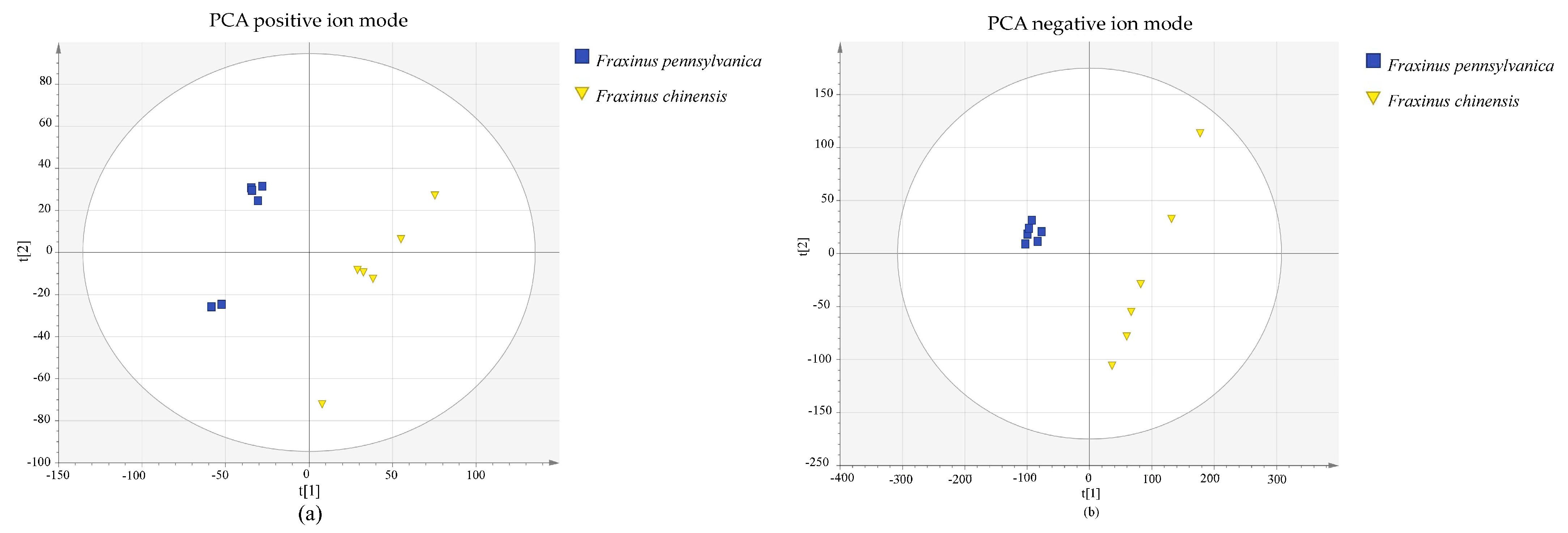
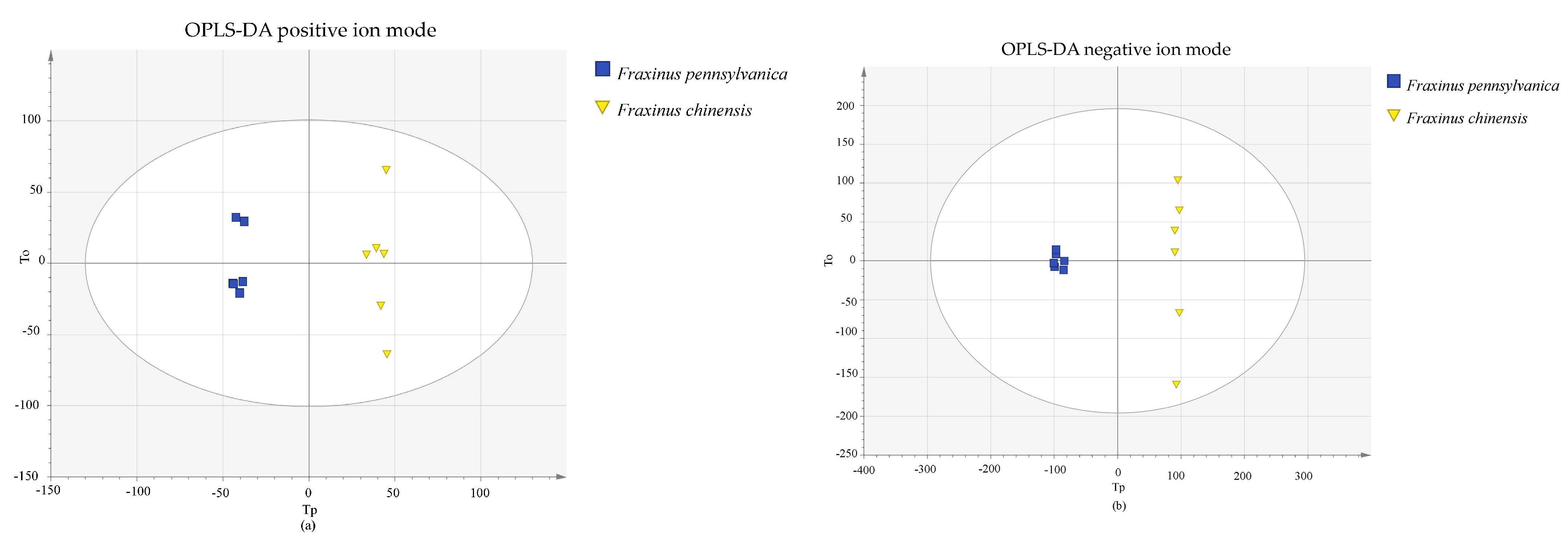
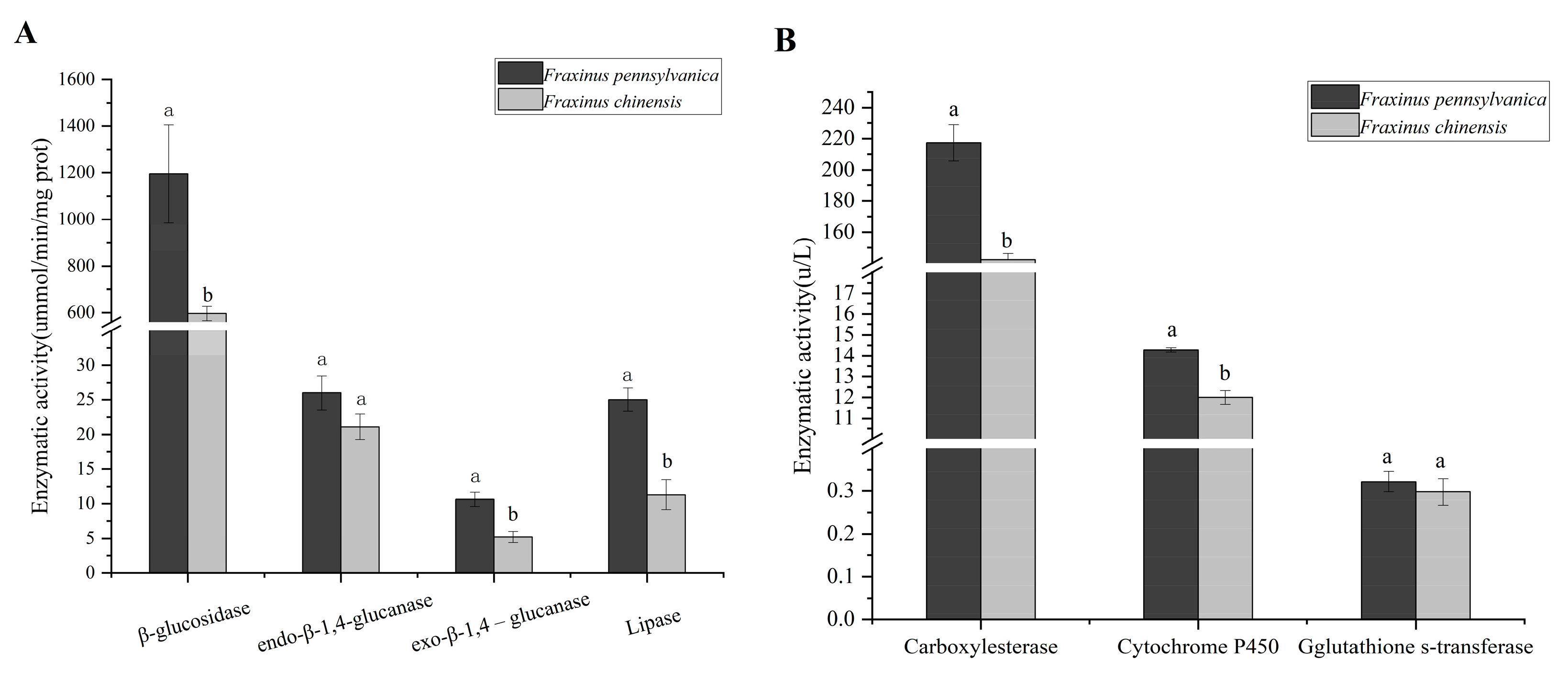
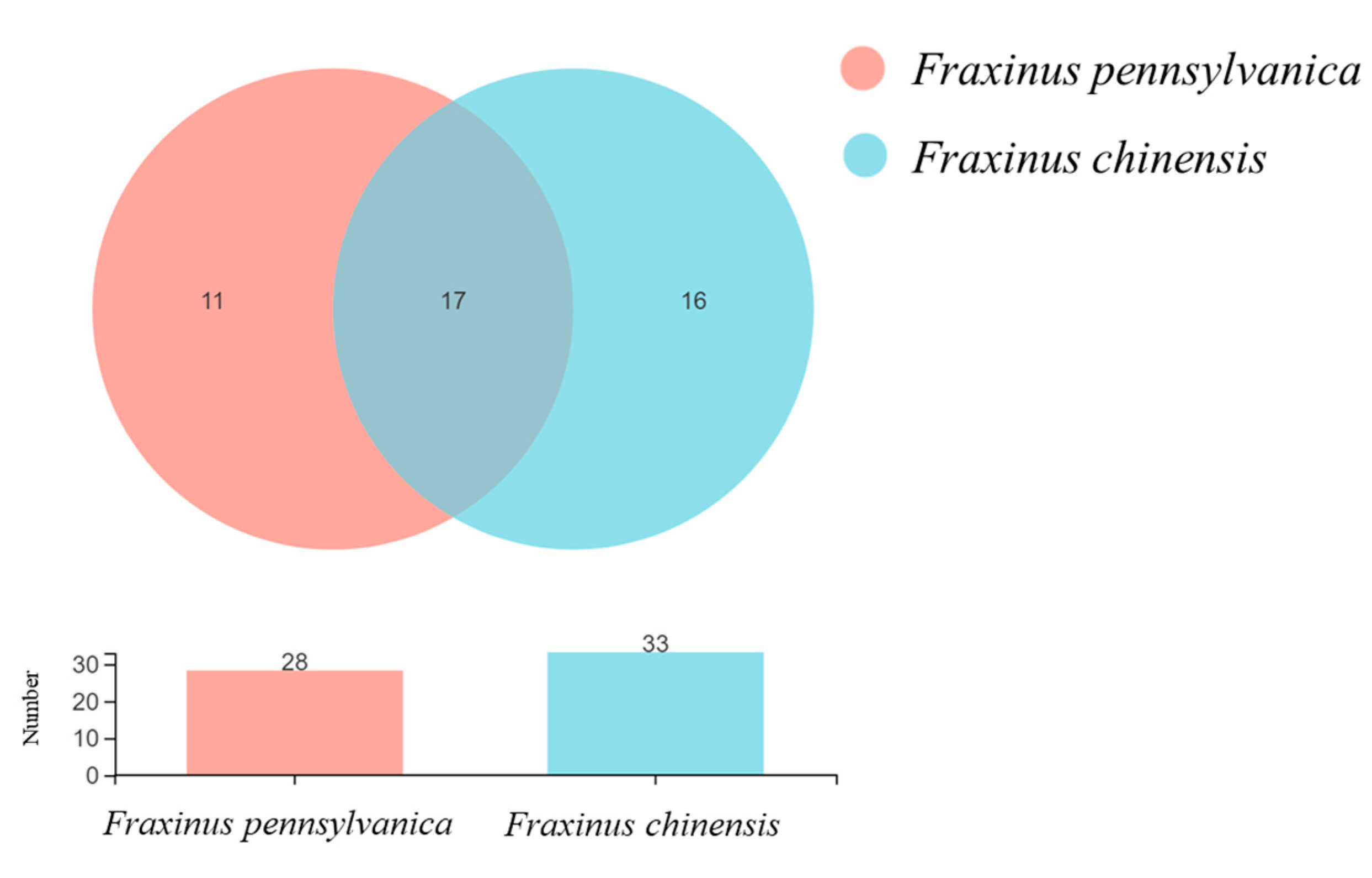
| Category | Name | Number | VIP | Fold Change |
|---|---|---|---|---|
| Isoprenoid derivative | Sesquiterpene | 3 | 12.26238 | 0.12282105 |
| Diterpenoid | 3 | 4.21144 | 0.27948403 | |
| Triterpene | 6 | 10.37481 | 0.60262061 | |
| Iridoids | 1 | 1.17893 | 2.01966533 | |
| Schicycloene ether | 1 | 6.37368 | 0.08465611 | |
| Phenols | Flavonoid | 20 | 20.97269 | 0.43348063 |
| Coumarin and derivatives | 13 | 23.13915 | 0.48444245 | |
| Polyphenol | 10 | 16.27028 | 0.39015662 | |
| Lignin | 1 | 3.48571 | 3.79855115 | |
| Isoflavone | 5 | 7.91847 | 0.43254758 | |
| Neoflavone | 1 | 2.13221 | 0.12592673 | |
| Phenolic acid | 5 | 15.17683 | 0.62583462 | |
| Nitrogenous compound | Alkaloids and derivatives | 5 | 8.33185 | 1.19046171 |
| Organic heterocyclic compounds | 21 | 50.01367 | 0.40141739 | |
| Amines | 1 | 2.88029 | 3.77167077 | |
| Benzenoids | 3 | 4.22763 | 0.2577388 | |
| Arylsulfates | 1 | 1.39372 | 0.08986068 | |
| Fatty acyls | 6 | 8.98549 | 1.16805049 | |
| Cinnamic acids and derivatives | 2 | 3.00178 | 1.14433432 | |
| Amino acids and derivatives | 17 | 29.6949 | 0.071145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Jia, R.; Guo, S.; Li, H.; Hao, E.; Yang, X.; Lu, P.; Qiao, H. Functional Role of Intestinal Symbiotic Microorganisms in Improving the Adaptability of Anoplophora glabripennis to Resistant Host Plants. Forests 2023, 14, 1573. https://doi.org/10.3390/f14081573
Gu Q, Jia R, Guo S, Li H, Hao E, Yang X, Lu P, Qiao H. Functional Role of Intestinal Symbiotic Microorganisms in Improving the Adaptability of Anoplophora glabripennis to Resistant Host Plants. Forests. 2023; 14(8):1573. https://doi.org/10.3390/f14081573
Chicago/Turabian StyleGu, Qi, Ruofeng Jia, Shuai Guo, Han Li, Enhua Hao, Xi Yang, Pengfei Lu, and Haili Qiao. 2023. "Functional Role of Intestinal Symbiotic Microorganisms in Improving the Adaptability of Anoplophora glabripennis to Resistant Host Plants" Forests 14, no. 8: 1573. https://doi.org/10.3390/f14081573
APA StyleGu, Q., Jia, R., Guo, S., Li, H., Hao, E., Yang, X., Lu, P., & Qiao, H. (2023). Functional Role of Intestinal Symbiotic Microorganisms in Improving the Adaptability of Anoplophora glabripennis to Resistant Host Plants. Forests, 14(8), 1573. https://doi.org/10.3390/f14081573






