Transcriptomic Response to Drought Stress in Populus davidiana Dode
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
2.2. Morphological and Physiological Analysis
2.3. Metal Ion Content
2.4. RNA Extraction, cDNA Library Building, and Transcriptome Sequencing
2.5. Sequencing Data Filtering
2.6. Reference Sequence Alignment Analysis
2.7. Gene Expression Analysis
2.8. Differential Expression Analysis
2.9. Gene Ontology (GO) Enrichment Analysis of DEGs
2.10. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
2.11. Quantification and Validation of Gene Expression Levels
2.12. Statistical Analysis
3. Results
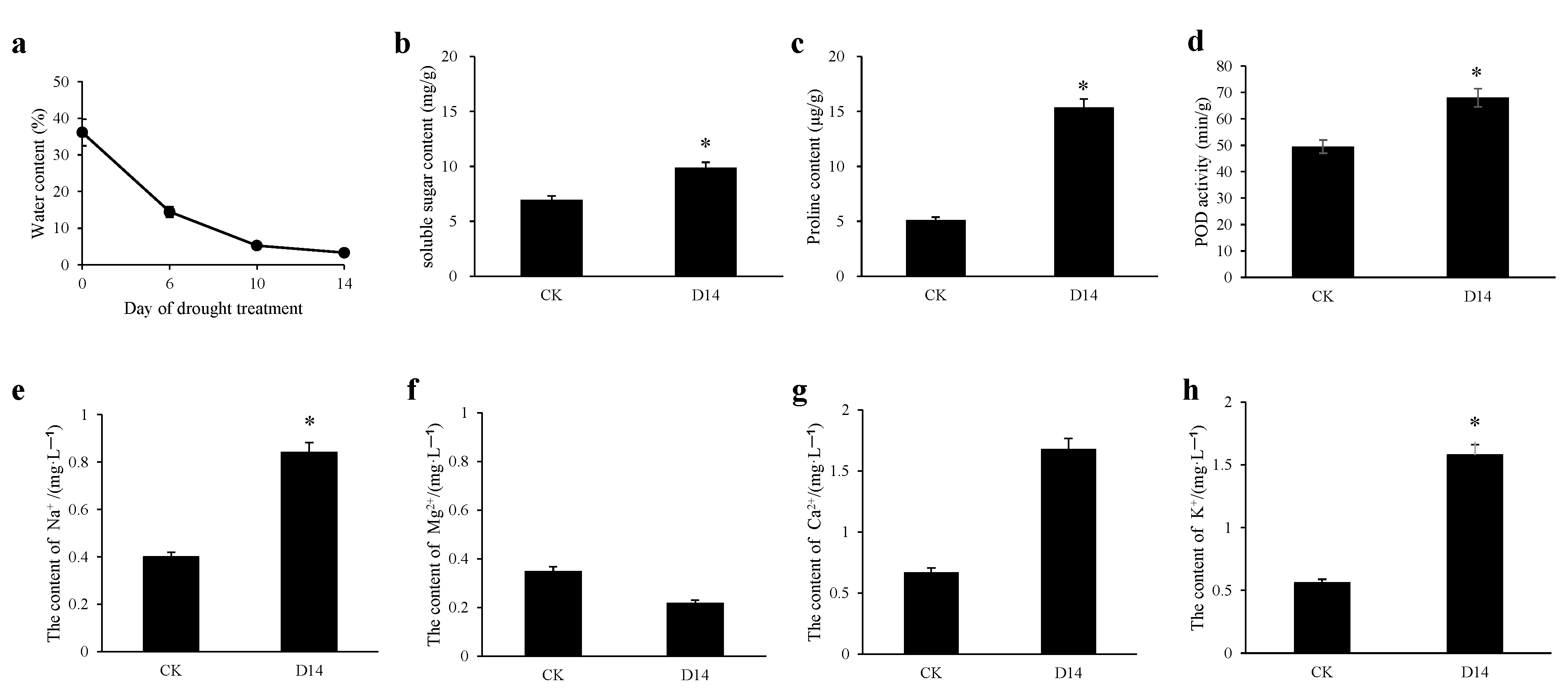
3.1. P. davidiana Phenotypic and Physiological Alterations in Response to Drought Stress
3.2. Changes in the Content of Metal Ions in P. davidiana under Drought Stress
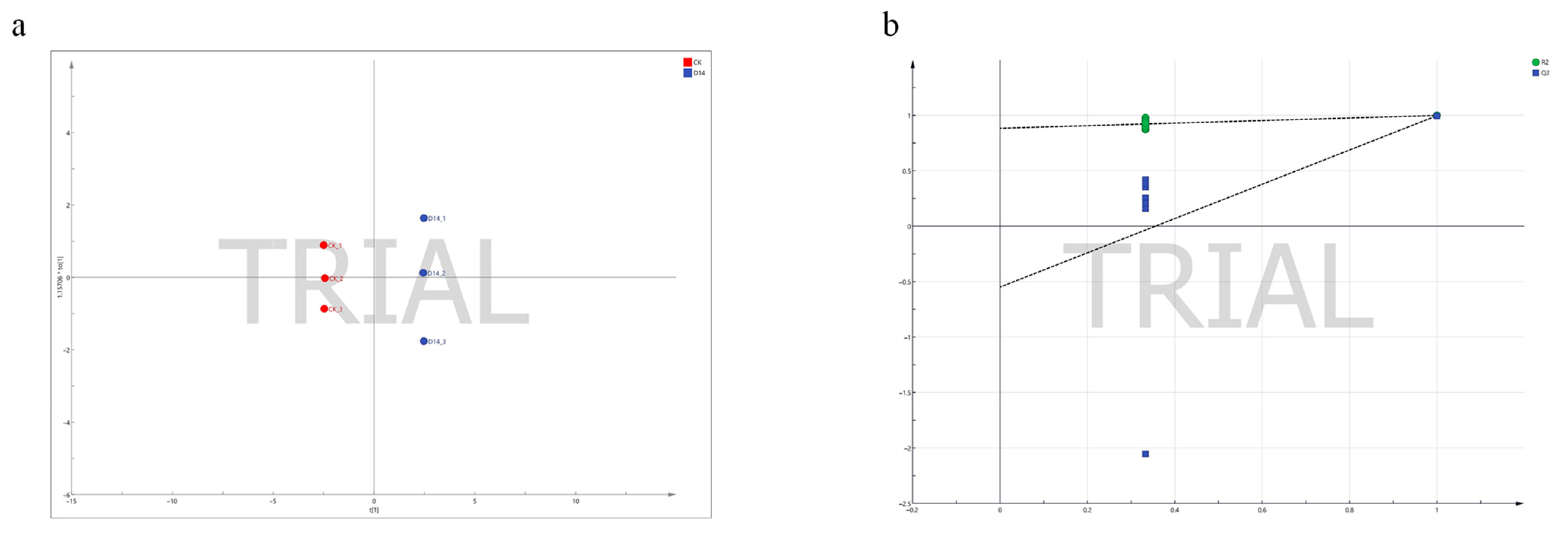
3.3. Analysis of Physiological Indicators of Two Groups of P. davidiana Based on OPLS-DA
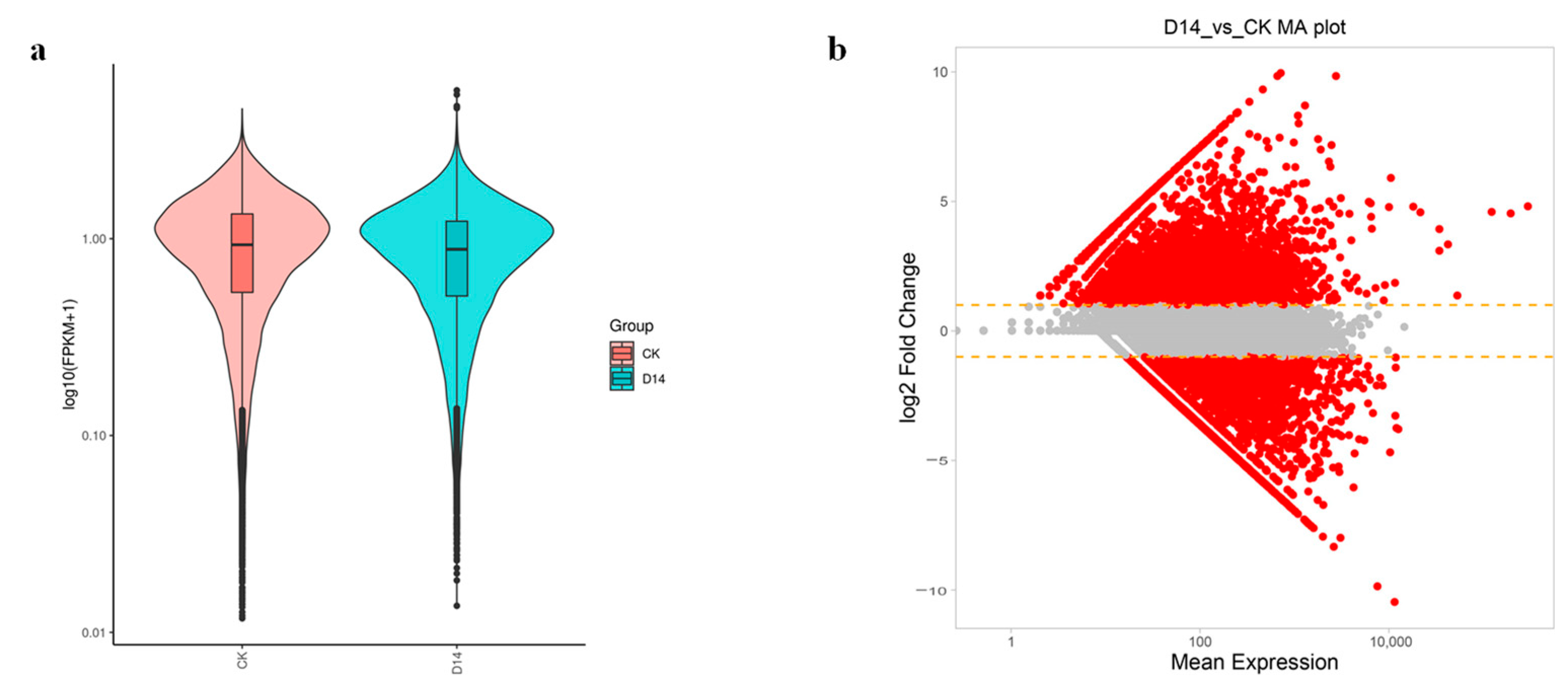
3.4. Sequencing of P. davidiana via Illumina HiSeq and RNA-Seq
3.5. Identification and Alignment of DEGs
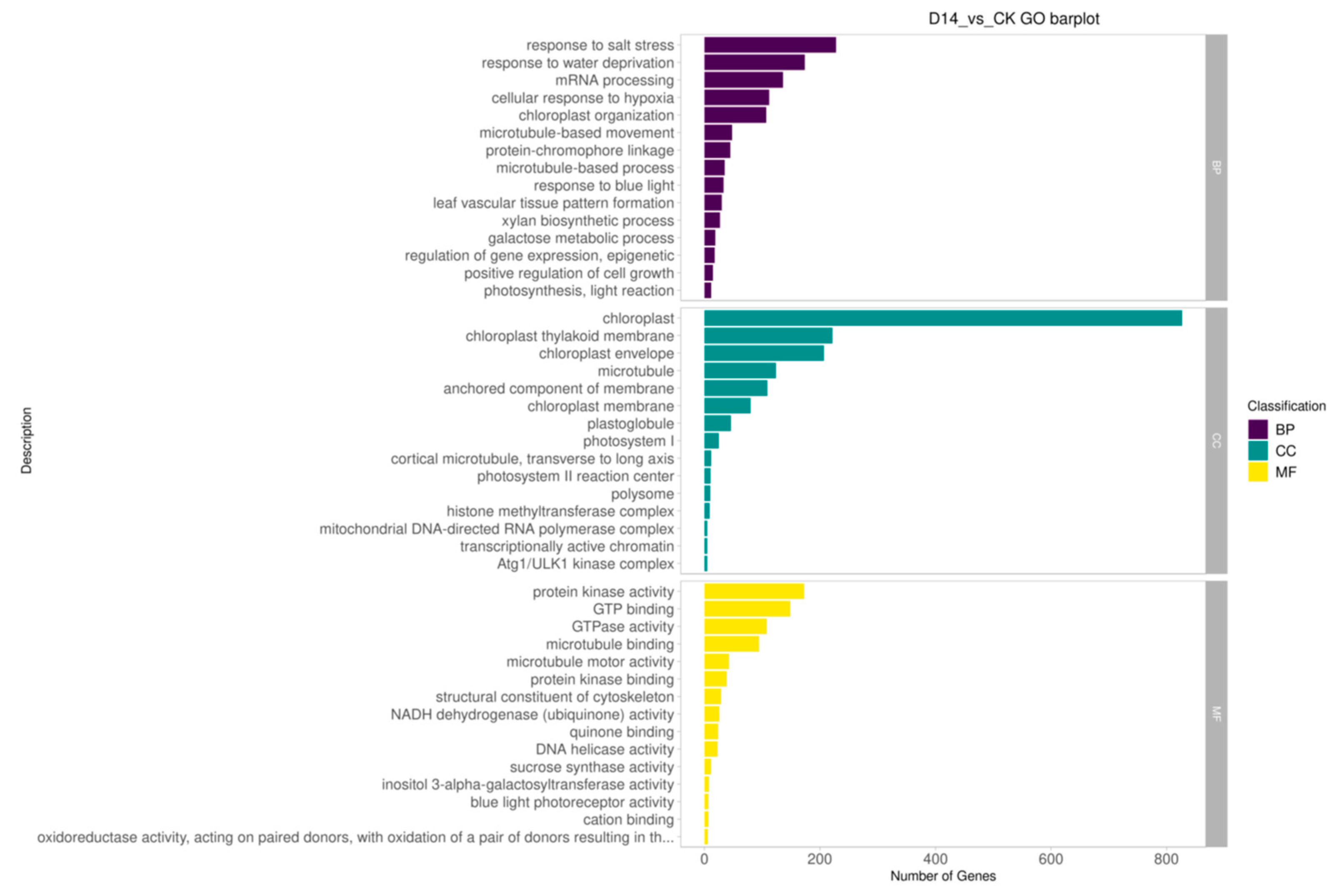
3.6. GO Enrichment Analysis and KEGG Enrichment Analysis
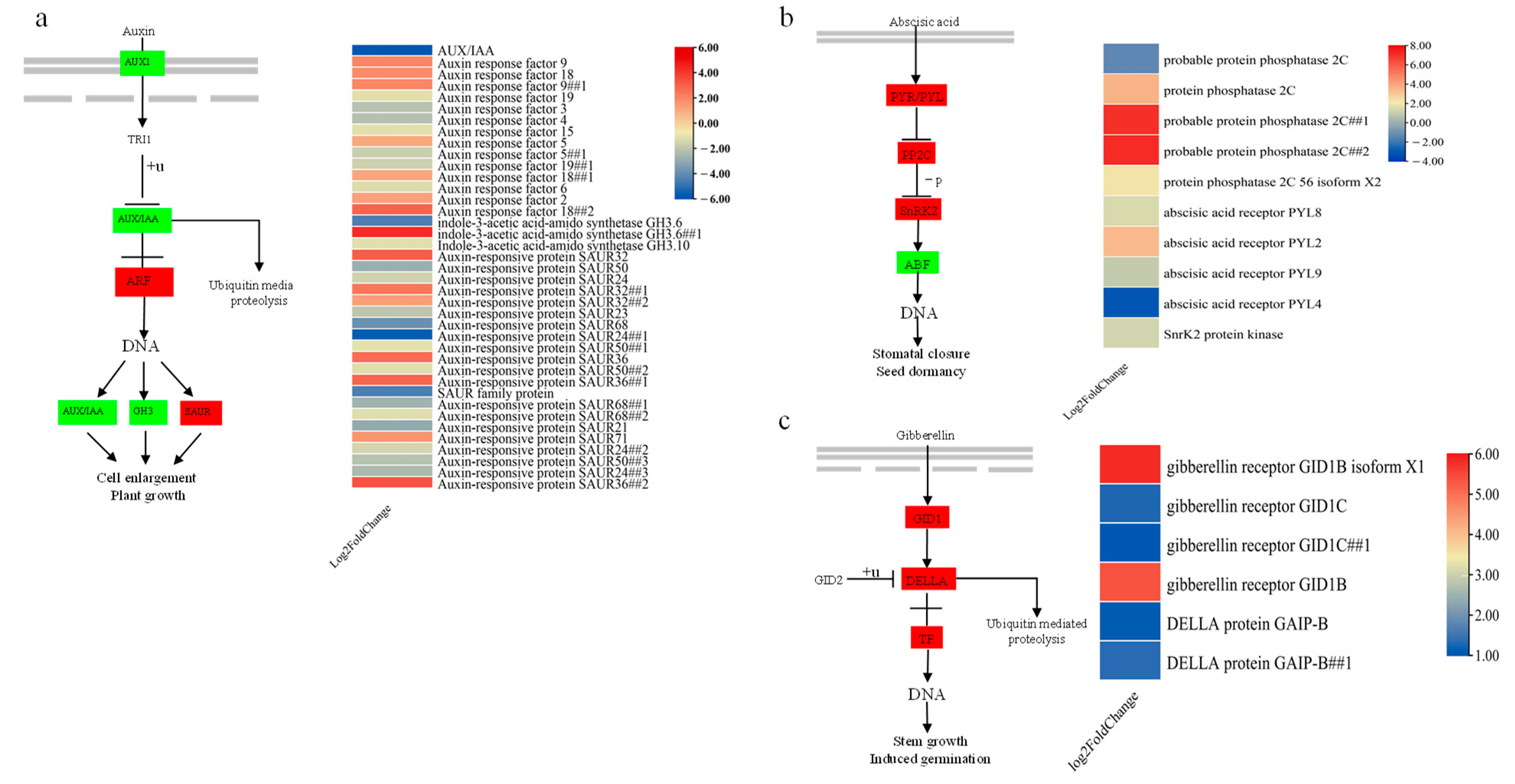
3.7. DEGs Involved in Phytohormone Signal Transduction under Drought Stress in P. davidiana
3.8. Transcription Factors (TFs) Involved in the Response to Drought Stress in P. davidiana
3.9. Responses of DEGs Involved in Antioxidative Mechanisms
3.10. Changes in Lignin under Drought Stress
3.11. Regulation of Glucose Metabolic Pathways
3.12. Regulation of Related Proteins
3.13. Evaluation of Gene Expression Levels Using Measurement
4. Discussion
4.1. Changes in Plant Hormone Signal Transduction Pathways under Drought Stress
4.2. The Role of Transcription Factors in Drought Stress in P. davidiana
4.3. Regulation of ROS in Plants under Drought Stress
4.4. Changes in Lignin Synthesis Pathways under Drought Stress
4.5. Changes in Starch and Sucrose Biosynthesis under Drought Stress
4.6. Changes in Proteins under Drought Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Zhou, G.; Yang, C.; Liu, G.; Xing, Y. Study on crossing breeding of Populus davidiana and P. tremuloides. Bull. Bot. Res. 2004, 24, 215–219. [Google Scholar]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef]
- Wu, H.; Yao, D.; Chen, Y.; Yang, W.; Zhao, W.; Gao, H.; Tong, C. De Novo genome assembly of Populus simonii further supports that Populus simonii and Populus trichocarpa belong to different sections. G3 Genes Genomes Genet. 2020, 10, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.; Nahal, H.; Foong, J.; Campbell, P.M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009, 149, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Guo, T.; Guo, B.; An, X. Comprehensive analysis of the R2R3 MYB transcription factor gene family in Populus trichocarpa. Ind. Crops Prod. 2021, 168, 113614. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomatal in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Schuster, S.C. Next-generation sequencing transforms today’s biology. Nat. Methods 2008, 5, 16–18. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Wang, X.J.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins are active repressors, and their stability and activity are modulated by Auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic Auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Balla, J.; Luschnig, C.; Wisniewska, J.; Reinöhl, V.; Friml, J.; Benková, E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006, 20, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Liscum, E.; Reed, J.W. Genetics of AUX/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef]
- Espasandin, F.D.; Maiale, S.J.; Calzadilla, P.; Ruiz, O.A.; Sansberro, P.A. Transcriptional regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gene by putrescine accumulation positively modulates ABA synthesis and drought tolerance in Lotus tenuis plants. Plant Physiol. Biochem. 2014, 76, 29–35. [Google Scholar] [CrossRef]
- Kalapos, B.; Dobrev, P.; Nagy, T.; Vítámvás, P.; Györgyey, J.; Kocsy, G.; Marincs, F.; Galiba, G. Transcript and hormone analyses reveal the involvement of ABA-signaling, hormone crosstalk and genotype-specific biological processes in cold-shock response in wheat. Plant Sci. 2016, 253, 86–97. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Binott, J.J.; Owuoche, J.O.; Bartels, D. Physiological and molecular characterization of Kenyan barley (Hordeum vulgare L.) seedlings for salinity and drought tolerance. Euphytica 2017, 213, 139. [Google Scholar] [CrossRef]
- Lee, H.; Cha, J.; Choi, C.; Choi, N.; Ji, H.S.; Park, S.R.; Lee, S.; Hwang, D.J. Rice WRKY11 plays a role in pathogen defense and drought tolerance. Rice 2018, 11, 5. [Google Scholar] [CrossRef]
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Liu, A.; Yang, Y.; Diouf, D.; You, J.; Zhang, X. Ectopic expression of the sesame MYB transcription factor SiMYB305 promotes root growth and modulates ABA-mediated tolerance to drought and salt stresses in Arabidopsis. AoB Plants 2019, 12, plz081. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Liu, D.; Wang, X.; Zhang, L. Transcription factor TabHLH49 positively regulates dehydrin WZY2 gene expression and enhances drought stress tolerance in wheat. BMC Plant Biol. 2020, 20, 259. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Zhang, D.F.; Fu, J.; Shi, Y.S.; Song, Y.C.; Wang, T.Y.; Li, Y. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 2012, 235, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef]
- Li, X.; Xiang, L.; Wang, X.; Wang, L.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Vishwakarma, R.K.; Arafat, Y.A.; Gupta, S.K.; Khan, B.M. Abiotic stress induces change in Cinnamoyl CoA Reductase (CCR) protein abundance and lignin deposition in developing seedlings of Leucaena leucocephala. Physiol. Mol. Biol. Plants 2015, 21, 197–205. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Wang, C.; Zhao, L.; Jin, Y.; Xing, Q.; Li, M.; Lv, T.; Qi, H. Lignin synthesized by CmCAD2 and CmCAD3 in oriental melon (Cucumis melo L.) seedlings contributes to drought tolerance. Plant Mol. Biol. 2020, 103, 689–704. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.P.; Batish, D.R.; Kaur, S. EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 2016, 253, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2019, 21, 144. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, M.; Wang, J. Over expressing heat shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef]
- Kalifa, Y.; Gilad, A.; Konrad, Z.; Zaccai, M.; Scolnik, P.A.; Bar-Zvi, D. The water-and salt-stress-regulated Asr1 (abscisic acid stress ripening) gene encodes a zinc-dependent DNA-binding protein. Biochem. J. 2004, 381, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kalifa, Y.; Perlson, E.; Gilad, A.; Konrad, Z.; Scolnik, P.A.; Bar-Zvi, D. Over-expression of the water and salt stress-regulated Asr1 gene confers an increased salt tolerance. Plant Cell Environ. 2004, 27, 1459–1468. [Google Scholar] [CrossRef]
- Dai, J.R.; Liu, B.; Feng, D.R.; Liu, H.Y.; He, Y.M.; Qi, K.B.; Wang, H.B.; Wang, J.F. MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep. 2011, 30, 1219–1230. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Kieffer, M.; Yu, H.; Kepinski, S.; Estelle, M. HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat. Commun. 2016, 7, 11677. [Google Scholar] [CrossRef]
- Xiang, D.J.; Man, L.L.; Zhang, C.L.; Liu, P.; Li, Z.G.; Zheng, G.C. A new Em-like protein from Lactuca sativa, LsEm1, enhances drought and salt stress tolerance in Escherichia coli and rice. Protoplasma 2018, 255, 1089–1106. [Google Scholar] [CrossRef]


| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Clean Bases Q30 (%) | Clean GC Content (%) | Mapped Rate |
|---|---|---|---|---|---|---|---|
| CK | 47.08 M | 7.06 G | 47.06 M | 7.02 G | 93.74 | 53.06 | 93.28% |
| D14 | 51.01 M | 7.65 G | 50.99 M | 7.62 G | 94.05 | 54.06 | 93.90% |
| Pathway | Pathway ID | p Value | Number of DEGs |
|---|---|---|---|
| Starch and sucrose metabolism | ko00500 | 4.05083 × 10−6 *** | 88 |
| Metabolic pathways | ko01100 | 1.00482 × 10−5 *** | 1055 |
| Photosynthesis | ko00195 | 1.20885 × 10−5 *** | 63 |
| MAPK signaling pathway—plant | ko04016 | 6.20075 × 10−5 ** | 91 |
| Circadian rhythm—plant | ko04712 | 7.72403 × 10−5 ** | 33 |
| Biosynthesis of secondary metabolites | ko01110 | 0.000483439 *** | 592 |
| Amino sugar and nucleotide sugar metabolism | ko00520 | 0.000758187 *** | 74 |
| Phagosome | ko04145 | 0.000792934 *** | 53 |
| Photosynthesis-antenna proteins | ko00196 | 0.00330978 ** | 14 |
| Plant hormone signal transduction | ko04075 | 0.003426549 ** | 137 |
| Galactose metabolism | ko00052 | 0.004983568 ** | 35 |
| Fructose and mannose metabolism | ko00051 | 0.005987693 ** | 37 |
| Peroxisome | ko04146 | 0.009642317 ** | 52 |
| Other types of O-glycan biosynthesis | ko00514 | 0.01501615 * | 11 |
| Glyoxylate and dicarboxylate metabolism | ko00630 | 0.01706609 * | 44 |
| beta-Alanine metabolism | ko00410 | 0.02395275 * | 31 |
| Anthocyanin biosynthesis | ko00942 | 0.0309142 * | 3 |
| Cysteine and methionine metabolism | ko00270 | 0.03889815 * | 59 |
| Ascorbate and aldarate metabolism | ko00053 | 0.03940826 * | 25 |
| Flavonoid biosynthesis | ko00941 | 0.04486245 * | 32 |
| Biosynthesis of unsaturated fatty acids | ko01040 | 0.04768018 * | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Wang, L.; Wang, X.; Li, Y.; Huang, H. Transcriptomic Response to Drought Stress in Populus davidiana Dode. Forests 2023, 14, 1465. https://doi.org/10.3390/f14071465
Yang M, Wang L, Wang X, Li Y, Huang H. Transcriptomic Response to Drought Stress in Populus davidiana Dode. Forests. 2023; 14(7):1465. https://doi.org/10.3390/f14071465
Chicago/Turabian StyleYang, Meng, Lili Wang, Xinyu Wang, Yijie Li, and Haijiao Huang. 2023. "Transcriptomic Response to Drought Stress in Populus davidiana Dode" Forests 14, no. 7: 1465. https://doi.org/10.3390/f14071465
APA StyleYang, M., Wang, L., Wang, X., Li, Y., & Huang, H. (2023). Transcriptomic Response to Drought Stress in Populus davidiana Dode. Forests, 14(7), 1465. https://doi.org/10.3390/f14071465




