Differences in the Growth of Seedlings and the Selection of Fast-Growing Species in the Gleditsia Genus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Analysis of Genome Size and Chromosome Quantity in the Gleditsia Genus
2.3. Determination of Photosynthetic Characteristics and Physiological Indicators
2.4. Determination of Hormone Content
2.5. RNA Extraction, Library Construction, and RNA-Seq Analysis
2.6. Statistical Analysis
3. Results
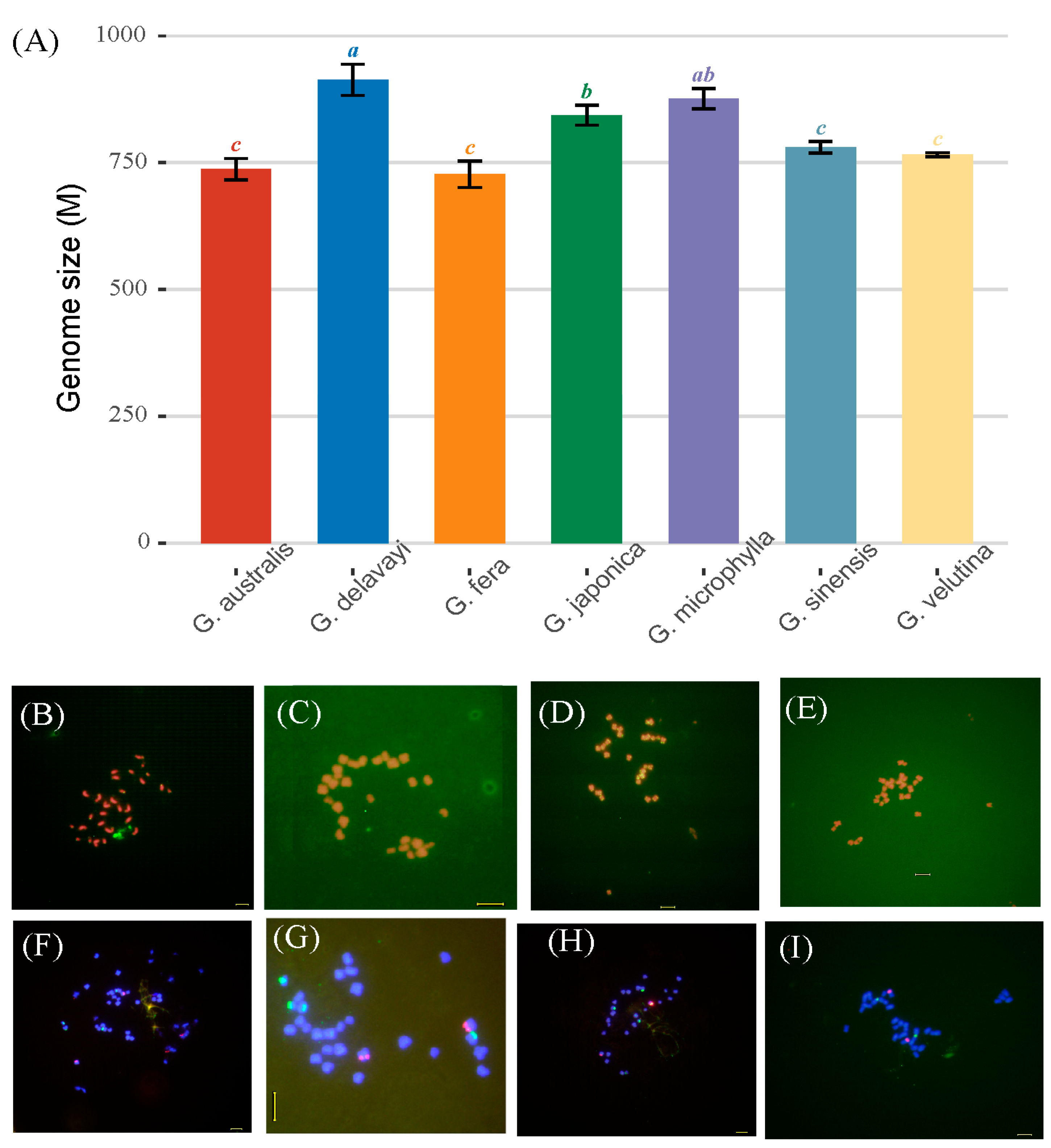
3.1. Genome Size and Chromosome Number Analysis
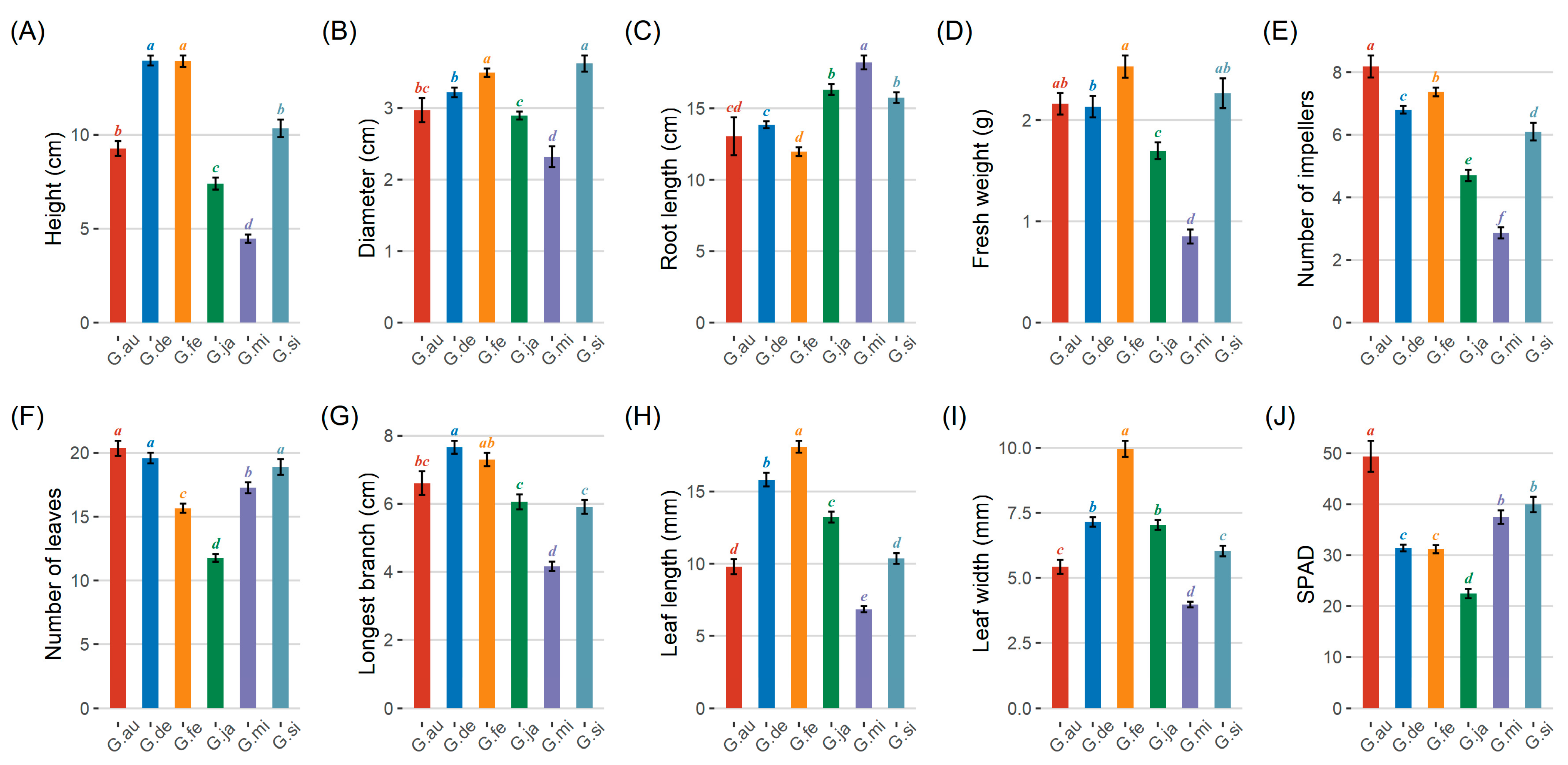
3.2. Growth Differences in Gleditsia Species
3.3. Differences in Photosynthetic Indicators and Physiological Characteristics
3.4. Differences in Endogenous Hormone Content
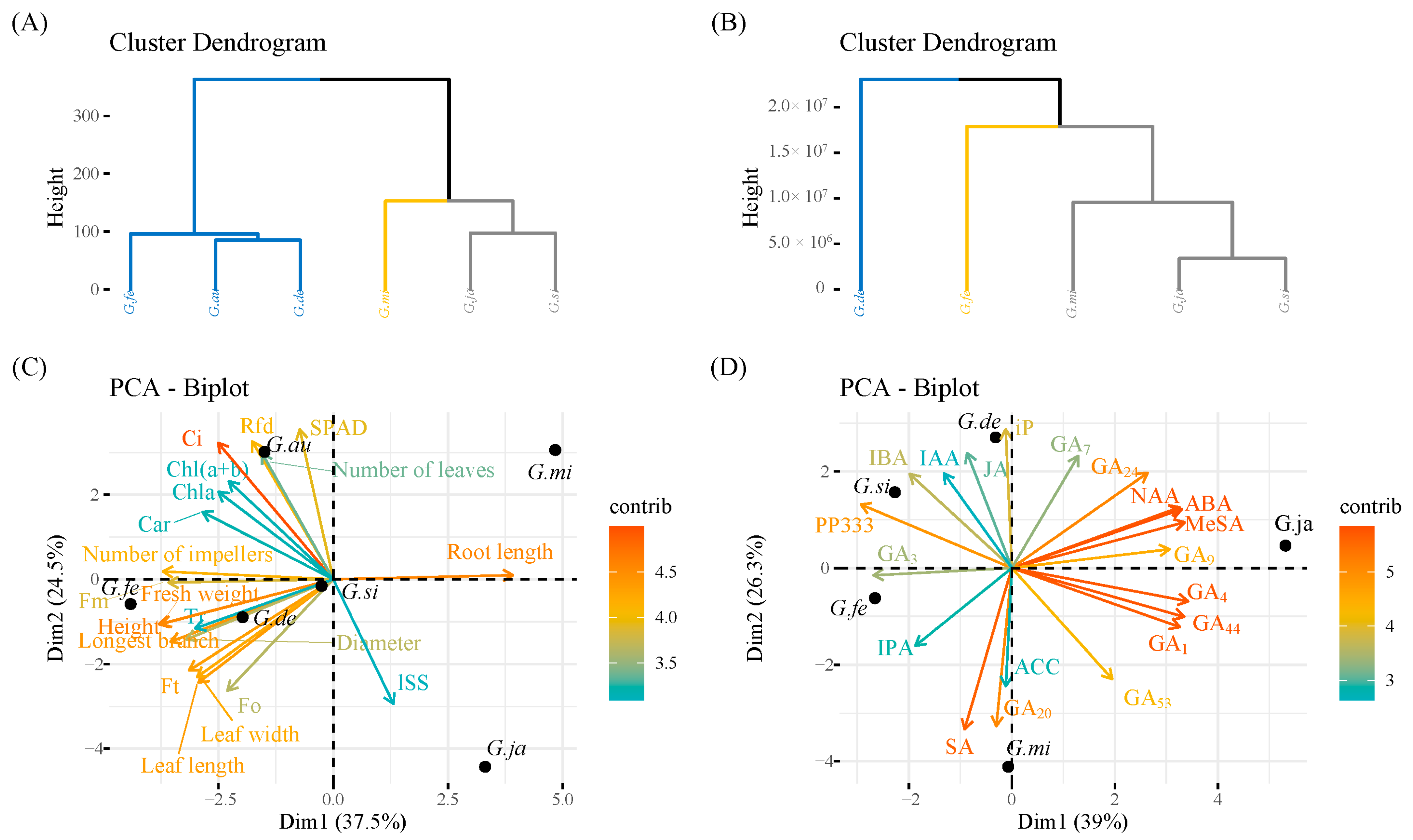
3.5. Cluster Analysis and Principal Component Analysis
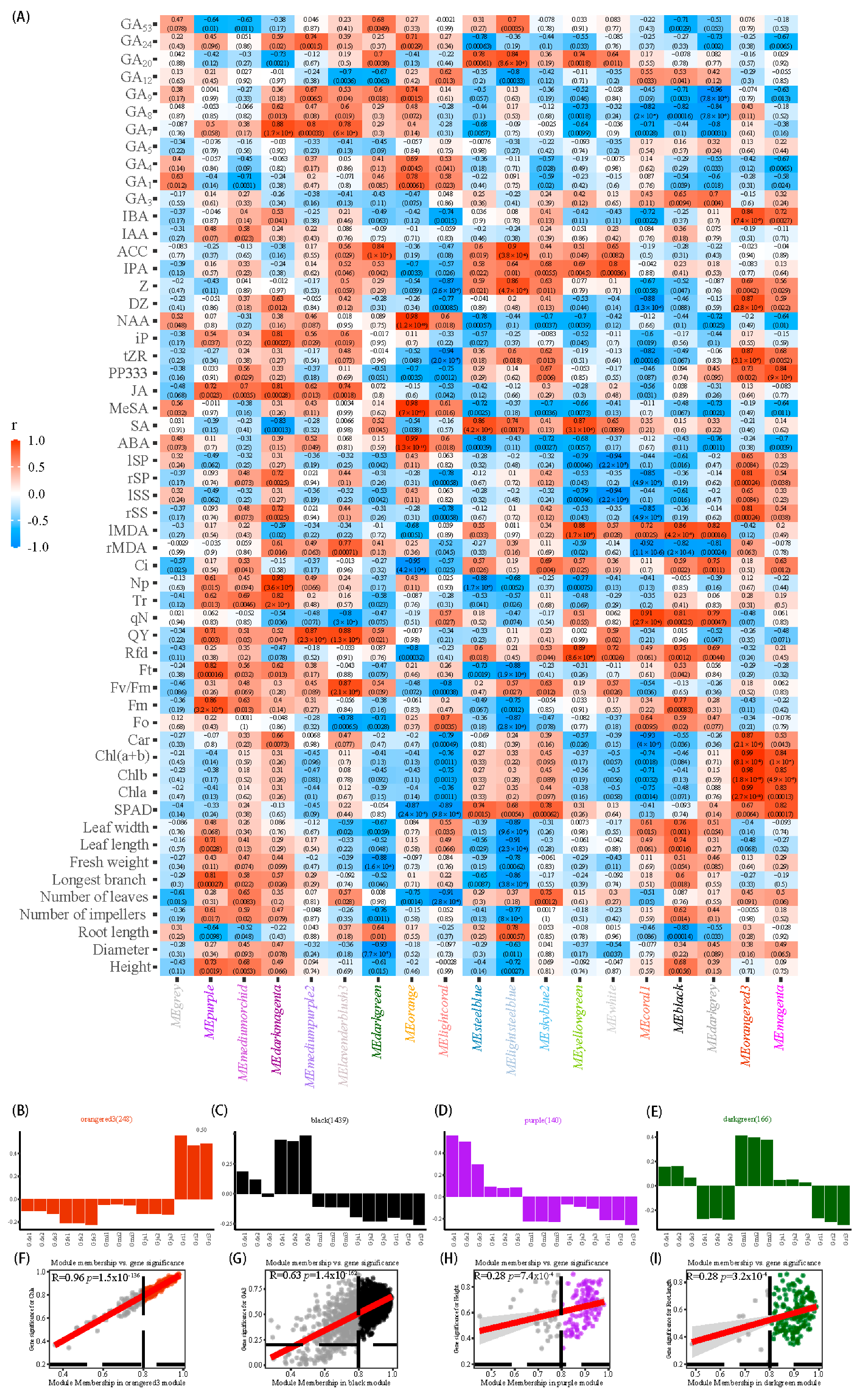
3.6. RNA-Seq Analysis and WGCNA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.P.; Tian, X.H.; Yang, Y.X.; Liu, Q.X.; Wang, Q.; Chen, L.P.; Li, H.L.; Zhang, W.D. Gleditsia species: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 178, 155–171. [Google Scholar] [CrossRef]
- Lan, Y.; Zhou, L.; Li, S.; Cao, Q.; Lan, W. Advances in research of Gleditsia and its prospect of industrializational development. World For. Res 2004, 6, 17–21. [Google Scholar]
- Gu, W.; Cuiling, S.; Yanping, L. Research advances and utilization development of Gleditsia sinensis in world. Sci. Silvae Sin. 2003, 39, 127–133. [Google Scholar]
- Xiao, F.; Zhao, Y.; Wang, X.; Sun, Y. Comparative Transcriptome Analysis of Gleditsia sinensis Thorns at Different Stages of Development. Plants 2023, 12, 1456. [Google Scholar] [CrossRef]
- Bai, J.; Jing, X.; Yang, Y.; Wang, X.; Feng, Y.; Ge, F.; Li, J.; Yao, M. Comprehensive profiling of chemical composition of Gleditsiae spina using ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2023, 37, e9467. [Google Scholar] [CrossRef]
- Li, J.; Ye, C. Genome-wide analysis of microsatellite and sex-linked marker identification in Gleditsia sinensis. BMC Plant Biol. 2020, 20, 338. [Google Scholar] [CrossRef]
- Ashraf, H.; Moussa, A.Y.; Eldahshan, O.A.; Singab, A.N.B. Genus Gleditsia: A Phytochemical and Biological Review (2015–2020). J. Biol. Act. Prod. Nat. 2022, 12, 1–23. [Google Scholar] [CrossRef]
- Han, S.; Wu, Z.; Wang, X.; Huang, K.; Jin, Y.; Yang, W.; Shi, H. De novo assembly and characterization of Gleditsia sinensis transcriptome and subsequent gene identification and SSR mining. Genet. Mol. Res. 2016, 15, gmr.15017740. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Lei, F.; Li, P.; Jiang, J. Comparison and characterization of galactomannan at different developmental stages of Gleditsia sinensis Lam. Carbohydr. Polym. 2019, 223, 115127. [Google Scholar] [CrossRef]
- Sun, M.; Sun, Y.; Li, Y.; Liu, Y.; Liang, J.; Zhang, Z. Physical properties and antidiabetic potential of a novel galactomannan from seeds of Gleditsia japonica var. delavayi. J. Funct. Foods 2018, 46, 546–555. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Wang, X.; Zhao, Y. Studies on Pollen Morphology, Pollen Vitality and Preservation Methods of Gleditsia sinensis Lam.(Fabaceae). Forests 2023, 14, 243. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Zhao, Y.; He, K. Effects of Different Temperatures on Growth and Physiological Characteristics of Gleditsia sinensis Seedlings. J. Mt. Agric. Biol. 2022, 41, 22–29. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Gómez-Plaza, E.; Bautista-Ortín, A.B.; Garde-Cerdán, T.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Rootstock effects on grape anthocyanins, skin and seed proanthocyanidins and wine color and phenolic compounds from Vitis vinifera L. Merlot grapevines. J. Sci. Food Agric. 2019, 99, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 47. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.J.; Carraway, E.R.; Hoffmann, M.R. Photocatalytic production of H2O2 and organic peroxides on quantum-sized semiconductor colloids. Environ. Sci. Technol. 1994, 28, 776–785. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. In Extract and Visualize the Results of Multivariate Data Analyses; Factoextra: Vienna, Austria, 2017; p. 76. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Vrána, J.; Cápal, P.; Bednářová, M.; Doležel, J. Flow cytometry in plant research: A success story. In Applied Plant Cell Biology: Cellular Tools and Approaches for Plant Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 395–430. [Google Scholar]
- Maragheh, F.P.; Janus, D.; Senderowicz, M.; Haliloglu, K.; Kolano, B. Karyotype analysis of eight cultivated Allium species. J. Appl. Genet. 2019, 60, 1–11. [Google Scholar] [CrossRef]
- Hemleben, V.; Volkov, R.A.; Zentgraf, U.; Medina, F.J. Molecular cell biology: Organization and molecular evolution of rDNA, nucleolar dominance, and nucleolus structure. In Progress in Botany: Genetics Physiology Systematics Ecology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 106–146. [Google Scholar]
- de Souza, T.B.; Gaeta, M.L.; Martins, C.; Vanzela, A.L.L. IGS sequences in Cestrum present AT- and GC-rich conserved domains, with strong regulatory potential for 5S rDNA. Mol. Biol. Rep. 2020, 47, 55–66. [Google Scholar] [CrossRef]
- Bersaglieri, C.; Santoro, R. Genome Organization in and around the Nucleolus. Cells 2019, 8, 579. [Google Scholar] [CrossRef]
- Ammiraju, J.S.; Zuccolo, A.; Yu, Y.; Song, X.; Piegu, B.; Chevalier, F.; Walling, J.G.; Ma, J.; Talag, J.; Brar, D.S. Evolutionary dynamics of an ancient retrotransposon family provides insights into evolution of genome size in the genus Oryza. Plant J. 2007, 52, 342–351. [Google Scholar] [CrossRef]
- Yan, H.; Martin, S.L.; Bekele, W.A.; Latta, R.G.; Diederichsen, A.; Peng, Y.; Tinker, N.A. Genome size variation in the genus Avena. Genome 2016, 59, 209–220. [Google Scholar] [CrossRef]
- Nandini, A.; Murray, B.; O’BRIEN, I.; Hammett, K. Intra-and interspecific variation in genome size in Lathyrus (Leguminosae). Bot. J. Linn. Soc. 1997, 125, 359–366. [Google Scholar] [CrossRef]
- Paula, A.D.P.D.O.; Santos, G.R.D.; Costa, L.; Pestana, R.; Souza, G.; Sousa, G.M.D.; Leite, A.V.; Carvalho, R.D. Karyotypic variability in Calliandra sect. Androcallis (Leguminosae–Caesalpinioideae). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 730–739. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, Y.; Wang, X.; Yang, Y. Targeted Metabolic and Transcriptomic Analysis of Pinus yunnanensis var. pygmaea with Loss of Apical Dominance. Curr. Issues Mol. Biol. 2022, 44, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.E.; King, K.E.; Ait-Ali, T.; Harberd, N.P. HOW Gibberellin Regulates Plant Growth and Development: A Molecular Genetic Analysis of Gibberellin Signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Cai, B.; Xie, Y.; Chen, Y.; Cao, M.; Feng, J.; Li, Y.; Yan, L.; Wei, Y.; Zhao, Y.; Xie, J.; et al. Transcriptome and Gene Co-Expression Network Analysis Identifying Differentially Expressed Genes and Signal Pathways Involved in the Height Development of Banana (Musa spp.). Int. J. Mol. Sci. 2023, 24, 2628. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Yang, H.; Liao, H.; Xu, F.; Zhang, W.; Xu, B.; Chen, X.; Zhu, B.; Pan, W.; Yang, X. Integrated transcriptomic and gibberellin analyses reveal genes related to branch development in Eucalyptus urophylla. Plant Physiol. Biochem. 2022, 185, 69–79. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Zhang, T.; Zhan, D.; Pang, Z.; Zhao, J.; Zhang, J. Comparative Transcriptomic, Anatomical and Phytohormone Analyses Provide New Insights Into Hormone-Mediated Tetraploid Dwarfing in Hybrid Sweetgum (Liquidambar styraciflua × L. formosana). Front. Plant Sci. 2022, 13, 2024. [Google Scholar] [CrossRef]
- Maharana, R.; Dobriyal, M.J.; Behera, L.; Gunaga, R.; Thakur, N. Effect of pre seed treatment and growing media on germination parameters of Gmelina arborea roxb. Indian J. Ecol. 2018, 45, 623–626. [Google Scholar]
- Kudo, T.; Kiba, T.; Sakakibara, H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 2010, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Flora of China Committee. Flora of China. 2018. Available online: http://www.iplant.cn (accessed on 1 March 2023).
- Yang, J.; Han, F.; Yang, L.; Wang, J.; Jin, F.; Luo, A.; Zhao, F. Identification of reference genes for RT-qPCR analysis in Gleditsia microphylla under abiotic stress and hormone treatment. Genes 2022, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ding, G.; Li, Z.; Fan, F. Full-Length Transcriptome Analysis of the Secondary-Growth-Related Genes of Pinus massoniana Lamb. with Different Diameter Growth Rates. Forests 2023, 14, 811. [Google Scholar] [CrossRef]
- Mo, X.; Chen, H.; Yang, X.; Mo, B.; Gao, L.; Yu, Y. Integrated Analysis of Transcriptome and Small RNAome Reveals the Regulatory Network for Rapid Growth in Mikania micrantha. Int. J. Mol. Sci. 2022, 23, 10596. [Google Scholar] [CrossRef]
- Han, X.; An, Y.; Zhou, Y.; Liu, C.; Yin, W.; Xia, X. Comparative transcriptome analyses define genes and gene modules differing between two Populus genotypes with contrasting stem growth rates. Biotechnol. Biofuels 2020, 13, 139. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Q.; Gao, J.; Wu, Y.; Feng, Z.; Huang, J.; Zou, H.; Zhu, X.; Chen, Y.; Yu, C.; et al. Identify of Fast-Growing Related Genes Especially in Height Growth by Combining QTL Analysis and Transcriptome in Salix matsudana (Koidz). Front. Genet. 2021, 12, 596749. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Y.; Li, J.; Jiao, N.; Zhang, X.; Zhang, Y.; Chen, J.; Tu, Z. RNA Sequencing Reveals Phenylpropanoid Biosynthesis Genes and Transcription Factors for Hevea brasiliensis Reaction Wood Formation. Front. Genet. 2021, 12, 763841. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.F.; Zhao, L.L.; Huang, L.J.; Wang, P.C. Morphological, transcriptomic and metabolomic analyses of Sophora davidii mutants for plant height. BMC Plant Biol. 2022, 22, 144. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Zhao, Y.; Wang, X.; Jian, X. Differences in the Growth of Seedlings and the Selection of Fast-Growing Species in the Gleditsia Genus. Forests 2023, 14, 1464. https://doi.org/10.3390/f14071464
Xiao F, Zhao Y, Wang X, Jian X. Differences in the Growth of Seedlings and the Selection of Fast-Growing Species in the Gleditsia Genus. Forests. 2023; 14(7):1464. https://doi.org/10.3390/f14071464
Chicago/Turabian StyleXiao, Feng, Yang Zhao, Xiurong Wang, and Xueyan Jian. 2023. "Differences in the Growth of Seedlings and the Selection of Fast-Growing Species in the Gleditsia Genus" Forests 14, no. 7: 1464. https://doi.org/10.3390/f14071464
APA StyleXiao, F., Zhao, Y., Wang, X., & Jian, X. (2023). Differences in the Growth of Seedlings and the Selection of Fast-Growing Species in the Gleditsia Genus. Forests, 14(7), 1464. https://doi.org/10.3390/f14071464




