Riparian Vegetation Conversion to an Oil Tea Plantation: Impacts on Small Mammals at the Community, Population, and Individual Level
Abstract
1. Introduction
2. Materials and Methods
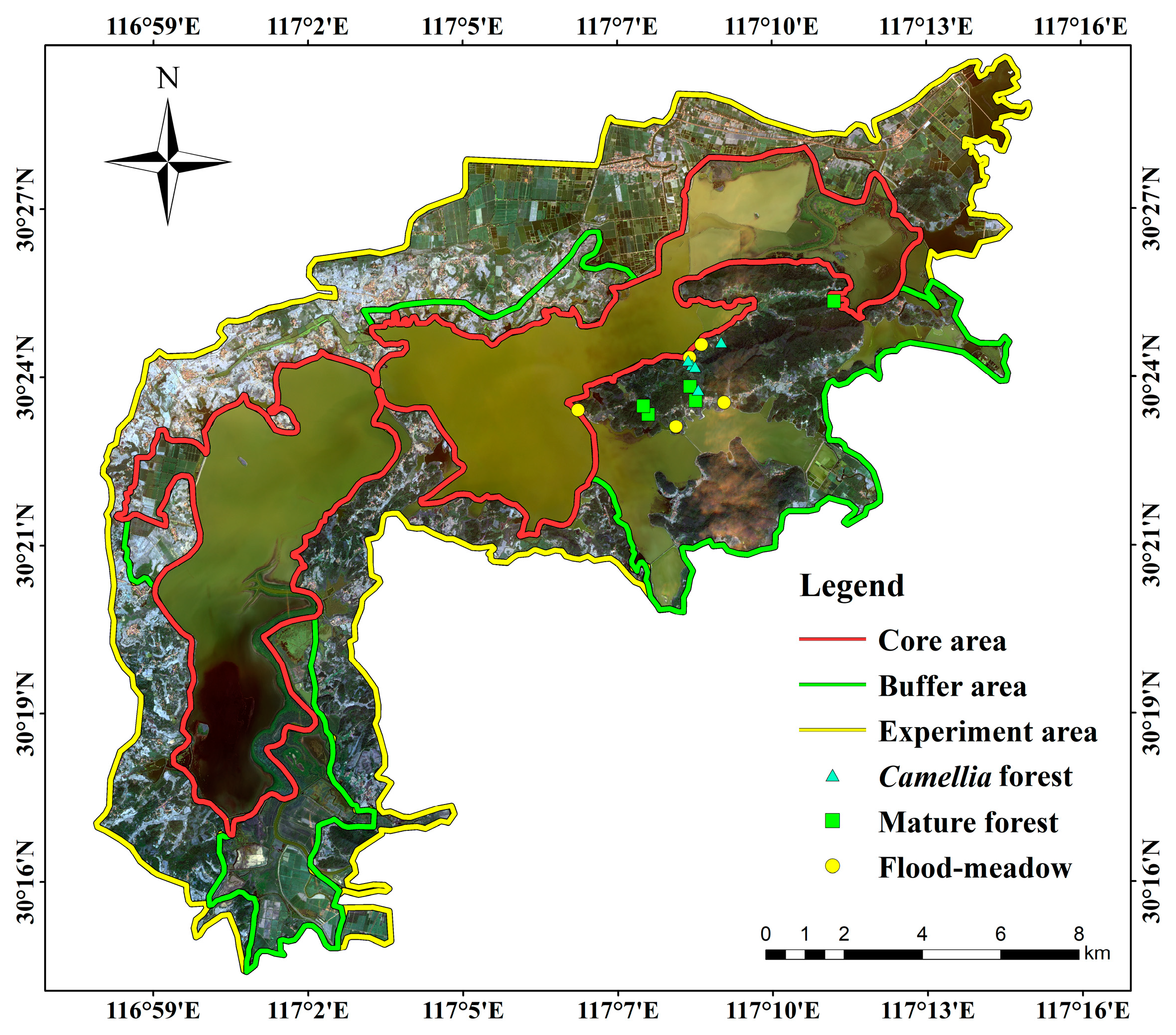
2.1. Study Area
2.2. Quantifying Habitat Characteristics
2.3. Sampling for Small Mammals
2.4. Data Analysis
3. Results
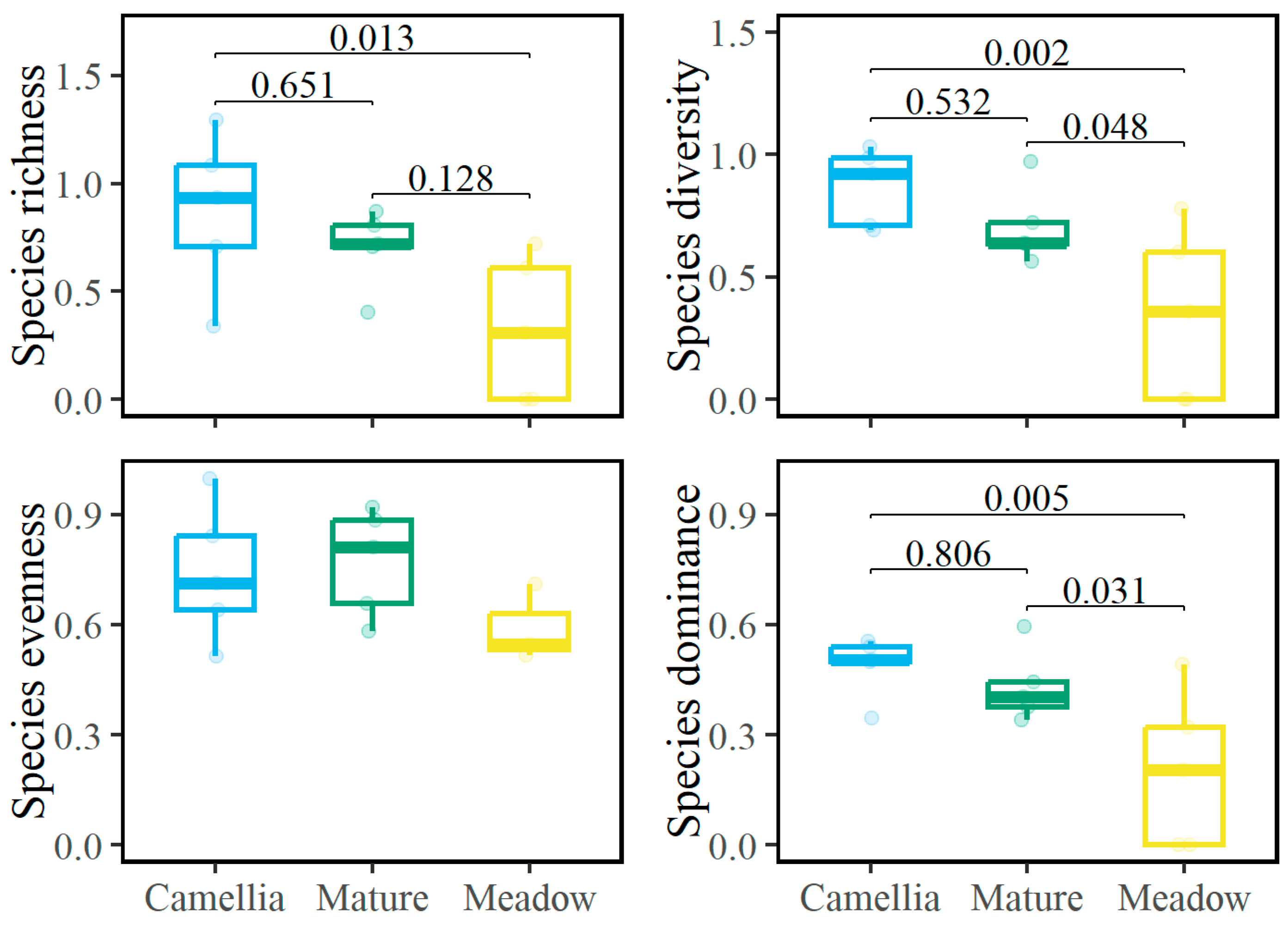
3.1. Community Diversity and Composition
3.2. Population Density and Sex Ratio
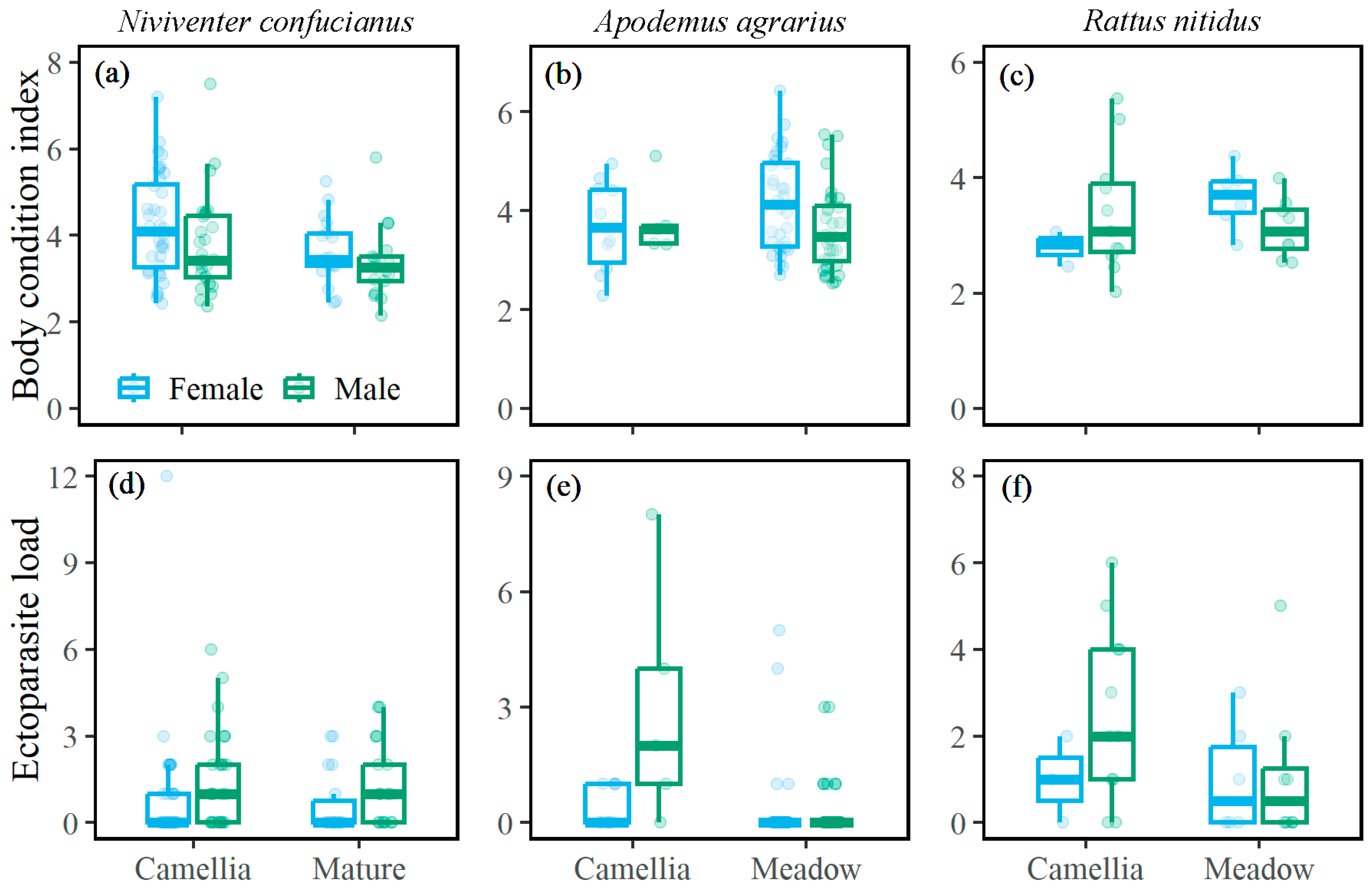
3.3. Body Condition Index and Ectoparasite Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naiman, R.J.; Decamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N. Global overview of ecosystem services provided by riparian vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ci, X.Q.; Hu, J.L.; Bai, Y.; Thornhill, A.H.; Conran, J.G.; Li, J. Riparian areas as a conservation priority under climate change. Sci. Total Environ. 2023, 858, 159879. [Google Scholar] [CrossRef]
- Dala-Corte, R.B.; Melo, A.S.; Siqueira, T.; Bini, L.M.; Martins, R.T.; Cunico, A.M.; Pes, A.M.; Magalhães, A.L.; Godoy, B.S.; Leal, C.G.; et al. Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J. Appl. Ecol. 2020, 57, 1391–1402. [Google Scholar] [CrossRef]
- Marczak, L.B.; Sakamaki, T.; Turvey, S.L.; Deguise, I.; Wood, S.L.; Richardson, J.S. Are forested buffers an effective conservation strategy for riparian fauna? An assessment using meta-analysis. Ecol. Appl. 2010, 20, 126–134. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible halophytes and halo-tolerant species in Apulia region (Southeastern Italy): Biogeography, traditional food use and potential sustainable crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Tonello, G.; Decian, V.S.; Restello, R.M.; Hepp, L.U. The conversion of natural riparian forests into agricultural land affects ecological processes in Atlantic forest streams. Limnologica 2021, 91, 125927. [Google Scholar] [CrossRef]
- Sheaves, M.; Johnston, R.; Miller, K.; Nelson, P.N. Impact of oil palm development on the integrity of riparian vegetation of a tropical coastal landscape. Agric. Ecosyst. Environ. 2018, 262, 1–10. [Google Scholar] [CrossRef]
- Espinoza-Toledo, A.; Mendoza-Carranza, M.; Castillo, M.M.; Barba-Macías, E.; Capps, K.A. Taxonomic and functional responses of macroinvertebrates to riparian forest conversion in tropical streams. Sci. Total Environ. 2021, 757, 143972. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Felipe-Lucia, M.R.; Bourgeois, B.; Boz, B.; Nilsson, C.; Palmer, G.; Sher, A.A. Integrative conservation of riparian zones. Biol. Conserv. 2017, 211, 20–29. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Hughes, F.M.; Rood, S.B. Allocation of river flows for restoration of floodplain forest ecosystems: A review of approaches and their applicability in Europe. Environ. Manag. 2003, 32, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Pisani, D.; Pazienza, P.; Perrino, E.V.; Caporale, D.; De Lucia, C. The economic valuation of ecosystem services of biodiversity components in protected areas: A review for a framework of analysis for the Gargano National Park. Sustainability 2021, 13, 11726. [Google Scholar] [CrossRef]
- Guo, X.B.; Tang, X.Z.; He, H.L. Data production and data product standards construction of long-term observation for ecosystem. China Sci. Technol. Resour. Rev. 2021, 53, 47–54. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Zhou, Y.L.; Zheng, Q.; Luo, S.Y.; Xiang, T.T.; Zhou, L.J.; Feng, S.L.; Yang, H.Y.; Ding, C.B. Characterization and comprehensive evaluation of phenotypic characters in wild Camellia oleifera germplasm for conservation and breeding. Front. Plant Sci. 2023, 14, 1052890. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Zhang, Z.B.; Wang, Y.S. Impacts of scatter-hoarding rodents on restoration of oil tea Camellia oleifera in a fragmented forest. For. Ecol. Manag. 2004, 196, 405–412. [Google Scholar] [CrossRef]
- Deng, Q.E.; Li, J.N.; Gao, C.; Cheng, J.Y.; Deng, X.Z.; Jiang, D.Z.; Li, L.; Yan, P. New perspective for evaluating the main Camellia oleifera cultivars in China. Sci. Rep. 2020, 10, 20676. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.L.; Su, E.Z.; Chen, Y.Z.; Cao, F.L. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Chowdhury, A.; Samrat, A.; Devy, M.S. Can tea support biodiversity with a few “nudges” in management: Evidence from tea growing landscapes around the world. Glob. Ecol. Conserv. 2021, 31, e01801. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, L.S.; Hu, Y.J.; Deng, C.H.; Sun, Q.; Deng, S.H.; Chen, X.B.; Mei, L.; Wu, J.S.; Su, Y.R. Soil microbial resource limitations and community assembly along a Camellia oleifera plantation chronosequence. Front. Microbiol. 2021, 12, 736165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lichao, W.; Sun, Q.; Chen, Y.Q.; Wang, C.Y.; Lu, S. Long-term Camellia oleifera cultivation influences the assembly process of soil bacteria in different soil aggregate particles. Land Degrad. Dev. 2023, 34, 441–452. [Google Scholar] [CrossRef]
- Chang, G.; Zhang, Z.B. Differences in hoarding behaviors among six sympatric rodent species on seeds of oil tea (Camellia oleifera) in Southwest China. Acta Oecol. 2011, 37, 165–169. [Google Scholar] [CrossRef]
- Wu, N.; Zhong, J.; Lei, B.Y.; Xie, Z.Q.; Zhou, Y.B. Community reestablishment and poor body conditions of small mammal assemblages in subtropical afforested ecosystems. Ecol. Eng. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Curtin, C.G.; Kelt, D.A.; Frey, T.C.; Brown, J.H. On the role of small mammals in mediating climatically driven vegetation change. Ecol. Lett. 2000, 3, 309–317. [Google Scholar] [CrossRef]
- Xiao, Z.S. Dual ecological functions of scatter-hoarding rodents: Pollinators and seed dispersers of Mucuna sempervirens (Fabaceae). Integr. Zool. 2022, 17, 918–929. [Google Scholar] [CrossRef]
- Surkova, E.; Popov, S.; Tchabovsky, A. Rodent burrow network dynamics under human-induced landscape transformation from desert to steppe in Kalmykian rangelands. Integr. Zool. 2019, 14, 410–420. [Google Scholar] [CrossRef]
- DB34/T3422-2019; Technical Specification for Ecological Monitoring of Wetlands of Provincial Importance and General Wetlands. Local Standards of Anhui Province: Hefei, China, 2019.
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Teixeira, D.; Carrilho, M.; Silva, M.; Nunes, M.; Vieira, M.L.; Novo, M.T.; Santos-Reis, M.; Rosalino, L.M. Mediterranean Eucalyptus plantations affect small mammal ectoparasites abundance but not individual body condition. Ecol. Res. 2019, 34, 415–427. [Google Scholar] [CrossRef]
- Li, C.L.; Zhou, L.Z.; Xu, L.; Zhao, N.N.; Beauchamp, G. Vigilance and activity time-budget adjustments of wintering hooded cranes, Grus monacha, in human-dominated foraging habitats. PLoS ONE 2015, 10, e0118928. [Google Scholar] [CrossRef]
- Wang, M.Z.; Chen, W.W.; Li, C.L.; Zhao, J.M. Definition of spatial positions within foraging Greater White-fronted Geese flocks from an individual’s perspective: Cost-benefit dynamics change with the distance to flock edge. Avian Res. 2022, 13, 100056. [Google Scholar] [CrossRef]
- Wan, W.J.; Zhou, L.Z.; Song, Y.W. Shifts in foraging behavior of wintering Hooded Cranes (Grus monacha) in three different habitats at Shengjin Lake, China. Avian Res. 2016, 7, 13. [Google Scholar] [CrossRef]
- Pan, C.; Zhou, L.Z.; Wang, X.H.; Xu, W.B.; Song, Y.W. Impact of artificial activities on the landscape patterns in Shengjin Lake National Nature Reserve. Ecol. Sci. 2021, 40, 116. [Google Scholar] [CrossRef]
- Peng, L.; Dong, B.; Wang, P.; Sheng, S.W.; Sun, L.; Fang, L.; Li, H.R.; Liu, L.P. Research on ecological risk assessment in land use model of Shengjin Lake in Anhui province, China. Environ. Geochem. Health 2019, 41, 2665–2679. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Zhou, Z.Z.; Chen, J.W.; Zheng, X.D.; Ye, X.Y. Effects of extreme flooding on aquatic vegetation cover in Shengjin Lake, China. Hydrol. Process. 2022, 36, e14459. [Google Scholar] [CrossRef]
- Smith, A.T.; Xie, Y.; Hoffmann, R.S.; Lunde, D.; MacKinnon, J.; Wilson, D.E.; Wozencraft, W.C.; Gemma, F. A Guide to the Mammals of China; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Chen, W.W.; Zhong, J.; Carson, W.P.; Tang, Z.H.; Xie, Z.Q.; Sun, S.C.; Zhou, Y.B. Proximity to roads disrupts rodents’ contributions to seed dispersal services and subsequent recruitment dynamics. J. Ecol. 2019, 107, 2623–2634. [Google Scholar] [CrossRef]
- Zhang, C. Statistical Modeling of Count Data with Over-Dispersion or Zero-Inflation Problems. Master’s Thesis, Montclair State University, Montclair, NJ, USA, 2019. [Google Scholar]
- Hardy, I.C. Sex Ratios: Concepts and Research Methods; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Schulte-Hostedde, A.I.; Zinner, B.; Millar, J.S.; Hickling, G.J. Restitution of mass–size residuals: Validating body condition indices. Ecology 2005, 86, 155–163. [Google Scholar] [CrossRef]
- Hillegass, M.A.; Waterman, J.M.; Roth, J.D. The influence of sex and sociality on parasite loads in an African ground squirrel. Behav. Ecol. 2008, 19, 1006–1011. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Stirkė, V.; Balčiauskienė, L. Rodent fertility in commercial orchards in relation to body mass and body condition. Agric. Ecosyst. Environ. 2022, 329, 107886. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends. Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef]
- Semenchuk, P.; Plutzar, C.; Kastner, T.; Matej, S.; Bidoglio, G.; Erb, K.-H.; Essl, F.; Haberl, H.; Wessely, J.; Krausmann, F. Relative effects of land conversion and land-use intensity on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Garden, J.G.; Mcalpine, C.A.; Possingham, H.P.; Jones, D.N. Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: A case study from Brisbane, Australia. Austral Ecol. 2007, 32, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.A.; Simonetti, J.A. Conservation opportunities in commercial plantations: The case of mammals. J. Nat. Conserv. 2011, 19, 351–355. [Google Scholar] [CrossRef]
- Carrilho, M.; Teixeira, D.; Santos-Reis, M.; Rosalino, L.M. Small mammal abundance in Mediterranean Eucalyptus plantations: How shrub cover can really make a difference. For. Ecol. Manag. 2017, 391, 256–263. [Google Scholar] [CrossRef]
- Ge, D.Y.; Lu, L.; Abramov, A.V.; Wen, Z.X.; Cheng, J.L.; Xia, L.; Vogler, A.P.; Yang, Q.S. Coalescence models reveal the rise of the white-bellied rat (Niviventer confucianus) following the loss of Asian megafauna. J. Mamm. Evol. 2019, 26, 423–434. [Google Scholar] [CrossRef]
- Morand, S.; Bordes, F.; Blasdell, K.; Pilosof, S.; Cornu, J.F.; Chaisiri, K.; Chaval, Y.; Cosson, J.F.; Claude, J.; Feyfant, T. Assessing the distribution of disease-bearing rodents in human-modified tropical landscapes. J. Appl. Ecol. 2015, 52, 784–794. [Google Scholar] [CrossRef]
- Zhang, M.W.; Wang, Y.; Li, B.; Guo, C.; Huang, G.X.; Shen, G.; Zhou, X.J. Small mammal community succession on the beach of Dongting Lake, China after the Three Gorges Project. Integr. Zool. 2014, 9, 294–308. [Google Scholar] [CrossRef]
- Prevedello, J.A.; Dickman, C.R.; Vieira, M.V.; Vieira, E.M. Population responses of small mammals to food supply and predators: A global meta-analysis. J. Anim. Ecol. 2013, 82, 927–936. [Google Scholar] [CrossRef]
- Gębczyńska, Z.; Sołtys, H.; Sienkiewicz, M. Food composition in striped field mice living at localities of various degrees of urban development. Acta Theriol. 1987, 32, 325–330. [Google Scholar] [CrossRef]
- Dyson, E.A.; Hurst, G.D. Persistence of an extreme sex-ratio bias in a natural population. Proc. Natl. Acad. Sci. USA 2004, 101, 6520–6523. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Grimm, K.M.; Livingston, K.A.; Brokman, A.M.; Lamberson, W.E.; Roberts, R.M. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc. Natl. Acad. Sci. USA 2003, 100, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Roberts, R.M. Maternal diet and other factors affecting offspring sex ratio: A review. Biol. Reprod. 2004, 71, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Lei, B.Y.; Newman, C.; Zhou, Y.B.; Wang, Z.P. Food resources and competition rather than eco-geographic rules explain trait variations in two contrasting rat species: Implications for future climate change. Glob. Ecol. Conserv. 2022, 40, e02339. [Google Scholar] [CrossRef]
- Côté, I.M.; Poulinb, R. Parasitism and group size in social animals: A meta-analysis. Behav. Ecol. 1995, 6, 159–165. [Google Scholar] [CrossRef]
- Stanko, M.; Miklisová, D.; Goüy de Bellocq, J.; Morand, S. Mammal density and patterns of ectoparasite species richness and abundance. Oecologia 2002, 131, 289–295. [Google Scholar] [CrossRef]
- Friggens, M.M.; Beier, P. Anthropogenic disturbance and the risk of flea-borne disease transmission. Oecologia 2010, 164, 809–820. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Morand, S.; Laurance, S.G.W.; Doggett, S.L.; Hahs, A.; Trinh, K.; Perera, D.; Firth, C. Rats and the city: Implications of urbanization on zoonotic disease risk in Southeast Asia. Proc. Natl. Acad. Sci. USA 2022, 119, e2112341119. [Google Scholar] [CrossRef]
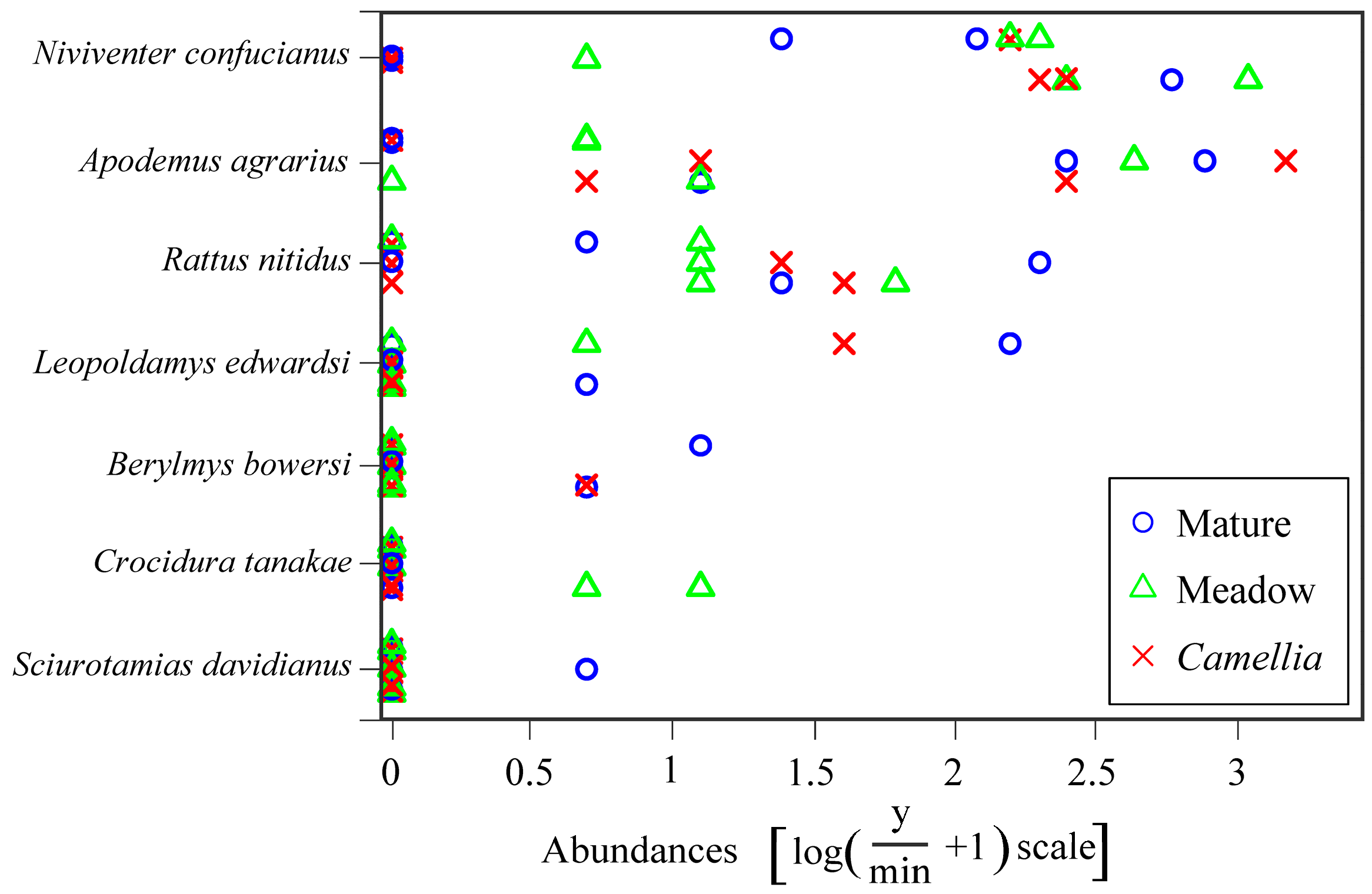

| Species | Family | Camellia Forest | Mature Forest | Flood-Meadow |
|---|---|---|---|---|
| Apodemus agrarius | Muridae | 15 | 2 | 65 |
| Berylmys bowersi | Muridae | 2 | 2 | 0 |
| Niviventer confucianus | Muridae | 64 | 35 | 1 |
| Rattus nitidus | Muridae | 14 | 3 | 14 |
| Leopoldamys edwardsi | Muridae | 1 | 13 | 0 |
| Sciurotamias davidianus | Sciuridae | 0 | 0 | 1 |
| Crocidura tanakae | Soricidae | 3 | 0 | 0 |
| Total | 99 | 55 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-L.; Tang, Y.-S.; Wang, Y.-J.; Wang, J.-N.; Wang, Z.; Zhang, B.-W.; Chen, W.-W.; Pan, Y.; Chen, X.-S. Riparian Vegetation Conversion to an Oil Tea Plantation: Impacts on Small Mammals at the Community, Population, and Individual Level. Forests 2023, 14, 1169. https://doi.org/10.3390/f14061169
Zhang L-L, Tang Y-S, Wang Y-J, Wang J-N, Wang Z, Zhang B-W, Chen W-W, Pan Y, Chen X-S. Riparian Vegetation Conversion to an Oil Tea Plantation: Impacts on Small Mammals at the Community, Population, and Individual Level. Forests. 2023; 14(6):1169. https://doi.org/10.3390/f14061169
Chicago/Turabian StyleZhang, Lei-Lei, Yun-Sheng Tang, Yu-Jue Wang, Jia-Neng Wang, Zheng Wang, Bao-Wei Zhang, Wen-Wen Chen, Ying Pan, and Xin-Sheng Chen. 2023. "Riparian Vegetation Conversion to an Oil Tea Plantation: Impacts on Small Mammals at the Community, Population, and Individual Level" Forests 14, no. 6: 1169. https://doi.org/10.3390/f14061169
APA StyleZhang, L.-L., Tang, Y.-S., Wang, Y.-J., Wang, J.-N., Wang, Z., Zhang, B.-W., Chen, W.-W., Pan, Y., & Chen, X.-S. (2023). Riparian Vegetation Conversion to an Oil Tea Plantation: Impacts on Small Mammals at the Community, Population, and Individual Level. Forests, 14(6), 1169. https://doi.org/10.3390/f14061169





