Abstract
The difficult propagation of shrub and tree species and their extensive exposure to grazing threaten their abundance and lead to the necessity to find alternative means of propagation for these species. In vitro micropropagation techniques, viz., tissue culture, offer a promising tool for the rapid, cost-effective, and efficient propagation of different plant species. In the current study, a rapid and efficient in vitro multiplication protocol was developed for the micropropagation of Maerua crassifolia Forssk. Our results revealed that Murashige and Skoog (MS) medium with 7.5 µM of 6-benzylaminopurine (BA) and 1.0 µM of 1-naphthaleneacetic acid (NAA) led to the highest shoot formation (13.9 shoots per explant in 85.7% of the cultivated hypocotyls) among all other treatments. The best in vitro root formation was obtained on half-strength MS medium with 1.0 µM of indole 3-butyric acid (IBA) as 94.1% of the cultivated shoots formed 6.8 roots per microshoot on average. Ninety percent of the rooted plantlets were successfully acclimatized and are currently growing in the botanical garden of the Botany and Microbiology Department, King Saud University, Riyadh, Saudi Arabia. The genetic fidelity of the micropropagated plants was authenticated via flow cytometry. The results of the current study explained a simple, cost-effective, and efficient protocol for the micropropagation of the endangered M. crassifolia trees.
1. Introduction
Maerua crassifolia Forssk. (Capparaceae) is a Sahelian species vulnerable to extinction due to the overexploitation of its flowers, fruits, and bark, grazing on young shoots, insect attacks on fruits, and soil erosion. This species is very characteristic of the arid and semi-arid landscapes of Saudi Arabia and is able to withstand drought for a long time. Its abundance and resistance to the harshest climatic conditions are mentioned [1]. The tree is also exposed to overgrazing because of the high value of the leaves for livestock, especially camels. It has historically been used to cure stomach ulcers, toothaches, and wounds [2]. The components of M. crassifolia have been examined by previous phytochemical research, which found the presence of tannins, steroids, flavonoids, cardiac glycosides, saponins, alkaloids, phenols, and terpenoids [3,4]. It was also reported that the leaves show analgesic, antipyretic, anti-inflammatory, and antimalarial properties [4,5]. The methanolic extract of M. crassifolia showed antibacterial activity against a variety of bacteria [6]. Furthermore, the aqueous extract of the leaves showed high free radical scavenging activity as confirmed by the DPPH (2,2-diphenyl-1-picrylhydrazyl) test [7]. With its high importance for grazing and medical uses and with no studies regarding the mass micropropagation of M. crassifolia, we designed this experiment to establish an efficient and rapid protocol for the in vitro propagation of this economically and medicinally important tree.
As a well-established technique for the propagation of difficult-to-propagate species, tissue culture now stands as one of the most prominent solutions to overcome the seed dormancy problems of tree species. The overexploitation of M. crassifolia for medicinal purposes and as fodder coupled with the slow growth of the tree raises the need for a rapid and efficient means of propagation. Tissue culture enables the controlled growth and significant multiplication of plants from very small explants of the plant, e.g., hypocotyls, segments of roots, leaves, or stems, on an artificial medium [8], and therefore becomes an integral part of plant biotechnology. This technique has been successfully applied to the propagation of several important and endangered plant species [8,9,10,11,12].
A previous study examined the in vitro propagation potential of M. crassifolia using leaves and immature fruits, with no success of regeneration [13], whereas there are two reports on the micropropagation of another species of the same genus, M. oblongifolia Forrsk. [14,15]. The authors of these studies used Murashige and Skoog (MS) medium supplemented with several combinations of cytokinins including 6-Benzylaminopurine (BA) and Kinetin (Kin) and auxins such as 1-Naphthaleneacetic acid (NAA), indole 3-butyric acid (IBA), and indole-3-acetic acid (IAA). In both reports, most of the regenerated plantlets were successfully acclimated [14,15] and survived when transferred to field conditions [15].
With no successful studies on the micropropagation of M. crassifolia, we designed this experiment with the aim to develop a rapid and efficient micropropagation protocol via the cultivation of hypocotyls. The genetic fidelity of the regenerated plants was examined using flow cytometric analysis to ensure the production of authentic in vitro propagated plants.
2. Materials and Methods
2.1. Plant Material and Surface Sterilization
Samples of Maerua crassifolia Forssk. seeds were collected from 10 randomly selected trees distributed across Dirab region (24°20′35″ and 24°20′51″ N; 46°31′41″ and 46°45′34″ E), Riyadh, Saudi Arabia. The seeds were immediately transported to the lab in the Botany and Microbiology Department, King Saud University, for further processing and cultivation. The seeds were washed for 15–20 min by running tap water followed by a 5 min wash using a commercial detergent solution (5%), and then rinsed 4–5 times using sterile Milli-Q ultrapure water. The seeds were then transferred to the laminar air flow hood (ESCO Labculture® Class II Type A2 Biological Safety Cabinet, Esco Micro Pte. Ltd., Singapore) and surface sterilized using 0.1% HgCl2 solution (prepared using commercial Clorox) for 3 min at room temperature. The treated seeds were finally washed five times using sterile Milli-Q ultrapure water and used for in vitro germination.
2.2. In Vitro Germination of Seeds
The sterilized seeds were germinated in vitro on MS medium [16] supplemented with 3% sucrose, 0.7% agar, 1.0 µM thidiazuron (TDZ), and 0.5 µM of gibberellic acid (GA3) in environmentally controlled growth chambers at a 16/8 h day/night cycle, a temperature of 24 ± 2 °C, and a relative humidity of 50–60%. The medium pH was adjusted to 5.8–5.9 using NaOH or HCl solutions. Each seed was cultivated in a glass culture tube containing 20 mL of media. After two weeks of cultivation, hypocotyls (explants) were transferred, after cutting with sterilized scalpel blades (No. 10) and removing their roots, to their designated cultivation media. Four hypocotyls were cultivated on each plate using sterilized forceps. The explants were arranged in a cross-like arrangement to provide the maximum possible space for each explant. The entire process was performed under aseptic conditions.
2.3. Cultivation Media, Growth Regulators, and Culture Conditions
The explants of M. crassifolia were cultivated on solid MS medium supplemented with 0.7% agar and 3% sucrose along with different combinations of cytokinins, namely, BA or Kin at various concentrations (2.5, 5.0, 7.5, and 10 µm) either alone or in combination with auxins, namely, IBA or NAA with different concentrations (0.1, 0.5, 1.0, and 2.0 µM). The explants were regularly subcultured on the same designated fresh medium every 3 weeks. All cultures were kept in environmentally controlled growth chambers at a 16/8 h day/night cycle with light intensity of 50 mol m−2 s−1 provided by cool LED tubes, a temperature of 24 ± 2 °C, and a relative humidity of 50–60%. Data on the number of shoots and average shoot length were recorded after 8 weeks of cultivation.
2.4. Rooting
The regenerated shoots of M. crassifolia were rooted in vitro. In detail, healthy regenerated microshoots (4–5 cm long) were transferred into culture tubes on ½ MS medium either without growth regulators (control) or supplemented with different concentrations (0.5, 1.0, and 2.0 µM) of IBA or NAA. The number of roots and average root length were recorded after 8 weeks of cultivation on the rooting medium. The number of roots was shown relative to the number of regenerated microshoots.
2.5. Acclimatization
Well-developed plantlets with healthy-looking shoots and roots were carefully removed from the cultivation tubes and rinsed with running tap water to remove any remains of the media. The plantlets were then transplanted into pots (15 cm diameter) filled with commercial potting soil (Planta Guard Potting Soil 40 L, Plantaflor Humus Verkaufs-GmbH, Vechta, Germany). To keep the highest possible humidity levels around the newly transplanted plantlets of M. crassifolia, the plantlets and pots were covered with transparent plastic film. The pots were then kept in the environmentally controlled growth rooms of the Botany and Microbiology Department, King Saud University, Riyadh, Saudi Arabia under a 16/8 h day/night cycle with light intensity of 50 mol m−2 s−1 and a temperature of 24 ± 2 °C. The relative humidity ranged from 70 to 80%.
2.6. Flow Cytometric (FCM) Analysis
For the flow cytometry analysis, fresh young leaves were randomly collected from healthy and fully developed regenerated plantlets. The cell nuclei of the collected leaf samples were isolated mechanically. The leaf sections of approximately 100 mg were gently chopped with new sharp surgical blades in plastic petri dishes containing 1.0 mL of precooled lysis buffer containing MOPS (20 mM), MgCl2 (45 mM), sodium citrate (30 mM) and Triton X-100 (0.1%, (v/v)) of pH 7.0 [17]. The lysate containing nuclei and cell fragments was filtered through a double-layered nylon membrane of 30 μm thickness with the help of a micro-syringe to eliminate cell debris from the mixture. The nuclei in the filtrate were stained after transferring into labeled plastic tubes containing 50 μg mL−1 of propridium iodide (PI) and 5 μL of 10 mg/mL RNAse. After 10 min incubation, the fluorescence intensity of the PI-stained samples was measured using a Muse Cell Analyzer (MuseTM Cell Analyzer, Merck KGaA, Darmstadt, Germany). The nuclei isolated from the young leaves of the donor plants were used as control to compare with the peaks of the regenerated plants.
2.7. Data Analysis
The experiment was completely randomized with 20 replicates for each treatment. The experiment was repeated three times. IBM® SPSS® Statistics version 28.0.0 (IBM-SPSS Inc., Chicago, IL, USA) was used for the statistical analysis of the collected data. One-way analysis of variance (ANOVA) was applied to examine the effect of the different studied factors. The statistical differences between the means (p ≤ 0.05) were examined via Duncan’s multiple range test.
3. Results
3.1. Effect of Cytokinins on the Morphogenetic Response of Hypocotyls
The morphogenetic response of the M. crassifolia hypocotyls to different concentrations of BA and Kin after 8 weeks of cultivation was examined (Table 1). Explants cultivated on MS basal media without any cytokinins (control) failed to grow, but the addition of any type or concentration of growth hormones led to a response (Table 1, Figure 1A). The addition of 7.5 µM BA showed the highest response percentage (71.2%), as well as the highest number of shoots (6.3 shoots/explant) and the highest average shoot length (2.5 cm) among all of the treatments (Figure 1B). Kinetin produced less growth compared to BA. The best Kin treatment (7.5 μM) produced significantly fewer shoots/explants (4.5), which were shorter (2.3 cm) and occurred in only 59.2% of the cultivated explants. The worst response was obtained with the lowest concentration of Kin (2.5 μM), as only 36.6% of the explants were reactive, producing 1.8 shoots per explant with an average shoot length of 1.8 cm (Table 1).

Table 1.
Effect of cytokinins, i.e., 6-benzylaminopurine (BA) and Kinetin (Kin), on shoot regeneration from hypocotyls of Maerua crassifolia.

Figure 1.
In vitro micropropagation of Maerua crassifolia. (A) An example of the hypocotyls (explants) that were cultured on solid MS medium supplemented with 0.7% agar and 3% sucrose with different combinations of cytokinins (BA or Kin) at various concentrations (2.5, 5.0, 7.5, and 10 µm) either alone or in combination with auxins (IBA or NAA) with different concentrations (0.1, 0.5, 1.0, and 2.0 µM) after 1 week of cultivation. (B) Explants cultivated on MS medium with 7.5 µM BA after 8 weeks of cultivation. Explants were regularly subcultured on the same designated fresh medium every 3 weeks to promote shoot growth. (C) Explants cultivated on MS media with 7.5 µM of BA and 1.0 µM of IBA after 8 weeks of cultivation. (D) Plantlets rooted on ½ MS medium containing 1.0 µM of IBA after 8 weeks of cultivation. (E) Rooted plants before transplantation. (F) A well-established micropropagated plant of M. crassifolia showing normal growth after 3–4 months of acclimatization.
3.2. Synergistic Effect of Cytokinins and Auxins on the Morphogenetic Response of Hypocotyls
The optimal concentration of BA (7.5 µM) was used in combination with different concentrations (0.1, 0.5, 1.0, and 2.0 µM) of two auxins (NAA, IBA) to examine the synergistic effects between the auxins and cytokinins (Table 2). MS medium supplemented with 7.5 µM of BA alone served as the control for this experiment. Our results revealed that 1.0 µM was the optimum concentration of both auxins as compared to other concentrations. Moreover, NAA (1.0 µM) showed the most potent synergistic effect with BA (7.5 µM). Out of all hypocotyls of M. crassifolia cultivated on MS medium supplemented with BA (7.5 µM) and NAA (1.0 µM), 85.7% formed, on average, 13.9 shoots per explant with an average shoot length of 3.1 cm after 8 weeks of cultivation (Figure 1C). The same dose of IBA (1.0 µM) with 7.5 µM of BA led to the formation of 10.0 shoots/explant with an average length of 2.9 cm in 80.9% of the cultivated hypocotyls after 8 weeks of culture. On the other hand, the lowest response percentage was observed in the control treatment (with no auxins). The addition of auxins increased the response gradually with the increasing concentration up to 1.0 µM; however, increasing the concentration further led to reduction in the percentage of responded explants, the average number of shoots, and the average shoot length. Furthermore, NAA was more effective in shoot induction than IBA at all concentrations except 2.0 µM (Table 2).

Table 2.
Synergistic effect of cytokinins, i.e., 6-benzylaminopurine (BA) and auxins, i.e., indole 3-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA), with the optimal concentration of BA (7.5 µM) on shoot regeneration from hypocotyls of Maerua crassifolia.
3.3. Rooting of the In Vitro Generated Shoots
For in vitro root formation, healthy shoots with a length of at least 5 cm were obtained from cultures after 8 weeks of cultivation and cultivated on ½ MS medium either without (control) or with auxins (IBA and NAA) at different concentrations (0.5, 1.0, and 2.0 µM). The results showed that IBA at all concentrations was more effective than NAA in inducing rhizogenesis (Table 3). In all of the examined treatments, the roots were induced within 2–3 weeks of cultivation (Figure 1D). However, the shoots cultivated on ½ MS medium without any auxins did not form any roots at all. After 8 weeks of cultivation on rooting media, the best response was observed on the media supplemented with 1.0 µM of IBA as 94.1% of the cultivated shoots formed an average of 6.8 roots per microshoots with an average root length of 4.3 cm. On the other hand, 0.5 µM of NAA showed the worst rooting induction effect with the lowest response percentage (67.2%), number of roots per microshoots (3.2), and root length (1.9 cm). For both kinds of auxins, increasing the concentration to 2.0 µM led to a decrease in the root formation response percentage compared to the concentration of 1.0 µM (Table 3).

Table 3.
Effect of auxins, i.e., indole 3-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA) on rooting of regenerated shoots of Maerua crassifolia.
3.4. Acclimatization
Healthy rooted plantlets of M. crassifolia with a well-developed root system and at least four full leaves (Figure 1E) were hardened in pots containing commercial potting soil inside the growth chamber and then transplanted into the outdoor garden. The hardened plants were transferred to the pots after 8 weeks of cultivation on rooting medium and roughly 90% of these plants survived the field conditions. The plants have so far maintained growth in the botanical garden of the department with no morphological abnormalities (Figure 1F).
3.5. Flow Cytometric Analysis
In this study, the ploidy levels of nuclei isolated from leaf samples of the in vitro plants and the donor plant were compared using a Muse Cell Analyzer. The FCM analysis of the stained nuclei of the donor plants and regenerated plants resulted in highly reproducible histograms with well-defined G0/G1 peaks and CV values usually below 2.5% (Figure 2A), ensuring the reliability of the methods used in this study. The histograms that were obtained from the nuclei of all of the leaf samples showed that there is a unimodal fluorescence peak of nuclear DNA with almost similar G0/G1 levels (Figure 2B). The experimental results showed that the fluorescence peaks of all leaf samples were consistent, suggesting that the ploidy level of the regenerated plants was maintained, guaranteeing the effective in vitro propagation of this plant without compromising its genetic reliability.
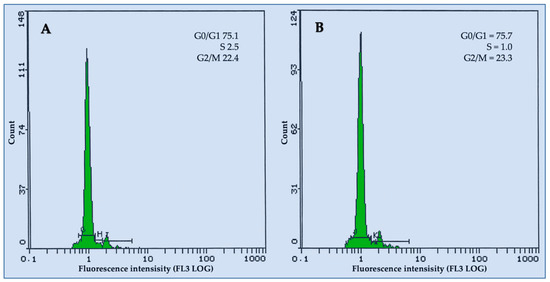
Figure 2.
Typical FCM histograms of Maerua crassifolia. (A) From leaf samples of in vitro propagated plants; (B) from leaf samples of the donor plants.
4. Discussion
The in vitro propagation of plants that face difficulties growing normally depends heavily on the type of growth medium and types and concentrations of the growth regulators added. A previous study on M. crassifolia failed to obtain fully acclimatized plants [13]. In the current study, we tried to improve the protocol by using hypocotyls as explants instead of leaves and immature fruits. The utilization of hypocotyl explants showed successful micropropagation in several plant species as they could be among the most totipotent plant cells [18,19]. Furthermore, a wider range of concentrations of cytokinins and auxins as well as a larger number of possible combinations were examined. MS medium supplemented with BA (7.5 µM) showed the highest response of cultivated hypocotyls along with the highest average number of shoots per explant and average shoot length. It is well established that the kind and concentration of cytokinins applied exogenously significantly affect the success of the in vitro propagation protocol [20,21]. Furthermore, several studies indicated the positive effects of cytokinins on the shoot proliferation from hypocotyl explants [22,23,24,25]. In the current study, the addition of a low concentration of NAA (1.0 µM) with the identified optimum concentration of BA (7.5 µM) increased the response of the hypocotyl explants of M. crassifolia. This result supports the assertion that a proper balance between cytokinins and auxins forms a synergistic effect that induces in vitro proliferation of shoots [26]. Previous studies demonstrated that a relatively higher concentration of cytokinins in combination with lower concentrations of auxins promote in vitro cell division and induce the formation of a greater number of shoots per explant compared to using cytokinins alone [8]. This effect could be explained by the aforementioned synergistic effect between auxins and cytokinins along with their roles in the cell cycle and their significant effects on different phases of cell division and multiplication cycle. As cytokinins influence DNA replication and control the processes that lead to mitosis and auxins affect mitosis, the application of both types of growth regulator increases the efficiency of culture and induces shoot proliferation and multiplication. Our results revealed that the highest multiplication potential of hypocotyls was obtained on MS medium supplemented with 7.5 µM BA and 1.0 µM NAA represented by the highest response (85.7%) and the maximum number of shoots per explant (13.92) among all other treatments. These results are consistent with previous studies on a wide range of species [8,27,28].
One of the most crucial steps in the success of any micropropagation protocol is the formation of a well-developed root system. In the current study, the best rhizogenesis response was achieved via cultivating the in vitro regenerated microshoots on ½ MS medium augmented with 1.0 µM of IBA exhibited by the rooting of 94.1% of the cultivated shoots with an average of 6.8 roots per microshoot. This result is consistent with the previous studies that used half-strength MS medium with low concentrations of IBA for rooting [8,28,29,30]. Plants absorb IBA quickly, retain it efficiently, and transport it through their tissues effectively. These characteristics support IBA’s ability to activate the genes responsible for rhizogenesis [31,32]. Previous reports indicated the higher efficiency of IBA in root induction compared to the other auxins, including NAA [8]. This efficiency could be attributed to the higher stability of IBA against photodegradation along with the higher resistance to deactivation via different biological pathways and/or adsorption to microshoots [33].
The utilization of FCM analysis is prevalent in the examination of in vitro regenerated plants through tissue culture. This is attributed to its capacity to ascertain the ploidy level, quantify DNA content, scrutinize the cell cycle, detect chimerism, and deliver quality control. The application of this methodology is of utmost importance in the assessment of the efficacy of tissue culture protocols. It enables cost-effective, expeditious, and dependable analyses for the detection of ploidy, nuclear DNA content, and the genome size of a given species, ensuring the genetic stability of the in vitro regenerated plants [34,35,36,37,38]. FCM is a considerably cheap, fast, and reliable technique that has been successfully employed to ensure the genetic stability of tissue culture plants [8,39,40,41,42]. This technique has been effectively employed to evaluate genetic stability in various in vitro regenerated woody plant species, such as Ulmus minor [43], Juniperus phoenicea [44], Olea maderensis and Olea europaea ssp. europaea var. sylvestris [39], Eucalyptus globulus [45], Neolamarkia cadamba [46], and Disanthus cercidifolius [47]. In this study, the histogram obtained from the leaf nuclei of the in vitro plants and the donor plants revealed that the fluorescence peaks of all of the samples were consistent, indicating that the ploidy level of the regenerated plants was maintained, thereby ensuring the efficacy this protocol plant without compromising its genetic integrity.
5. Conclusions
Maerua crassifolia is one of the most important trees in Saudi Arabia; however, it is vulnerable to extinction because of several factors, e.g., grazing and soil erosion. Tissue culture techniques enable the mass and rapid micropropagation of endangered species. In the current study, we developed a well-established protocol for the in vitro multiplication of M. crassifolia trees via the cultivation of hypocotyls on MS medium with 7.5 µM BA and 1.0 µM NAA. The regenerated shoots were rooted on ½ MS medium with 1.0 µM IBA. Finally, the rooted plantlets were acclimatized and able to survive in field conditions. These plants were genetically similar to their donor plant based on genetic fidelity analyses via flow cytometry. Further studies are recommended to verify the adaptability of the proposed protocol in large-scale setups.
Author Contributions
Conceptualization, A.A.A., A.A.Q. and E.M.A.-S.; data curation, A.A.Q. and E.M.A.-S.; formal analysis, A.A.A., A.A.Q. and E.M.A.-S.; funding acquisition, A.A.A.; investigation, A.A.A., A.A.Q., E.M.A.-S., M.F. and M.A.E.-S.; methodology, A.A.A., A.A.Q., E.M.A.-S., M.F. and M.A.E.-S.; project administration, A.A.A.; resources, A.A.A.; software, A.A.Q. and E.M.A.-S.; supervision, A.A.A., M.F. and M.A.E.-S.; validation, A.A.A., M.F. and M.A.E.-S.; visualization, A.A.Q., E.M.A.-S., and M.F.; writing—original draft, A.A.A., A.A.Q. and E.M.A.-S.; writing—review and editing, M.A.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3–295–1).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elaidarous, A.A.; Osman, H.E.; Galal, T.M.; El-Morsy, M.H. Vegetation–environment relationship and floristic diversity of Wadi Al-Sharaea, Makkah Province, Saudi Arabia. Rend. Lincei. Sci. Fis. Nat. 2022, 33, 169–184. [Google Scholar] [CrossRef]
- Yonbawi, A.R.; Abdallah, H.M.; Alkhilaiwi, F.A.; Koshak, A.E.; Heard, C.M. Anti-Proliferative, Cytotoxic and Antioxidant Properties of the Methanolic Extracts of Five Saudi Arabian Flora with Folkloric Medicinal Use: Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.; Kourková, P.; Zelený, V. Healing war wounds and perfuming exile: The use of vegetal, animal, and mineral products for perfumes, cosmetics, and skin healing among Sahrawi refugees of Western Sahara. J. Ethnobiol. Ethnomed. 2012, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.G.; Akanimo, E.G.; Ahunna, A.G.; Nwakaego, E.M.; Chimsorom, C.K. Antimalarial potency of the methanol leaf extract of Maerua crassifolia Forssk (Capparaceae). Asian Pac. J. Trop. Dis. 2014, 4, 35–39. [Google Scholar] [CrossRef]
- Akuodor, G.C.; Essien, A.D.; Akpan, J.L.; Chilaka, K.C.; Uwaezuoke, N.J.I.; Nwadike, K.I.; Nwobodo, N.N.; Ezeokpo, B.C. Analgesic, antiinflammatory and antipyretic activities of methanolic leaf extract of Maerua crassifolia. J. Coast. Life Med. 2016, 4, 225–230. [Google Scholar] [CrossRef]
- Kingsley Chimsorom Ckilaka, G.C.A.; Akpan, J.L.; Ogiji, E.D.; Eze, C.O.; Ezeokpo, B.C. Antibacterial and antioxidant activities of methanolic leaf extract of Maerua crassifolia. J. Appl. Pharm. Sci. 2015, 5, 147–150. [Google Scholar] [CrossRef]
- Chaib, F.; Sahki, R.; Sabaou, N.; Rached, W.; Bennaceur, M. Phytochemical investigation and biological activities of some Saharan plants from Hoggar. J. Agric. Sci. 2015, 7, 163–173. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Ruta chalepensis L. Plants 2021, 10, 2820. [Google Scholar] [CrossRef]
- Bakhtiar, Z.; Mirjalili, M.H.; Sonboli, A. In vitro callus induction and micropropagation of Thymus persicus (Lamiaceae), an endangered medicinal plant. Crop Breed. Appl. Biotechnol. 2016, 16, 48–54. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, K.; Saxena, G.; Kumar, L.; Bharadvaja, N. Effect of different media and growth hormones on shoot multiplication of in vitro grown Centella asiatica accessions. Adv. Tech. Biol. Med. 2016, 4, 1–4. [Google Scholar] [CrossRef]
- Hussain, S.A.; Anis, M.; Alatar, A.A. Efficient In Vitro Regeneration System for Tecoma stans L., Using Shoot Tip and Assessment of Genetic Fidelity Among Regenerants. Proc. Natl. Acad. Sci. India Sect. Biol. Sci. 2020, 90, 171–178. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Hakeem, K.R. Development of an efficient micropropagation system for Tecoma stans (L.) Juss. ex Kunth using thidiazuron and effects on phytochemical constitution. Vitr. Cell. Dev. Biol. Plant 2019, 55, 442–453. [Google Scholar] [CrossRef]
- Saad, A.A.M.; Asalam, A.A. Culture of leaves and immature fruits of Maerua crassifolia in vitro. Int. J. Inf. Res. Rev. 2015, 2, 496–498. [Google Scholar]
- Al-Qurainy, F.; Nadeem, M.; Khan, S.; Alansi, S.; Tarroum, M.; Al-Ameri, A.; Gaafar, A.; Alshameri, A. Micropropagation and evaluation of genetic fidelity of Maerua oblongifolia (FORSSK.) A. RICH: A rare medicinal plant from Saudi Arabia. Fresenius Environ. Bull. 2018, 27, 165–171. [Google Scholar]
- Rathore, M.S.; Shekhawat, N.S. Micropropagation of Maerua oblongifolia: A rare ornamental from semi arid regions of Rajasthan, India. J. Dev. Biol. Tissue Eng. 2011, 3, 92–98. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid Flow Cytometric Analysis of the Cell Cycle in Intact Plant Tissues. Science 1983, 220, 1049. [Google Scholar] [CrossRef]
- Liu, R.; Xue, Y.; Ci, H.; Gao, J.; Wang, S.; Zhang, X. Establishment of highly efficient plant regeneration of Paeonia ostii ‘Fengdan’ through optimization of callus, adventitious shoot, and rooting induction. Hortic. Plant J. 2022, 8, 777–786. [Google Scholar] [CrossRef]
- de Oliveira, L.S.; Brondani, G.E.; Molinari, L.V.; Dias, R.Z.; Teixeira, G.L.; Gonçalves, A.N.; de Almeida, M. Optimal cytokinin/auxin balance for indirect shoot organogenesis of Eucalyptus cloeziana and production of ex vitro rooted micro-cuttings. J. For. Res. 2022, 33, 1573–1584. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Naz, R.; Anis, M.; Alatar, A.A. Successful plant regeneration system via de novo organogenesis in Syzygium cumini (L.) Skeels: An important medicinal tree. Agrofor. Syst. 2019, 93, 1285–1295. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Teixeira da Silva, J.A.; Bulley, S.M.; Hudák, I. The role of cytokinins in shoot organogenesis in apple. Plant Cell Tiss. Org. Cult. 2010, 101, 251–267. [Google Scholar] [CrossRef]
- Tomasiak, A.; Zhou, M.; Betekhtin, A. Buckwheat in Tissue Culture Research: Current Status and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 2298. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.H.; Naseri, M.; Younesikelaki, F.S.; Yusefi Javan, I.; Swamy, N.R. Influence of Plant Growth Regulators on Callus Induction, Silymarin Production and Antioxidant Activity in Silybum marianum L. Gaertn. by Tissue Culture. Int. J. Hortic. Sci. Technol. 2023, 10, 223–236. [Google Scholar]
- Doğru, S.M.; Balkaya, A.; Kurtar, E.S. In vitro Micropropagation of Maintainer White Head Cabbage Lines Using Cotyledon and Hypocotyl Explants. Black Sea J. Agric. 2022, 5, 180–188. [Google Scholar] [CrossRef]
- Bedir, H.; Ari, E.; Vural, G.E.; Seguí-Simarro, J.M. Effect of the genotype, explant source and culture medium in somatic embryogenesis and organogenesis in Vaccaria hispanica (Mill.) Rauschert. Plant Cell Tiss. Org. Cult. 2022, 150, 329–343. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. Plant Growth Regulators I: Introduction; Auxins, their Analogues and Inhibitors. In Plant Propagation by Tissue Culture: Volume 1. The Background; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 175–204. [Google Scholar] [CrossRef]
- Hesami, M.; Daneshvar, M.H.; Yoosefzadeh-Najafabadi, M.; Alizadeh, M. Effect of plant growth regulators on indirect shoot organogenesis of Ficus religiosa through seedling derived petiole segments. J. Genet. Eng. Biotechnol. 2018, 16, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Nathar, V.N. In Vitro Method of High-Frequency Plant Regeneration Through Internodal Callus of Ruta graveolens L. In Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation; Khasim, S.M., Long, C., Thammasiri, K., Lutken, H., Eds.; Springer: Singapore, 2020; pp. 761–768. [Google Scholar] [CrossRef]
- Patricia, D.; Stephen, B.; John, A. Shoot organogenesis from leaf discs of the African ginger (Mondia whitei (Hook.f.) Skeels), an endangered medicinal plant. Vitr. Cell. Dev. Biol. Plant 2021, 57, 493–498. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Zhang, Q.; Lin, H.; Xia, T.; Akhtar, K.; Liu, J.; Miao, R.; Xu, F.; Xu, W. In Vitro Regeneration Potential of White Lupin (Lupinus albus) from Cotyledonary Nodes. Plants 2020, 9, 318. [Google Scholar] [CrossRef]
- Estrella-Maldonado, H.; Talavera-May, C.R.; Fuentes Ortìz, G.; Desjardins, Y.; Santamarìa, J.M. Rhizogenesis on in-vitro plantlets of Carica papaya L.: Identification and expression profiling of transcription repressors of response to auxin (Aux/IAA) and auxin response factor (ARF) genes. Vitr. Int. Symp. Papaya 2019, 1250, 153–158. [Google Scholar] [CrossRef]
- Fogaça, C.M.; Fett-Neto, A.G. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regul. 2005, 45, 1–10. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Pasqual, M.; Pio, L.A.S.; Oliveira, A.C.L.; Soares, J.D.R. Flow cytometry applied in tissue culture. In Recent Advances in Plant In Vitro Culture; Leva, A., Laura, M., Rinaldi, R., Eds.; InTech: Rijeka, Croatia, 2012; pp. 109–122. [Google Scholar]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Evaluation of genetic fidelity of in vitro raised plants of Dendrocalamus asper (Schult. & Schult. F.) Backer ex K. Heyne using DNA-based markers. Acta Physiol. Plant 2013, 35, 419–430. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Sliwinska, E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss. Org. Cult. 2013, 114, 197–206. [Google Scholar] [CrossRef]
- Nybom, H.; Weising, K.; Rotter, B. DNA fingerprinting in botany: Past, present, future. Investig. Genet. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crops Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Brito, G.; Lopes, T.; Loureiro, J.; Rodriguez, E.; Santos, C. Assessment of genetic stability of two micropropagated wild olive species using flow cytometry and microsatellite markers. Trees 2010, 24, 723–732. [Google Scholar] [CrossRef]
- Liu, L.-S.; Li, R.; Zhao, Y.; Wen, C.-L.; Ren, S.; Guo, Y.-D. High efficiency regeneration and genetic stability analysis of somatic clones of Gynura bicolor DC. Afr. J. Biotechnol. 2011, 10, 10380–10386. [Google Scholar]
- Konar, S.; Karmakar, J.; Ray, A.; Adhikari, S.; Bandyopadhyay, T.K. Regeneration of plantlets through somatic embryogenesis from root derived calli of Hibiscus sabdariffa L. (Roselle) and assessment of genetic stability by flow cytometry and ISSR analysis. PLoS ONE 2018, 13, e0202324. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Dash, B.; Kar, B.; Nayak, S. Rapid plant regeneration in industrially important Curcuma zedoaria revealing genetic and biochemical fidelity of the regenerants. 3 Biotech. 2019, 10, 17. [Google Scholar] [CrossRef]
- Conde, P.; Loureiro, J.; Santos, C. Somatic embryogenesis and plant regeneration from leaves of Ulmus minor Mill. Plant Cell Rep. 2004, 22, 632–639. [Google Scholar] [CrossRef]
- Loureiro, J.; Capelo, A.; Brito, G.; Rodriguez, E.; Silva, S.; Pinto, G.; Santos, C. Micropropagation of Juniperus phoenicea from adult plant explants and analysis of ploidy stability using flow cytometry. Biol. Plant. 2007, 51, 7–14. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Xavier, A.; Otoni, W.C.; Campos, J.M.S.; Viccini, L.F.; Takahashi, E.K. Assessment of genetic stability of micropropagated Eucalyptus globulus Labill hybrid clones by means of flow cytometry and microsatellites markers. Rev. Árvore 2017, 41, e410114. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Zhai, Y.; Ouyang, K.; Chen, X.; Bai, L. High frequency regeneration of plants via callus-mediated organogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia Cadamba). Sci. Rep. 2020, 10, 4558. [Google Scholar] [CrossRef] [PubMed]
- Ulvrova, T.; Vitamvas, J.; Cepkova, P.H.; Eliasova, K.; Janovska, D.; Bazant, V.; Viehmannova, I. Micropropagation of an ornamental shrub Disanthus cercidifolius Maxim. and assessment of genetic fidelity of regenerants using ISSR and flow cytometry. Plant Cell Tiss. Org. Cult. 2021, 144, 555–566. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).