Abstract
Insect outbreaks are major drivers of natural disturbances in forest ecosystems. Outbreaks can have both direct and indirect effects on the composition of soil arthropod communities through canopy opening, nutrient addition and predator-prey interactions. In this study, we aimed to understand the effects of forest tent caterpillar (Malacosoma disstria; FTC) outbreaks through cascading effects on ant communities in both temperate and boreal forests in Canada. Pitfall traps and Berlese funnels were used to compare the ant communities, as well as the surrounding arthropod communities, between control and outbreak sites in boreal and temperate forests (in Quebec, Canada). Using the Sørensen dissimilarity index, we determined the alpha and beta diversity of the ant community. Other arthropods collected in the traps were counted to evaluate the richness and abundance of potential prey for the ants and other potential predators of the FTC. We used an indicator species analysis to examine the species associated with sites defoliated by the outbreak. In the boreal forest, we found that FTC outbreaks caused decreases in species richness and increases in the evenness of ant communities in defoliated sites. In the boreal forest sites, species composition varied significantly between control and outbreak sites. This pattern was driven in part by the presence of other predators. A similar, but weaker pattern was observed in the temperate forest. We saw no changes in the beta diversity in the boreal forest, but did see a significant decrease in the temperate forest between the outbreak sites and the control sites. Ant species in the boreal forest tended to exhibit a more marked preference for either control or previously defoliated sites than species in the temperate forest. Our study showed that disturbances such as insect outbreaks can drive changes in the ant community. While we saw small effects of outbreaks, manipulation experiments using resource addition could help us validate the mechanisms behind these relationships.
1. Introduction
Insect outbreaks cause major natural disturbance events in forests, and have had an impact on these ecosystems for a long time [1,2,3]. Defoliation, hereby meaning the loss of leaves, by insect herbivores not only slows tree growth, but it can also promote nutrient cycling and accelerate succession [4]. Caterpillars are prey, and their population explosions affect trophic webs [5,6]. Ecosystem effects of insect outbreaks can happen through various direct and indirect mechanisms: leaf removal increases light and temperature on the forest floor; exploding caterpillar populations increase prey availability for many predators; and frass, insect corpses and dropped foliage constitute nutrient transfers from trees to soils [5,7,8,9,10,11]. Together, these effects can stimulate decomposition, enhance nutrient mineralization, increase soil respiration, promote plant growth and alter brown food webs, defined by the consumption of decaying biomass by detritivores [12,13,14,15,16,17]. However, the full complexity of underground food webs, referring to any trophic interaction taking place in and under the layer of litter, remains poorly understood, and the role of soil arthropods in mediating the effects of leaf-eating insect outbreaks on ecosystem functioning is not clear [18].
Caldéron-Sanou et al. [18] found that direct and indirect effects of caterpillar outbreaks increase the diversity of other arthropods in the underground food web at different trophic levels. They showed that the magnitude of the effects of defoliation did not decrease at higher trophic levels, contrary to what was assumed. The researchers expected the effect of defoliation to be diluted, the higher the trophic level. Instead, they observed a more diverse food web and a greater proportion of high-trophic level taxa in defoliated than in control forests. In this kind of study in temperate and boreal regions, it is expected that the effects of disturbance will be higher for primary decomposers and producers and lessen for higher trophic levels such as predators. This finding does not support a mitigation hypothesis related to disturbances where the effects of disturbances would be lower for species at the top of the food web. Additionally, because so many of the species making up the soil community are responsible for structural and functional characteristics of the ecosystem, a change in their assemblage could have large impacts on ecosystems [19,20,21,22,23,24]. The functional roles of ants are also important to take into account, as they will mitigate the ants’ responses to perturbations, even in the environment [25].
Ants are particularly important in forests, since they are one of the few large-scale ecological engineers [26,27,28]. Ants make up a large part of the insect biomass and can play multiple ecological roles such as predators, soil engineers, nutrient cyclers and regulators of plant growth and reproduction [29,30,31,32], thus shaping both in-ground and above-ground trophic webs. In these northern forests, ants play a crucial role in arthropod communities [33,34] and nutrient fluxes [32,35]. The social organization of ant colonies means they can respond rapidly and dramatically to changes in the environment, and hence can mediate ecosystem effects of disturbance [36].
The prey availability for ants can increase with defoliator outbreaks, both directly due to the presence of caterpillars and indirectly due to the stimulation of brown food webs. Indeed, soil detrivores and fungivores respond to an increase in microbial biomass and activity induced by high-quality inputs, leading to increased soil respiration and nutrient cycling, especially if the nutrient inputs are sustained for a few years [5,37,38,39,40]. Many of these soil microarthropods, such as collembola, acarina and isopoda, can be prey for ants [41,42], but this is not the only way in which changes in brown food webs can influence ants. Indeed, ants are deeply interconnected with boreal and temperate forest trophic webs [33,34], but their responses to changes in these communities are not well understood. For instance, increases in other arthropod predators could constrain ant responses. Ants are well-understood to exert significant predation pressure on forest defoliators [43,44,45,46]. However, the reverse, namely the effects of outbreaks on ant communities, has received less attention. Considering the keystone role of ants, this knowledge gap constrains our understanding of the cascading effects of defoliator outbreaks.
The impacts of forest canopy opening on ants have mostly been studied through the effects of forest management [47,48,49,50]. Multiple studies have shown an increase in ant abundance and diversity with moderate management intensity, where there is some increase in clearing area, canopy opening and edges [49,51,52]. The main driver appears to be a change in the microclimate on the forest floor that results from canopy opening. Grevé et al. [53] found that forest management (with a moderate proportion of harvested tree volume and even-aged stands) increases abundance, species richness and functional diversity in ant communities in temperate forests, and that this was due to reduced canopy cover and stand structural complexity. They also found that shade-intolerant ant species (pioneer or gap species needing environments with high availability of light to establish and grow [54]) were more likely to be favored, and that this was likely due to warm conditions in the stands. In Japanese temperate forests, both open habitat specialists and generalists were abundant in managed forests, but shade-tolerant species (persistent or mature forest species, able to thrive in shaded environments [54]) declined [48,55]. In boreal forests, similar trends have been observed in Europe [51,56]. In general, open habitats in temperate and boreal forests seem to have positive effects on ant diversity and abundance. A decrease in shade-tolerant ant species was especially correlated with lower canopy coverage in European studies [51,56,57]. The forest tent caterpillar (Malacosoma disstria Hübner, 1820) is an important forest defoliator of hardwoods across much of North America. Outbreaks usually last 3–5 years and, while they slow the growth of host trees, seldom lead to widespread tree death [58]. Defoliation during forest tent caterpillar outbreaks increases canopy openness, leading to an increase in sun exposure to the forest floor, resulting in higher soil temperatures, drier soil, and an increased growth of saplings and understory plants [59]. Similar canopy opening has been shown to increase ant abundance and species richness in managed or recovering forests [49,51].
However, insect outbreaks, unlike most forest management practices, also involve an increase in prey abundance for ants, both directly from the outbreaking caterpillars and indirectly via the stimulation of brown food webs. Indeed, ants are common predators of caterpillars [60], and have been shown to have substantial impacts on the abundance of caterpillars [43,61,62] and other leaf-chewing herbivores [63]. Ant predation has been suggested to show a density-dependent response to caterpillar availability, increasing during an outbreak and playing a role in controlling the outbreak [64,65]. Ants could alter trophic cascades by lowering herbivory damage [66,67,68]. While thinking of trophic cascades, it is also important to consider that ants prey on soil micro-organisms, such as springtails as well, with certain groups such as the ground-dwelling Dacetini being specialized predators [69], thus acting on different parts of the food web associated with caterpillar outbreaks.
In this study, we investigated the dynamics between ant communities and defoliation at the ecosystem level, in both a boreal and a temperate forest. Specifically, we evaluated the effects of a forest tent caterpillar outbreak on ant communities, examining drivers that are related to both environmental conditions on the forest floor and changes in soil arthropod communities. We hypothesized that canopy opening, the presence of high numbers of caterpillars, and an increase in soil arthropods driven by nutrient inputs, will lead to higher ant species diversity. Increased energy and nutrient flow, combined with increased heterogeneity of the forest floor was predicted to open niches, thus promoting higher ant diversity. In terms of evenness, there are two possibilities: either many species are favored, thus leading to a more homogenous community (higher evenness), or only a few species can take advantage of novel conditions, thus leading to a more heterogenous community (lower evenness). We also examined associations of the ant species with control or outbreak sites. Finally, we evaluated the role of three potential drivers related to outbreaks that could affect ant populations, namely canopy opening, increased soil microarthropod populations (using collembola as a representative group), and changes in soil arthropod predator populations.
2. Materials and Methods
2.1. Study Area and Experimental Design
This study was conducted in two forest stands that were both affected by the most recent FTC outbreak. In both regions, an FTC outbreak was detected in 2016 [70] and continued in 2017 [71], but no defoliation was observed in 2018 or 2019.
The Forêt d’enseignement et de recherche du lac Duparquet (FERLD; N48.513, W79.369) is within the boreal mixed-wood forests of eastern Canada, in post-fire (1923) regenerated stands dominated by trembling aspen (Populus tremuloides Michx, 1803). The forest was mostly made up of mature trees reaching 10–15 m and some saplings between 1–3 m. Due to the vertical structure of aspens (very few branches until the canopy), the trees are closer together, and thus at a relatively high density. The climate is cold-temperate, with a (1961–1990) mean annual temperature of 0.9 °C and mean annual precipitation of 642 mm of rain and 215 mm of snow. Fire drives the disturbance regime, and large even-age trembling aspen stands arise from post-fire regeneration [72]. The soils are Grey Luvisols [73] originating from glaciolacustrine clay deposits [74]. The region was affected by an FTC outbreak for 3 years, starting in 2015 [75] and continuing in 2016 and 2017 [70,71]. No FTC defoliation was observed in either 2018 or 2019 [76]. Spruce budworm [77] attack the conifers in the area, and the birch tubemaker and the lesser-eyed sallow [78] can also attack deciduous trees in this forest, both through defoliation.
The Kenauk Nature Reserve (N45.712, W74.887) is within a temperate deciduous forest composed of stands dominated by sugar maple (Acer saccharum Marsh, 1785) and American beech (Fagus grandifolia Ehrh, 1787). The forest was mostly made up of mature trees reaching 10–15 m and several saplings between 1–3 m. Since maple trees tend to have branches along the trees, the density was slightly lower than for aspen forests due to more shade. The climate is cold-temperate, with a mean annual temperature of 5 °C, and has a mean total annual rainfall of 807.4 mm and a total annual snowfall of 178.1 cm [79]. The disturbance regime is driven by a mix of insect pests, mostly defoliators such as the forest tent caterpillar, and more recently, the spongy moth, as well as weather events. The soils in the study area are classified as Dystric Brunisols with a moder-type humus [73,80].
2.2. Experimental Design
In the boreal forest, we selected 28 sites in trembling aspen-dominated stands (generally over 50% of aspen and where other trees were conifers), including 14 that were heavily defoliated in 2016–2017 (outbreak sites), and 14 control sites. This variable will be referred to as defoliation history for the rest of the study. The sites were classified using both data from the MFFP reports cited previously, as well as from on-site observations from other researchers. As both sampled sites are part of research institutes and have had different projects involved with forest and insect monitoring over the years, we used the resources they provided to choose the different sites. During sampling, we also selected 20 trees at random within 20 m of the focal tree, and sampled for wild colonies to confirm the state of the sites.
In the temperate forest, twelve (12) sites in sugar maple stands that were heavily defoliated in 2016 and 2017 (outbreak sites), and 12 control sites in similar age stands having escaped defoliation were sampled in 2018 and 2019. In both cases, each site was characterized by a focal tree around which we sampled the arthropod fauna. The arthropod sampling was conducted within 5 m of the focal tree at each site. We sampled all sites, 2 times per field season, over two years. We then pooled the traps, sampling period and year for each site.
2.3. Environmental Variables
To confirm the defoliation history of control and outbreak sites, we examined twenty saplings of the respective focus tree species in each region, between 1 and 3 m tall in a 100 m radius around the focal tree, for forest tent caterpillar colonies in 2017, 2018 and 2019. At each site, we also measured canopy openness using a densiometer in 2018 and 2019 (every two weeks during the months of May, June and July), and used an average of four measurements taken on the densiometer, as per the methodology recommended with the tool. We then averaged the four measurements taken at the same sites since the bud burst, and averaged the measurements per site taken during the different periods. Unfortunately, the 2017 data used in this study came from the dataset of a previous student, and did not contain the canopy data.
2.4. Ant Survey
The ants were sampled in control stands at FERLD in 2016 by Despland and Lessard [65], and these data were only included in the species list (Table S1). Since the sampling method was quite different from the ones used the following year, we could not use these data in the analysis; however, they helped us obtain a snapshot of the ant fauna. Standardized ant sampling in control and outbreak sites was conducted in 2017–2019 at FERLD, and in 2018–2019 at Kenauk.
At each site, five pitfall traps, 50 mm in diameter filled with propylene glycol and one drop of both ethanol and unscented liquid soap, were set in the ground for 48 h [81]. Pitfall traps in cold temperate climates usually attract bigger ants, but are readily used to sample ants of most functional groups [82]. Pitfall traps were positioned in a radius around the focal tree, 5 m from one another. The process was only repeated two times, once in late May and once in late June, to coincide with the period during which FTC is most active. Note that ants are not very active in May, but late June is near the peak activity period.
All of the ants sampled in the pitfall traps were identified to species level and confirmed by Dr. André Francoeur [83,84]. Morphospecies were used for the specimens that were either too deteriorated or could not be identified using taxonomic keys. Ant abundance was transformed to presence–absence data to account for bias that could come from nest proximity. Ant occurrence was evaluated as the number of traps in which a given species of ant was collected.
For further analyses, we pooled data from all of the traps at each site. We calculated ant species richness as the number of ant species found per plot.
2.5. Survey of Other Arthropods
We collected collembolas to use them as a proxy of other potential food resources for ants beyond the FTC larvae. Collembolas were sampled as well in a subset (N = 8) of outbreak and control sites. The samples were taken within a diameter of 1 m around the focal tree. At each tree, two replicates of litter were taken within a 20 × 20 cm quadrant, two soil samples from 0–5 cm and two samples from 5–10 cm, using a 5 cm corer. The two replicates for each sampling type were combined and conserved at 4 °C, and extracted less than 48 h after collecting in the laboratory. The soil fauna was extracted using Berlese–Tullgren extractors running for 7 days, with temperatures gradually increasing from 20 °C to 50 °C. The specimens were conserved in 70% ethanol and sorted to separate collembolas from other soil organisms. Collembolas were prepared and identified using published keys [85,86,87,88], as well as collembola.org and ecotaxonomy.org. The specimens were identified to the lowest taxonomic level, if possible, but were at least identified to family. The specimens were also grouped into morphospecies for hard-to-identify species [89].
Additionally, all other potential predators and/or parasitoids found in the pitfall traps were counted, such as spiders, wasps, beetles and stinkbugs, that have been previously defined as predators and/or parasitoids of the forest tent caterpillar [90,91,92,93,94], and the total predator abundance was included in statistical analyses.
2.6. Statistical Analysis
An ANOVA was performed to evaluate the effect of defoliation history (independent variable) on ant species richness and evenness (dependent variables). All of the years were pooled together in each region (2017–2019 for the boreal forest and 2018–2019 for the temperate forest) in all analyses, since a preliminary analysis showed no effect or interaction of year.
To investigate the differences in community composition between sites with different defoliation histories, we calculated a dissimilarity matrix based on the Sørensen dissimilarity index [95,96]. This index was used for the community analysis (for both ordination and beta diversity analyses) because it is one of the widely used indices for presence–absence datasets, and examines the number of species shared by two sites and the number of species unique to each. The maximum and minimum values of Sørensen are 0 (the same species composition) and 1 (no shared species). We used nonparametric multidimensional scaling (NMDS) plots, via the metaMDS function in the vegan package for R [97], to visualize the dissimilarity matrices. Then, we tested the differences in the taxonomic position of the community centroids (multivariate location) between defoliation histories using ‘Permutational Multivariate Analysis of Variance’ (PERMANOVA), the adonis2 function in the vegan package, with 999 permutations. These vectors of our chosen variables (defoliation history, canopy openness, predator abundance, prey abundance and prey species richness) were tested for significant effects in shaping the observed ant communities. Since we could not use AICs or similar methods for model selection with PERMANOVAs, we retained variables that had an R2 of more than 0.02 when conducting a PERMANOVA in our analysis.
To further explore the community changes due to the different regimes of defoliation, we performed an indicator species analysis [98]. This method allows us to quantitatively assess the association of specific species of ants to either of our two groups, in this case, the control and outbreak sites. It assesses the predictive values of species as indicators of the conditions prevailing under different defoliation regimes [99]. Component ‘A’ is a conditional probability referring to the positive predictive value of the species as an indicator of the defoliation history. Component ‘B’ is another conditional probability referring to the probability of finding a species in sites experiencing the defoliation history. Both components vary from 0 to 1 [98]. All of the analyses were carried out using R version 4.2.0 [100].
3. Results
We collected and identified a total of 2944 individual worker ants belonging to 54 species and morphospecies from 3 subfamilies and 10 genera across our 28 sites in the boreal forest and 24 sites in the temperate forest.
Ants were not present in all pitfall traps, even though other arthropods were collected. From the sites sampled in the boreal forest, we were able to collect ants from 100% of the control sites and 92% of the outbreak sites. In the temperate forest, we collected ants from 83% of the control sites and 50% of the outbreak sites. Species accumulation curves validated our experimental design in terms of species sampling for both defoliation histories and both regions.
Forest tent caterpillar colonies were observed in 2017 in the outbreak sites (3.36 ± 1.86 (mean ± SD) colonies of 20 saplings) but not in the control sites, and none were observed at all in 2018 and 2019 in the boreal forest. In the temperate forest, we observed colonies both in the outbreak sites (5.5 ± 2.85 (mean ± SD) colonies of 20 saplings) and in the control sites (4.01 ± 2.50 (mean ± SD) colonies of 20 saplings).
3.1. Species Richness and Evenness
Three species were shared between the two regions. We found a total of 30 species in the boreal forest sites, with the species occurring most often being Camponotus novaeboracensis (Fitch, 1855) (23% of traps), Myrmica alaskensis (Wheeler, 1917) and Formica subaenescens (Emery 1893). In the temperate forest sites, we found a total of 18 species, with the species occurring most often being Aphaenogaster picea (Wheeler, 1908) (25% of traps), Lasius americanus (Emery 1893) and Stenamma diecki (Emery 1895). Many rare species, with only one occurrence, were observed.
In the boreal forest, we observed significantly lower species richness (df =1, F = 9.901, p = 0.003) and significantly higher evenness in the outbreak than in the control sites (df = 1, F = 8.667, p = 0.005). We identified similar trends in the temperate forest sites; however, the high proportion of traps that did not collect ants (90%) reduced the sample size and, hence, the power of the analyses. At least one trap per site had ants; therefore, when pooled, we had ants in 83% of the outbreak sites and 50% of the control sites. The species richness did not differ between the control and outbreak sites (df = 1, F = 1.53, p = 0.234), but the evenness was significantly higher in the outbreak sites (df = 1, F = 5.008, p = 0.056, residuals df = 47, residuals F = 0.008) (Figure 1).
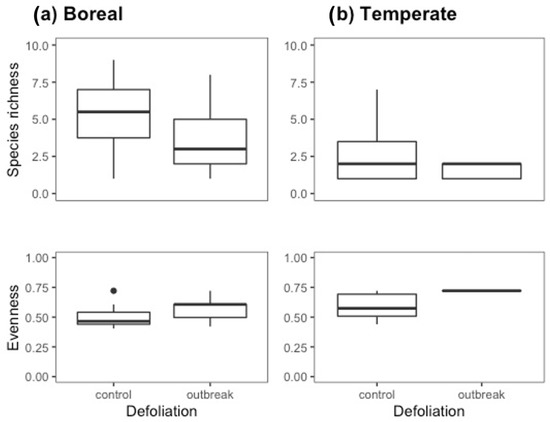
Figure 1.
Species richness and evenness. The two panels on the right (b) refer to the boreal forest data, while the two left panels (a) refer to the temperate forest data. Box plots represent data from pitfall traps only, and from a subset of years for the boreal forest samples (2017–2018) to limit the variation in sampling method from 2016 and 2019, respectively. In the box plots, the lower boundary of the box indicates the 25th percentile, the bold line within the box marks the median, and the upper boundary of the box indicates the 75th percentile. The whiskers indicate the 10th and 90th percentiles. Dots are outliers (>Q3 + 1.5 × interquartile range).
3.2. Species Composition
Ordination
In the boreal forest sites, the species composition varied significantly between control and outbreak sites (F = 5.391, p = 0.002, R2 = 0.091 df = 1) (Figure 2; Table 1). Canopy openness, collembola abundance and diversity all aligned with defoliation, showing that they all increased with increased defoliation, but did not contribute significantly to predicting ant communities. Predators also increased with defoliation, and were significantly associated with changes in the ant community.
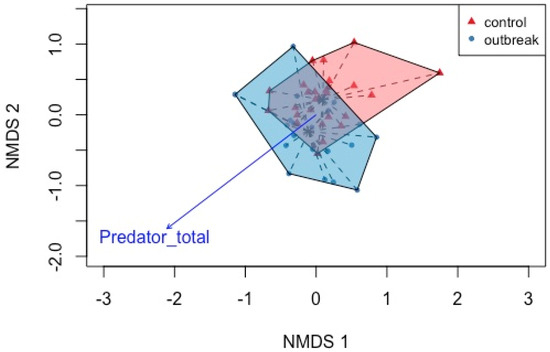
Figure 2.
NMDS ordination of boreal forest sites within control and outbreak stands based on the Sørensen dissimilarity index using taxonomic data. The abundance of predators was the only covariate that aligned significantly with ant community composition.

Table 1.
Summary of statistics for PERMANOVAs of the effect of defoliation history on taxonomic compositional turnover of ant communities. Significant values (p < 0.05) are highlighted in bold.
3.3. Beta Diversity
The taxonomic multivariate dispersion (i.e., homogenization) did not differ significantly between defoliation histories in the boreal forest (F = 1.021, df = 1, p = 0.27), but did in the temperate forest (F = 7.60, df = 1, p = 0.01) (Figure 3). However, it is important to note that homogenization was quite low (i.e., beta diversity is high) for all of our sites, since the distance to the centroid was high above the null expectation.
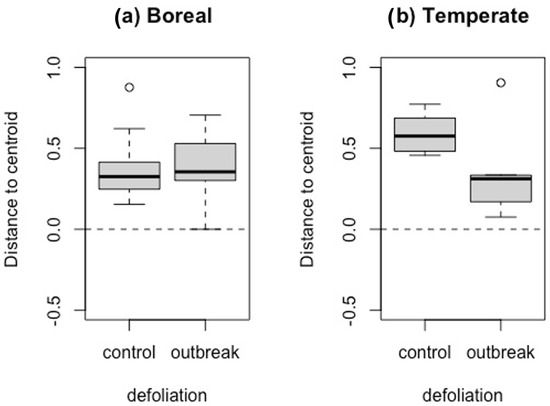
Figure 3.
Boxplot of the standardized effect size of the distance to multivariate space centroid (SES Dcentroid) of each site in (a) boreal and (b) temperate forests. The black dashed line represents null expectations. In the box plots, the lower boundary of the box indicates the 25th percentile, the bold line within the box marks the median, and the upper boundary of the box indicates the 75th percentile. The whiskers indicate the 10th and 90th percentiles. Dots are outliers (>Q3 + 1.5 × interquartile range).
3.4. Indicator Species
The indicator value (IndVal) index measures the association between a species and a site group, in our case, the defoliation history. Out of the 30 species found in the selected sites in the boreal forest, 26% (5 species) were significantly associated with one group, with 4 represented the control sites and 1 represented the outbreak sites (Table 2); another 4 species were found to be as likely to occur in both groups.

Table 2.
Indicator species analysis for the boreal forest data only at alpha = 0.05. The “Indicator value index” measures the association between a species and a site group. “A” is the positive predictive value of the species as an indicator of the site group, and “B” is the sensitivity of the species as an indicator of the target site group.
In the temperate forest, however, we found no significant pattern of association with the defoliation history groups. Out of the 15 species found in the selected sites in the temperate forest, 4 species were as likely to occur in both groups, but even for others showing a preference for one of the two groups, none was strong enough to be considered as an indicator.
4. Discussion
Contrary to our hypothesis that disturbance would increase the diversity present at defoliated sites by creating new ecological niches, the sites where the outbreak occurred did not contain a more diverse ant community. In the boreal forest, defoliation slightly lowered the ant alpha diversity, but did not alter the beta diversity or the proportion of singletons. The increase in evenness was due to no species being overwhelmingly dominant. The only significant trend identified in the temperate forest was a decrease in the beta diversity with defoliation. In the boreal forest, observed changes in ant communities were not significantly linked to changes in canopy openness, prey abundance or diversity, but did correlate with an overall increase in predator abundance in the defoliated sites. In the boreal forest, four ant species were identified as indicators of the control sites, and one as an indicator of the outbreak sites.
4.1. Ant Community Responses to Disturbance
In both the temperate and boreal forests, the sites that had experienced defoliation due to an outbreak were more even and less species-rich than the control sites; however, this effect only attained statistical significance in the boreal forest. Disturbances generally have a greater impact on arthropod communities in structurally simple habitats than in complex ones [101,102]. In our case, the intensity of the disturbance, referred to the intensity of defoliation as measured by the MFFP protocol of outbreak detection and damages where stands are categorized with light (defoliation on only the top of the canopy in some trees), was moderate (defoliation on the top 2/3 in most of the trees) and severe (defoliation on most of the branches in most of the trees) [70].
Ants are typically impacted indirectly by disturbances through effects on habitat structure, microclimate, resource availability and competitive interactions [103,104,105,106,107,108,109,110]. In our study, we knew (Lafleur, pers. obs. (boreal) and Nowell, pers. obs. (temperate)) that there was a large amount of light reaching the understory during the outbreak, therefore creating a disturbance in the light regime. Outbreaks of species such as the FTC are what could be considered a moderate chronic disturbance [111], meaning that an outbreak event in itself causes moderate damage in an area; this is because leaves can reflush during the summer and that the epidemic cycle is relatively short (10 years), in contrast to the timescale on which a forest operates. However, since these outbreaks are not static geographically nor transient in time [112], the impacts may be less dramatic, especially since impacts on ant communities are mostly driven by long-term disturbance regimes [110,113,114,115,116]. While FTC outbreaks are part of this disturbance regime and can alter forest succession in the boreal forest [59], they are relatively short-lived compared to those of the spruce budworm [117].
Research suggests that ant foraging in temperate regions is sensitive to sun exposure on the forest floor [1]. This influence on foraging could then translate to changes in communities, as foraging and competition are some of the main drivers of community assemblage [65,118]. Canopy openness was higher in defoliated sites, but it was not a significant contributor to predicting the ant communities in our study, suggesting that the observed canopy opening was not significant enough to directly affect the ant communities. However, it is important to mention that we did not have the canopy openness data for the years of the outbreak; thus, this measure relates to the canopy 1- and 2 years post-outbreak. Additionally, aspens and maples have the capacity to reflush during the same summer; hence, we qualified the long-term loss of canopy as being due to defoliation events. In the boreal region, rarer shade species, however, were excluded from the outbreak sites and replaced with more common species. Therefore, while canopy openness was not retained as an indicator in our model, we nonetheless saw a change in the community that was consistent with a role for opening. Our study system, therefore, resembled uneven-aged forest management with a strong vertical structure and small gaps that closed quickly. These small gaps were less favorable to shade-intolerant ants than large open areas created by forest harvesting.
4.2. Indicator Species
Taxa that favor open habitats, such as the generalist Myrmicinae, usually do well in disturbed habitats; groups that favor closed habitats, such as specialist predators, are often at a disadvantage [25,119,120,121]. In our study, the species that were only present in the outbreak sites are known from the literature to be associated with more open habitats such as managed forests, bogs and open areas [122,123,124,125,126,127].
In the boreal forest, Camponotus novaeboracensis was the species that occurred most often in both the control and defoliated stands. C. novaeboracensis is a behaviorally dominant ant [128], and has been observed to successfully attack forest tent caterpillars during the outbreak preceding our study [65]. However, our results did not show this species becoming more dominant in the outbreak sites.
The main species that were retained as indicators of control sites in the boreal forest were Camponotus herculeanus (Linnaeus, 1758) and Myrmica detritinodis (Emery, 1921). C. herculeanus is a very common species in boreal forests [129,130]. M. detritinodis is classified as a shade species that prefers shelter under moss and lichens, preferably in high-moisture conditions [131]. It was expected to prefer a high canopy cover. C. herculeanus was more surprising, since it was seen to be previously associated with either closed or open habitats [129,130]. In our system, we concluded that they may be more associated with closed habitats, but this may depend on other environmental characteristics.
One species that was associated with the outbreak sites is Formica integra (Nylander, 1856), but limited resources are available on their ecology due to their low occurrences. However, they are part of the Formica rufa group (Red wood ants), whose ecology at large has been well-studied in European boreal and temperate forests. In particular, red wood ants feed on invertebrate prey from both the canopy and forest floor [28,31,132], and have been suggested to have the potential to control outbreaks of insect pests [133]. They also build large mounds on the forest floor for their nests. The distribution of these mounds is governed by multiple factors (climate, ecosystem productivity, food resources) [134,135], but light availability stood out as the most important factor [136], signifying the importance of canopy openness. In our study, their increase in the outbreak sites suggests that this ant species is uniquely positioned to benefit from a short-term canopy opening and resource pulse associated with a defoliator outbreak to carry it over temporally.
Since these mounds concentrate nutrients and can affect the biotic and abiotic components of the surrounding forest, they can increase the presence of associated species (e.g., spiders, beetles and millipedes) living near them, such as parasites, predators and scavengers, as they provide a stable habitat with consistently higher temperatures than the rest of the environment [137].
Formica and other Camponotus species have been observed to attack other Lepidopterans in eastern Canada [65]. While studying ants’ predation of the spongy moth, Weseloh [138] found that both Formica and Camponotus spp. workers attacked caterpillars, especially at the first instar, and this decreased as the larvae grew. Thus, the use of forest tent caterpillars as prey could contribute to populations of F. integra and C. novaeboracensis in outbreak sites.
In the temperate forest, the species that occurred most were smaller-bodied ants from the Aphaenogaster and Lasius genera. However, no species were found to be indicators of either the control or outbreak sites. Aphaenogaster species from the species complex A. picea are omnivorous feeders, and generally feed on small invertebrates as well as some mushrooms [139]. Lasius americanus is omnivorous and feeds on seeds as well as live and dead insects [84]. Both the Aphaenogaster and Lasius genera were widely distributed in the sites studied, but their distribution did not appear to be influenced by a history of defoliation.
4.3. Prey, Predators, and Outbreaks
The results from Grevé et al. [53] support our finding that overall prey abundance did not influence ant species composition through increased food resources. However, our results show a positive relationship between ants and other predators in the boreal forests; the basis for this relationship is not clear. These arthropods could be responding to disturbance, similarly to ants, but without any direct trophic interactions between them [53]. Generalist predators tend to increase slowly in response to increased prey availability during a caterpillar outbreak, thus showing up as delayed density-dependent scenarios [140]. Thus, high numbers can be maintained in the year following an outbreak crash [141,142].
Previous research also confirmed that increased prey abundance does not drive ant community responses to disturbance [53], and that ants do not respond to resource pulses of arthropod prey [143]. Resource pulses tend to satiate predators and aboveground consumers [144,145,146]. This pulse can also lead to changes in both the structure and dynamics of communities [147] This phenomenon is particularly present in boreal forest communities that are affected by outbreaks of the spruce budworm. This outbreak and the resulting pulse increase the relative abundance of mobile predators and parasitoids [148]. It is, however, important to note that spruce budworm outbreaks, such as the ones in the cited studies, last much longer than the FTC. Therefore, it is not surprising that the effects are larger. The decrease in ant diversity at the outbreak sites did correlate with an increase in other arthropod predators, suggesting that these predators may have been more effective than ants in responding to the prey pulse generated by the insect outbreak [53]. This trend may have emphasized an indirect effect of other predators on the ant population, but this was not directly studied.
5. Conclusions
Changes in the ant community were only observed in the boreal forest sites. Indeed, less ecologically complex ecosystems are often less resilient to disturbances such as FTC defoliation [53]. In the boreal forest, the ant community composition did differ depending on the defoliation history, albeit with the opposite effect that we expected, with lower species richness and no clear association with either increased sunlight or prey availability. The increase in other potential predators of the FTC showed a path toward the hypothesis of the resource pulse, since this functional group also increased with defoliation. The effect of resource addition to ants and other predators could provide us with a better understanding of the large disturbance event that are insect outbreaks. Further research exploring microclimatic changes and resources related to FTC outbreaks and ants is needed, in order to decipher the mechanisms at play. More natural history knowledge for specific ant species is also needed to be able to make light of these mechanisms. Additionally, as mentioned by Kristensen, Rousk and Metcalfe [149], finding a distinction between low-intensity chronic herbivory and intense pulses such as those present during outbreaks in similar systems would be beneficial in deciphering the underlying mechanisms leading to changes in food webs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14061147/s1, Table S1: Species list and occurrence in traps of ants in the boreal forest sites along with functional traits and shade-tolerance; Table S2: Species list and occurrence in traps of ants in the temperate forest sites along with functional traits and shade tolerance; Table S3: Potential predator abundance. Collected from pitfall traps. References [150,151,152] are cited in the supplementary materials.
Author Contributions
A.-S.C., E.G.K., I.T.H. and E.D. made contributions to the conception and design. A.-S.C. and E.G.K. acquired the data, and A.-S.C. analyzed and interpreted the data with the help of E.D., M.M.G., E.G.K. and I.T.H.; A.-S.C. wrote the manuscript and M.M.G. and E.D. revised it critically. M.M.G. and E.D. provided resources and supervision. All authors gave final approval and agreed to be accountable for all aspects of the research, such as the accuracy and integrity of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministère de la Forêt de la Faune et des Parcs (Quebec) (N3-2054427), Norbord Inc., Kenauk Institute, the Natural Sciences and Engineering Research Council (Canada)(522722-17), and SERG-international (2019/12-2019-171).
Data Availability Statement
Data can be found in Spectrum, Concordia University’s open-access research repository.
Acknowledgments
We want to acknowledge that this research was conducted on the treaty and traditional territory of the Abitibiwinni and Algonquin Anishinabeg people in the boreal forest, as well as in Kanien’kehá꞉ka, Omàmìwininìwag (Algonquin) and Anishinabewaki territories in the temperate forest. We thank Liane Nowell from The Kenauk Institute for access to the Kenauk territory and the invaluable support provided during data collection from Liane Nowell and the Kenauk Institute. We would like to thank Gaspar Legendre, who set up the experiment and collected data in 2017; André Francoeur for his help with the identification of the ant specimens; Jean-Philippe Lessard for his guidance through the set-up and analysis of the study, as well as valuable comments along with Carly Ziter; Javier Ibarra Isassi and Eric Pedersen for their help with the statistical analysis; Pamela Yataco Marquez for the sampling; as well as April Mansfield, Sarah Farhat and Andrew Cormier for their help with sorting the samples and identifying the samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aakala, T.; Remy, C.C.; Arseneault, D.; Morin, H.; Girardin, M.P.; Gennaretti, F.; Navarro, L.; Kuosmanen, N.; Ali, A.A.; Boucher, É.; et al. Millennial-scale disturbance history of the boreal zone. In Boreal Forests in the Face of Climate Change: Sustainable Management; Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 53–87. ISBN 978-3-031-15988-6. [Google Scholar]
- Montoro Girona, M.; Navarro, L.; Morin, H. A Secret Hidden in the Sediments: Lepidoptera Scales. Front. Ecol. Evol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Lavoie, J.; Montoro Girona, M.; Grosbois, G.; Morin, H. Does the type of silvicultural practice influence spruce budworm defoliation of seedlings? Ecosphere 2021, 12, e03506. [Google Scholar] [CrossRef]
- De Grandpré, L.; Marchand, M.; Kneeshaw, D.D.; Paré, D.; Boucher, D.; Bourassa, S.; Gervais, D.; Simard, M.; Griffin, J.M.; Pureswaran, D.S. Defoliation-induced changes in foliage quality may trigger broad-scale insect outbreaks. Commun. Biol. 2022, 5, 463. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Jarry, J.J.; Despland, E. Early instar mortality of a forest pest caterpillar: Which mortality sources increase during an outbreak crash? Entomol. Exp. Appl. 2022, 170, 268–276. [Google Scholar] [CrossRef]
- Swank, W.T.; Waide, J.B.; Crossley, D.A.; Todd, R.L. Insect defoliation enhances nitrate export from forest ecosystems. Oecologia 1981, 51, 297–299. [Google Scholar] [CrossRef]
- Hunter, M.D. Insect population dynamics meets ecosystem ecology: Effects of herbivory on soil nutrient dynamics. Agric. For. Entomol. 2001, 3, 77–84. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Asner, G.P.; Martin, R.E.; Silva Espejo, J.E.; Huasco, W.H.; Farfán Amézquita, F.F.; Carranza-Jimenez, L.; Galiano Cabrera, D.F.; Baca, L.D.; Sinca, F.; et al. Herbivory makes major contributions to ecosystem carbon and nutrient cycling in tropical forests. Ecol. Lett. 2014, 17, 324–332. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Crutsinger, G.M.; Kumordzi, B.B.; Wardle, D.A. Nutrient fluxes from insect herbivory increase during ecosystem retrogression in boreal forest. Ecology 2016, 97, 124–132. [Google Scholar] [CrossRef]
- Lovett, G.M.; Christenson, L.M.; Groffman, P.M.; Jones, C.G.; Hart, J.E.; Mitchell, M.J. Insect Defoliation and Nitrogen Cycling in Forests. BioScience 2002, 52, 335. [Google Scholar] [CrossRef]
- Gravel, D.; Albouy, C.; Thuiller, W. The meaning of functional trait composition of food webs for ecosystem functioning. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150268. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy flux: The link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Kristensen, J.A.; Metcalfe, D.B.; Rousk, J. The biogeochemical consequences of litter transformation by insect herbivory in the Subarctic: A microcosm simulation experiment. Biogeochemistry 2018, 138, 323–336. [Google Scholar] [CrossRef]
- Lovett, G.M.; Ruesink, A.E. Carbon and nitrogen mineralization from decomposing gypsy moth frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef]
- Debaly, Z.M.; Marchand, P.; Girona, M.M. Autoregressive models for time series of random sums of positive variables: Application to tree growth as a function of climate and insect outbreak. Ecol. Model. 2022, 471, 110053. [Google Scholar] [CrossRef]
- Odum, E.P. The Strategy of Ecosystem Development. Ekistics 1970, 29, 234–238. [Google Scholar]
- Calderón-Sanou, I.; Münkemüller, T.; Zinger, L.; Schimann, H.; Yoccoz, N.G.; Gielly, L.; Foulquier, A.; Hedde, M.; Ohlmann, M.; Roy, M.; et al. Cascading effects of moth outbreaks on subarctic soil food webs. Sci. Rep. 2021, 11, 15054. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Caruso, T. Soil microbial community responses to climate extremes: Resistance, resilience and transitions to alternative states. Philos. Trans. R. Soc. B 2020, 375, 20190112. [Google Scholar] [CrossRef]
- De Ruiter, P.C.; Neutel, A.-M.; Moore, J.C. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 1995, 269, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Saravesi, K.; Aikio, S.; Wäli, P.R.; Ruotsalainen, A.L.; Kaukonen, M.; Huusko, K.; Suokas, M.; Brown, S.P.; Jumpponen, A.; Tuomi, J. Moth outbreaks alter root-associated fungal communities in subarctic mountain birch forests. Microb. Ecol. 2015, 69, 788–797. [Google Scholar] [CrossRef]
- Vindstad, O.P.L.; Schultze, S.; Jepsen, J.U.; Biuw, E.M.; Kapari, L.T.; Sverdrup-Thygeson, A.; Ims, R.A. Numerical responses of saproxylic beetles to rapid increases in dead wood availability following geometrid moth outbreaks in sub-arctic mountain birch forest. PLoS ONE 2014, 9, e99624. [Google Scholar] [CrossRef]
- Sandén, H.; Mayer, M.; Stark, S.; Sandén, T.; Nilsson, L.O.; Jepsen, J.U.; Wäli, P.R.; Rewald, B. Moth outbreaks reduce decomposition in subarctic forest soils. Ecosystems 2020, 23, 151–163. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Andersen, A.N. Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral Ecol. 2003, 28, 444–464. [Google Scholar] [CrossRef]
- Jurgensen, M.F.; Finér, L.; Domisch, T.; Kilpeläinen, J.; Punttila, P.; Ohashi, M.; Niemelä, P.; Sundström, L.; Neuvonen, S.; Risch, A.C. Organic mound-building ants: Their impact on soil properties in temperate and boreal forests. J. Appl. Entomol. 2008, 132, 266–275. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F. Ants in the soil system-a hydrological, chemical and biological approach. J. Appl. Entomol. 2008, 132, 265. [Google Scholar] [CrossRef]
- Domisch, T.; Finér, L.; Neuvonen, S.; Niemelä, P.; Risch, A.C.; Kilpeläinen, J.; Ohashi, M.; Jurgensen, M.F. Foraging activity and dietary spectrum of wood ants (Formica rufa group) and their role in nutrient fluxes in boreal forests. Ecol. Entomol. 2009, 34, 369–377. [Google Scholar] [CrossRef]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Wardle, D.A.; Hyodo, F.; Bardgett, R.D.; Yeates, G.W.; Nilsson, M.-C. Long-term aboveground and belowground consequences of red wood ant exclusion in boreal forest. Ecology 2011, 92, 645–656. [Google Scholar] [CrossRef]
- Laine, K.J.; Niemelä, P. The influence of ants on the survival of mountain birches during an Oporinia autumnata (Lep., Geometridae) outbreak. Oecologia 1980, 47, 39–42. [Google Scholar] [CrossRef]
- Punttila, P.; Niemelä, P.; Karhu, K. The impact of wood ants (Hymenoptera: Formicidae) on the structure of invertebrate community on mountain birch (Betula pubescens ssp. czerepanovii). In Proceedings of the Annales Zoologici Fennici; JSTOR. Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2004; pp. 429–446. [Google Scholar]
- Finér, L.; Jurgensen, M.F.; Domisch, T.; Kilpeläinen, J.; Neuvonen, S.; Punttila, P.; Risch, A.C.; Ohashi, M.; Niemelä, P. The Role of Wood Ants (Formica rufa group) in Carbon and Nutrient Dynamics of a Boreal Norway Spruce Forest Ecosystem. Ecosystems 2013, 16, 196–208. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of ant communities to disturbance: Five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 2019, 88, 350–362. [Google Scholar] [CrossRef]
- Kaukonen, M.; Ruotsalainen, A.L.; Wäli, P.R.; Männistö, M.K.; Setälä, H.; Saravesi, K.; Huusko, K.; Markkola, A. Moth herbivory enhances resource turnover in subarctic mountain birch forests? Ecology 2013, 94, 267–272. [Google Scholar] [CrossRef]
- Mikola, J.; Yeates, G.W.; Barker, G.M.; Wardle, D.A.; Bonner, K.I. Effects of defoliation intensity on soil food-web properties in an experimental grassland community. Oikos 2001, 92, 333–343. [Google Scholar] [CrossRef]
- Pitman, R.M.; Vanguelova, E.I.; Benham, S.E. The effects of phytophagous insects on water and soil nutrient concentrations and fluxes through forest stands of the Level II monitoring network in the UK. Sci. Total Environ. 2010, 409, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.K.; Hart, S.C.; Cobb, N.S.; Whitham, T.G.; Koch, G.W. Insect herbivory increases litter quality and decomposition: An extension of the acceleration hypothesis. Ecology 2003, 84, 2867–2876. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Wise, D.H. Direct and indirect effects of ants on a forest-floor food web. Ecology 2007, 88, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Çakır, M. The negative effect of wood ants (Formica rufa) on microarthropod density and soil biological quality in a semi-arid pine forest. Pedobiologia 2019, 77, 150593. [Google Scholar] [CrossRef]
- Karhu, K.J.; Neuvonen, S. Wood ants and a geometrid defoliator of birch: Predation outweighs beneficial effects through the host plant. Oecologia 1998, 113, 509–516. [Google Scholar] [CrossRef]
- Way, M.J.; Khoo, K.C. Role of Ants in Pest Management. Annu. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Tilman, D. Cherries, Ants and Tent Caterpillars: Timing of Nectar Production in Relation to Susceptibility of Caterpillars to Ant Predation. Ecology 1978, 59, 686–692. [Google Scholar] [CrossRef]
- Gösswald, K. Die Waldameise. Band 2. Die Waldameise in Okosystem Wald, ihr Nutzen und ihre Hege; Aula Verlag: Totnes, UK, 1990; Volume 510S. [Google Scholar]
- Carvalho, K.S.; Vasconcelos, H.L. Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biol. Conserv. 1999, 91, 151–157. [Google Scholar] [CrossRef]
- Maeto, K.; Sato, S. Impacts of forestry on ant species richness and composition in warm-temperate forests of Japan. For. Ecol. Manag. 2004, 187, 213–223. [Google Scholar] [CrossRef]
- Palladini, J.D.; Jones, M.G.; Sanders, N.J.; Jules, E.S. The recovery of ant communities in regenerating temperate conifer forests. For. Ecol. Manag. 2007, 242, 619–624. [Google Scholar] [CrossRef]
- Ewers, R.M.; Boyle, M.J.; Gleave, R.A.; Plowman, N.S.; Benedick, S.; Bernard, H.; Bishop, T.R.; Bakhtiar, E.Y.; Chey, V.K.; Chung, A.Y. Logging cuts the functional importance of invertebrates in tropical rainforest. Nat. Commun. 2015, 6, 6836. [Google Scholar] [CrossRef] [PubMed]
- Punttila, P.; Haila, Y.; Niemelä, J.; Pajunen, T. Ant communities in fragments of old-growth taiga and managed surroundings. In Proceedings of the Annales Zoologici Fennici; JSTOR; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 1994; pp. 131–144. [Google Scholar]
- Véle, A.; Holuša, J.; Horák, J. Ant abundance increases with clearing size. J. For. Res. 2016, 21, 110–114. [Google Scholar] [CrossRef]
- Grevé, M.E.; Hager, J.; Weisser, W.W.; Schall, P.; Gossner, M.M.; Feldhaar, H. Effect of forest management on temperate ant communities. Ecosphere 2018, 9, e02303. [Google Scholar] [CrossRef]
- Farji-Brener, A. Why are leaf-cutting ants more common in early secondary forests than in old-growth tropical forests? An evaluation of the palatable forage hypothesis. Oikos 2001, 92, 169–177. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Pajunen, T.; Tukia, H. Colonisation of clearcut forests by ants in the southern Finnish taiga: A quantitative survey. Oikos 1991, 61, 250–262. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Fitzgerald, T. The Tent Caterpillars; Cornell University Press: Ithaca, NY, USA, 1995. [Google Scholar]
- Moulinier, J. Impacts de la défoliation par la livrée des forêts sur la mortalité du peuplier faux-tremble et la dynamique forestière post-épidémie en forêt boréale. Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda, QC, Canada, 2013. [Google Scholar]
- Lach, L.; Parr, C.; Abbott, K. Ant Ecology; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Montllor, C.B.; Bernays, E.A. Invertebrate Predators and Caterpillar Foraging; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Piñol, J.; Espadaler, X.; Cañellas, N.; MARTÍNEZ-VILALTA, J.; Barrientos, J.A.; Sol, D. Ant versus bird exclusion effects on the arthropod assemblage of an organic citrus grove. Ecol. Entomol. 2010, 35, 367–376. [Google Scholar] [CrossRef]
- Clark, R.E.; Farkas, T.E.; Lichter-Marck, I.; Johnson, E.R.; Singer, M.S. Multiple interaction types determine the impact of ant predation of caterpillars in a forest community. Ecology 2016, 97, 3379–3388. [Google Scholar] [CrossRef]
- Green, G.; Sullivan, C. Ants Attacking Larvae of the Forest Tent Caterpillar, Malacosoma disstria Hbn. (Lepidoptera: Lasiocampidae). Can. Entomol. 1950, 82, 94–195. [Google Scholar] [CrossRef]
- Despland, E.; Lessard, J.-P. Social predation by ants as a mortality source for an arboreal gregarious forest pest. Basic Appl. Ecol. 2022, 59, 82–91. [Google Scholar] [CrossRef]
- Rosumek, F.B.; Silveira, F.A.O.; Neves, F.D.S.; Barbosa, N.P.D.U.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef]
- Romero, G.Q.; Antiqueira, P.A.; Koricheva, J. A meta-analysis of predation risk effects on pollinator behaviour. PLoS ONE 2011, 6, e20689. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Zas, R.; Sampedro, L. Bottom-up effects of host-plant species diversity and top-down effects of ants interactively increase plant performance. Proc. R. Soc. B Biol. Sci. 2012, 279, 4464–4472. [Google Scholar] [CrossRef]
- Cerdá, X.; Dejean, A. 3. Predation by ants on arthropods and other animals. In Predation in the Hymenoptera: An Evolutionary Perspective; Transworld Research Network: Trivandrum, India, 2011. [Google Scholar]
- MFFP. Aires Infestées par la Livrée des Forêts au Québec en 2016; Gouvernement du Québec, Direction de la protection des Forêts: Québec, QC, Canada, 2016; p. 10. [Google Scholar]
- MFFP. Aires Infestées par la Livrée des Forêts au Québec en 2017; Gouvernement du Québec, Direction de la protection des forêts: Québec, QC, Canada, 2017; p. 14. [Google Scholar]
- Bergeron, Y. Species and stand dynamics in the mixed woods of Quebec’s southern boreal forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Agriculture Canada Expert Committee on Soil Survey. The Canadian System of Soil Classification (Second Edition). Available online: https://sis.agr.gc.ca/cansis/publications/manuals/1987-cssc-ed2/index.html (accessed on 3 November 2022).
- Vincent, J.-S.; Hardy, L. L’évolution et l’extension des lacs glaciaires Barlow et Ojibway en territoire québécois. Géographie Phys. Quat. 1977, 31, 357–372. [Google Scholar] [CrossRef]
- MFFP. Aires Infestées par la Livrée des Forêts au Québec en 2015; Gouvernement du Québec, Direction de la protection des Forêts: Québec, QC, Canada, 2015; p. 8. [Google Scholar]
- MFFP. Aires Infestées par la Livrée des Forêts au Québec en 2018; Gouvernement du Québec, Direction de la protection des Forêts: Québec, QC, Canada, 2019; p. 14. [Google Scholar]
- Morin, H.; Laprise, D.; Bergeron, Y. Chronology of spruce budworm outbreaks near Lake Duparquet, Abitibi region, Quebec. Can. J. For. Res. 1993, 23, 1497–1506. [Google Scholar] [CrossRef]
- Dubuc, J.-F. La Performance Biologique et le Comportement Alimentaire de trois espèces de Lépidoptères Après Défoliation du Bouleau Blanc (Betula papyrifera marsh.); Université du Québec à Montréal: Montreal, QC, Canada, 1996. [Google Scholar]
- Environment Canada. National Climate Data and Information Archive; Environment Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Lajoie, P.G. Étude pédologique des comtés de Hull, Labelle et Papineau; Québec Ottawa Ministère Iagriculture: Ottawa, ON, Canada, 1967. [Google Scholar]
- Bestelmeyer, B.T.; Agosti, D.; Alonso, L.E.; Brandão, C.R.F.; Brown, W.L.; Delabie, J.H.; Silvestre, R. Field techniques for the study of ground-dwelling ant: An overview, description, and evaluation. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: Washington, DC, USA, 2000. [Google Scholar]
- Mahon, M.B.; Campbell, K.U.; Crist, T.O. Effectiveness of Winkler Litter Extraction and Pitfall Traps in Sampling Ant Communities and Functional Groups in a Temperate Forest. Environ. Entomol. 2017, 46, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Francœur, A. Les fourmis de la forêt boréale du Québec (Formicidae, Hymenoptera). Nat. Can. 2001, 125, 8. [Google Scholar]
- Ellison, A.M.; Gotelli, N.J.; Farnsworth, E.J.; Alpert, G.D. A Field Guide to the Ants of New England; Yale University Press: London, UK, 2012; ISBN 978-0-300-16930-0. [Google Scholar]
- Hopkin, S.P. A Key to the Collembola (Springtails) of Britain and Ireland; FSC Publications: Shrewsbury, UK, 2007. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part 1: Poduromorpha Fauna. In Entomologica Scandinavica; Brill Academic: Leiden, The Netherlands, 1998. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part II: Entomobryomorpha and Symphypleona; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Christiansen, K.; Bellinger, P. The Collembola of North America North of the Rio Grande. A Taxonomic Analysis. Part 1. Introduction. General. Families Poduridae and Hypogastruridae. Part 2. Families Onychiuridae and Isotomidae. Part 3. Family Entomobrydae. Part 4. Families Neelidae and Sminthuridae. Glossary. Bibliography. Index; Grinnell College: Grinnell, IA, USA, 1980. [Google Scholar]
- Koudji, E.G.; Despland, E.; Caron, A.; Handa, I.T. Soil Springtail Communities Are Resilient to Forest Tent Caterpillar Defoliation in Quebec Mixed Hardwood Forests. Forests, 2023; submitted. [Google Scholar]
- Evans, E. Influence of weather on predator/prey relations: Stinkbugs and tent caterpillars. J. N. Y. Entomol. Soc. 1982, 90, 241–246. [Google Scholar]
- Fitzgerald, T.; Costa, J. Collective behavior in social caterpillars. In Information Processing in Social Insects; Birkhäuser: Basel, Switzerland, 1999; pp. 379–400. [Google Scholar]
- McClure, M.; Despland, E. Collective foraging patterns of field colonies of Malacosoma disstria caterpillars. Can. Entomol. 2010, 142, 473–480. [Google Scholar] [CrossRef]
- Ronnås, C.; Larsson, S.; Pitacco, A.; Battisti, A. Effects of colony size on larval performance in a processionary moth. Ecol. Entomol. 2010, 35, 436–445. [Google Scholar] [CrossRef]
- Cobbold, C.A.; Lewis, M.A.; Lutscher, F.; Roland, J. How parasitism affects critical patch-size in a host–parasitoid model: Application to the forest tent caterpillar. Theor. Popul. Biol. 2005, 67, 109–125. [Google Scholar] [CrossRef]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skar. 1948, 5, 1–34. [Google Scholar]
- Jaccard, P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull. Soc. Vaud. Sci. Nat. 1901, 37, 547–579. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 1.17-4. Acesso Em 2010, 23, 2010. Available online: http://cran.r-project.org> (accessed on 5 December 2022).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Caceres, M.; Jansen, F. Package ‘indicspecies’. Indicators 2016, 8. [Google Scholar]
- Team R Development Core. A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing, Department of Agronomy, Faculty: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 2 February 2023).
- Broza, M.; Izhaki, I. Post-fire arthropod assemblages in Mediterranean forest soils in Israel. Int. J. Wildland Fire 1997, 7, 317–325. [Google Scholar] [CrossRef]
- Gardner, S.M.; Cabido, M.R.; Valladares, G.R.; Diaz, S. The influence of habitat structure on arthropod diversity in Argentine semi-arid Chaco forest. J. Veg. Sci. 1995, 6, 349–356. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef]
- Hedlund, K.; Griffiths, B.; Christensen, S.; Scheu, S.; Setälä, H.; Tscharntke, T.; Verhoef, H. Trophic interactions in changing landscapes: Responses of soil food webs. Basic Appl. Ecol. 2004, 5, 495–503. [Google Scholar] [CrossRef]
- Anderson, K.E.; Russell, J.A.; Moreau, C.S.; Kautz, S.; Sullam, K.E.; Hu, Y.I.; Basinger, U.; Mott, B.M.; Buck, N.; Wheeler, D.E. Highly similar microbial communities are shared among related and trophically similar ant species. Mol. Ecol. 2012, 21, 2282–2296. [Google Scholar] [CrossRef]
- Souza, R.F.; Anjos, D.V.; Carvalho, R.; Del-Claro, K. Availability of food and nesting-sites as regulatory mechanisms for the recovery of ant diversity after fire disturbance. Sociobiology 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Frizzo, T.L.; Campos, R.I.; Vasconcelos, H.L. Contrasting effects of fire on arboreal and ground-dwelling ant communities of a Neotropical savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Silveira, J.M.; Louzada, J.; Barlow, J.; Andrade, R.; Mestre, L.; Solar, R.; Lacau, S.; Cochrane, M.A. A multi-taxa assessment of biodiversity change after single and recurrent wildfires in a Brazilian Amazon forest. Biotropica 2016, 48, 170–180. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelissen, T. Effects of fire disturbance on ant abundance and diversity: A global meta-analysis. Biodivers. Conserv. 2017, 26, 177–188. [Google Scholar] [CrossRef]
- Calizza, E.; Rossi, L.; Careddu, G.; Sporta Caputi, S.; Costantini, M.L. Species richness and vulnerability to disturbance propagation in real food webs. Sci. Rep. 2019, 9, 19331. [Google Scholar] [CrossRef]
- Cooke, B.J.; Sturtevant, B.R.; Robert, L.-E. The Forest Tent Caterpillar in Minnesota: Detectability, Impact, and Cycling Dynamics. Forests 2022, 13, 601. [Google Scholar] [CrossRef]
- Andersen, A.N.; Penman, T.D.; Debas, N.; Houadria, M. Ant community responses to experimental fire and logging in a eucalypt forest of south-eastern Australia. For. Ecol. Manag. 2009, 258, 188–197. [Google Scholar] [CrossRef]
- York, A. Long-term effects of frequent low-intensity burning on ant communities in coastal blackbutt forests of southeastern Australia. Austral Ecol. 2000, 25, 83–98. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African savanna ants to long-term fire regimes. J. Appl. Ecol. 2004, 41, 630–642. [Google Scholar] [CrossRef]
- Maravalhas, J.; Vasconcelos, H.L. Revisiting the pyrodiversity–biodiversity hypothesis: Long-term fire regimes and the structure of ant communities in a N eotropical savanna hotspot. J. Appl. Ecol. 2014, 51, 1661–1668. [Google Scholar] [CrossRef]
- Johns, R.C.; Bowden, J.J.; Carleton, D.R.; Cooke, B.J.; Edwards, S.; Emilson, E.J.S.; James, P.M.A.; Kneeshaw, D.; MacLean, D.A.; Martel, V.; et al. A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks be Stopped? Forests 2019, 10, 910. [Google Scholar] [CrossRef]
- Lessard, J.-P.; Dunn, R.R.; Sanders, N.J. Temperature-mediated coexistence in temperate forest ant communities. Insectes Sociaux 2009, 56, 149–156. [Google Scholar] [CrossRef]
- Fotso Kuate, A.; Hanna, R.; Tindo, M.; Nanga, S.; Nagel, P. Ant Diversity in Dominant Vegetation Types of Southern Cameroon. Biotropica 2015, 47, 94–100. [Google Scholar] [CrossRef]
- Leal, I.R.; Filgueiras, B.K.C.; Gomes, J.P.; Iannuzzi, L.; Andersen, A.N. Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Biodivers. Conserv. 2012, 21, 1687–1701. [Google Scholar] [CrossRef]
- Solar, R.R.D.C.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity consequences of land-use change and forest disturbance in the Amazon: A multi-scale assessment using ant communities. Biol. Conserv. 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Fairweather, A.D.; Lewis, J.H.; Hunt, L.; McAlpine, D.F.; Smith, M.A. Ants (Hymenoptera: Formicidae) of Rockwood Park, New Brunswick: An Assessment of Species Richness and Habitat. Northeast. Nat. 2020, 27, 576. [Google Scholar] [CrossRef]
- Milford, E.R. Ant communities in flooded and unflooded riparian forest of the middle Rio Grande. Southwest. Nat. 1999, 44, 278–286. [Google Scholar]
- Ellison, A.M.; Farnsworth, E.J.; Gotelli, N.J. Ant diversity in pitcher-plant bogs of Massachusetts. Northeast. Nat. 2002, 9, 267–284. [Google Scholar] [CrossRef]
- Francoeur, A. Deux nouvelles fourmis néarctiques: Leptothorax retractus et L. sphagnicolus (Formicidae, Hymenoptera). Can. Entomol. 1986, 118, 1151–1164. [Google Scholar] [CrossRef]
- Francoeur, A. Révision taxonomique des espèces néarctiques du groupe Fusca, genre Formica (Formicidae, Hymenoptera). Mem. Soc. ent. Queb. 3. 316 pp. 1979. Formicoidea. Can. Its Insect Fauna Mem. Ent. Soc. Can. 1973, 108, 502–503. [Google Scholar]
- Francoeur, A. Extension de l’aire connue de la fourmi Myrmica quebecensis (Formicidae, Hymenoptera). 2011. Available online: https://www.erudit.org/fr/revues/natcan/2018-v142-n1-natcan03265/1042014ar.pdf (accessed on 2 February 2023).
- Oberg, E.; Del Toro, I.; Pelini, S. Characterization of the thermal tolerances of forest ants of New England. Insectes Sociaux 2012, 59, 167–174. [Google Scholar] [CrossRef]
- Wheeler, G.C.; Wheeler, J. The Ants of North Dakota; University of North Dakota Grand Forks: Grand Forks, ND, USA, 1963. [Google Scholar]
- MacKay, W.P.; Mackay, E. The Ants of New Mexico (Hymenoptera: Formicidae); Edwin Mellen Press: Lewiston, NY, USA, 2002. [Google Scholar]
- Sirois, L. Impact of fire on Picea mariana and Pinus banksiana seedlings in subarctic lichen woodlands. J. Veg. Sci. 1993, 4, 795–802. [Google Scholar] [CrossRef]
- Rosengren, R. The interaction between red wood ants, Cinara aphids, and pines. A ghost of mutualism past? In Ant-Plant Interactions; Oxford University Press: Oxford, UK, 1991; pp. 80–91. [Google Scholar]
- Parmentier, T.; Dekoninck, W.; Wenseleers, T. A highly diverse microcosm in a hostile world: A review on the associates of red wood ants (Formica rufa group). Insectes Sociaux 2014, 61, 229–237. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Finér, L.; Niemelä, P.; Domisch, T.; Neuvonen, S.; Ohashi, M.; Risch, A.C.; Sundström, L. Carbon, nitrogen and phosphorus dynamics of ant mounds (Formica rufa group) in managed boreal forests of different successional stages. Appl. Soil Ecol. 2007, 36, 156–163. [Google Scholar] [CrossRef]
- Serttaş, A.; Bakar, Ö.; Alkan, U.M.; Yılmaz, A.; Yolcu, H.I.; Ipekdal, K. Nest Survival and Transplantation Success of Formica rufa (Hymenoptera: Formicidae) Ants in Southern Turkey: A Predictive Approach. Forests 2020, 11, 533. [Google Scholar] [CrossRef]
- Stockan, J.A.; Rao, S.; Pakeman, R. Nesting preferences of the threatened wood ant Formica exsecta (Hymenoptera: Formicidae); implications for conservation in Scotland. J. Insect Conserv. 2010, 14, 269–276. [Google Scholar] [CrossRef]
- Rosengren, R.; Fortelius, W.; Lindström, K.; Luther, A. Phenology and causation of nest heating and thermoregulation in red wood ants of the Formica rufa group studied in coniferous forest habitats in southern Finland. In Proceedings of the Annales Zoologici Fennici; JSTOR; Finnish Academy of Sciences, Societas Scientiarum Fennica, Societas pro Fauna et Flora Fennica and Societas Biologica Fennica Vanamo: Helsinki, Finland, 1987; pp. 147–155. [Google Scholar]
- Weseloh, R.M. Simulation of predation by ants based on direct observations of attacks on gypsy moth larvae. Can. Èntomol. 1989, 121, 1069–1076. [Google Scholar] [CrossRef]
- Mokadam, C. Native and Non-Native Ant Impacts on Native Fungi. Ph.D. Thesis, Buffalo State College, Buffalo, NY, USA, 2021. [Google Scholar]
- Sabelis, M.W. Predatory arthropods. Nat. Enemies Popul. Biol. Predat. Parasites Dis. 1992, 225–264. [Google Scholar] [CrossRef]
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Symondson, W.; Sunderland, K.; Greenstone, M. Can Generalist Predators Be Effective Biocontrol Agents. Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Yang, L.H. Interactions between a detrital resource pulse and a detritivore community. Oecologia 2006, 147, 522–532. [Google Scholar] [CrossRef]
- Karban, R. Increased reproductive success at high densities and predator satiation for periodical cicadas. Ecology 1982, 63, 321–328. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Vannote, R.L. Population synchrony in mayflies: A predator satiation hypothesis. Evolution 1982, 36, 810–821. [Google Scholar] [CrossRef]
- Williams, K.S.; Smith, K.G.; Stephen, F.M. Emergence of 13-Yr periodical cicadas (Cicadidae: Magicicada): Phenology, mortality, and predators satiation. Ecology 1993, 74, 1143–1152. [Google Scholar] [CrossRef]
- Yang, L.H.; Edwards, K.F.; Byrnes, J.E.; Bastow, J.L.; Wright, A.N.; Spence, K.O. A meta-analysis of resource pulse–consumer interactions. Ecol. Monogr. 2010, 80, 125–151. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef]
- Kristensen, J.Å.; Rousk, J.; Metcalfe, D.B. Below-ground responses to insect herbivory in ecosystems with woody plant canopies: A meta-analysis. J. Ecol. 2020, 108, 917–930. [Google Scholar] [CrossRef]
- Banschbach, V.S.; Herbers, J.M. Nest Movements and Population Spatial Structure of the Forest Ant Myrmica Punctiventris (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 1999, 92, 414–423. [Google Scholar] [CrossRef]
- Francoeur, A. The Ant Fauna near the Tree-Line in Northern Québec (Formicidae, Hymenoptera). Nordicana 1983, 47, 177–180. [Google Scholar]
- Francoeur, A. Ants (Hymenoptera: Formicidae) of the Yukon. 1997. Available online: https://www.researchgate.net/publication/237749861_Ants_Hymenoptera_Formicidae_of_the_Yukon (accessed on 2 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).