Efficacy of Fungus Comb Extracts Isolated from Indo-Malayan Termite Mounds in Controlling Wood-Decaying Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungus Comb Preparation and Extraction Process
2.2. Extract Compounds Analysis Using UPLC-HRMS
2.3. Preparation of Extract Solutions
2.4. In-Vitro Antimicrobial Susceptibility Test of the Extract Solutions
2.5. Wood Samples and the Vacuum-Pressure Treatment
2.6. Anti-Fungal Test of Extract Solutions According to EN-113 Standard
2.7. Wood Degradation Monitoring
2.8. Data Analysis
3. Results
3.1. Compounds Identified in All Extracts
3.2. Bioactivity of Fungus Comb Extract against S. commune
3.2.1. Results of In-Vitro Antimicrobial Susceptibility Test
3.2.2. Results of the Anti-Fungal Test According to EN-113 Standard
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priadi, T. Wood Decay Hazard Analyses of Residential Buildings in Java Island. Ph.D. Dissertation, Bogor Agricultural University, Bogor, Indonesia, 2011. [Google Scholar]
- Herliyana, E.N.; Maryam, L.F.; Hadi, Y.S. Schizophyllum commune Fr. As Indonesian National Standard Wood Resistance Test Fungi on Four Kinds of Community Wood: Sengon, Rubber, Tusam, and Mangium. J. Silvikult. Trop. 2011, 2, 805. [Google Scholar] [CrossRef]
- Djarwanto, D.; Suprapti, S.; Rulliaty, S. Service Life of Railway Wood Sleepers in Indonesia. Wood Res. J. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Ohm, R.A.; De Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; De Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome Sequence of the Model Mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–964. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-32138-5. [Google Scholar]
- Bureau of Meteorology. Climatology and Geophysics (BMKG) The Republic of Indonesia. Central Database of BMKG; Bureau of Meteorology: Jakarta, Indonesia, 2023.
- Drewello, R.; Weissmann, R. Microbially Influenced Corrosion of Glass. Appl. Microbiol. Biotechnol. 1997, 47, 337–346. [Google Scholar] [CrossRef]
- Müller, E.; Drewello, U.; Drewello, R.; Weißmann, R.; Wuertz, S. In Situ Analysis of Biofilms on Historic Window Glass Using Confocal Laser Scanning Microscopy. J. Cult. Herit. 2001, 2, 31–42. [Google Scholar] [CrossRef]
- Corrêa Pinto, A.M.; Palomar, T.; Alves, L.C.; da Silva, S.H.M.; Monteiro, R.C.; Macedo, M.F.; Vilarigues, M.G. Fungal Biodeterioration of Stained-Glass Windows in Monuments from Belém Do Pará (Brazil). Int. Biodeterior. Biodegrad. 2019, 138, 106–113. [Google Scholar] [CrossRef]
- Statistics Indonesia. Statistics of Forestry Production 2018; BPS: Jakarta, Indonesia, 2018.
- Hadi, Y.S.; Massijaya, M.Y.; Abdillah, I.B.; Pari, G.; Arsyad, W.O.M. Color Change and Resistance to Subterranean Termite Attack of Mangium (Acacia mangium) and Sengon (Falcataria moluccana) Smoked Wood. J. Korean Wood Sci. Technol. 2020, 48, 1–11. [Google Scholar] [CrossRef]
- Nandika, D.; Karlinasari, L.; Arinana, A.; Batubara, I.; Sitanggang, P.S.; Santoso, D.; Witasari, L.D.; Rachmayanti, Y.; Firmansyah, D.; Sudiana, I.K.; et al. Chemical Components of Fungus Comb from Indo-Malayan Termite Macrotermes gilvus Hagen Mound and Its Bioactivity against Wood-Staining Fungi. Forests 2021, 12, 1591. [Google Scholar] [CrossRef]
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the World. Bull. Am. Mus. Nat. Hist. 2013, 377, 6430. [Google Scholar]
- Nandika, D.; Rismayadi, Y.; Diba, F. Rayap: Biologi Dan Pengendaliannya [Termite: Biology and Their Control]; Surakarta Muhammadiyah University Press: Surakarta, Indonesia, 2015. [Google Scholar]
- Subekti, N. Distribution Patterns, Architecture and Population Density of Subterranean Termites Macrotermes gilvus Hagen (Blattodea: Termitidae) in Yanlappa Nature Reserve, West Java; IPB University: Bogor, Indonesia, 2010. [Google Scholar]
- Nandika, D.; Syamsu, K.; Arinana, A.; Kusumawardhani, D.T.; Fitriana, Y. Bioactivities of Catechin from Gambir (Uncaria gambir Roxb.) against Wood-Decaying Fungi. BioResources 2019, 14, 5646–5656. [Google Scholar] [CrossRef]
- Simeto, S.; Held, B.W.; Blanchette, R.A. Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer. Forests 2023, 14, 576. [Google Scholar] [CrossRef]
- Syafii, W. Antitermitic Compounds from the Heartwood of Sonokeling Wood (Dalbergia latifolia Roxb.). Indones. J. Trop. Agric. IJTA 2000, 9, 55–58. [Google Scholar]
- Hidayah, N.; Hisan, A.K.; Solikin, A.; Irawati, I.; Mustikaningtyas, D. Uji Efektivitas Ekstrak Sargassum muticum Sebagai Alternatif Obat Bisul akibat Aktivitas Staphylococcus aureus [Effectiveness Test of Sargassum muticum Extract as an Alternative Medicine for Ulcers due to Staphylococcus aureus]. J. Creat. Stud. 2016, 1, 7794. [Google Scholar]
- Labagu, R.; Naiu, A.S.; Yusuf, N. Levels of Saponin in Mangrove Fruit (Sonneratia alba) Extract and Its Inhibition Against DPPH Free Radical. Jambura Fish Process. J. 2022, 4, 9344. [Google Scholar] [CrossRef]
- Dia, S.P.S.; Nurjanah, J.A.M.; Jacoeb, A. Komposisi Kimia dan Aktivitas Antioksidan Akar, Kulit Batang dan Daun Lindur [Chemical Composition and Antioxidant Activity of Lindur Root, Stem Bark and Leaves]. J. Pengolah. Has. Perikan. Indones. 2015, 18, 205–219. [Google Scholar]
- Arsa, A.K.; Achmad, Z. Ekstraksi Minyak Atsiri Dari Rimpang Temu Ireng (Curcuma aeruginosa Roxb) Dengan Pelarut Etanol Dan N-Heksana [Extraction of Essential Oils from the Rhizomes of Temu Ireng (Curcuma aeruginosa Roxb) Using Ethanol and N-Hexane Solvents]. J. Teknol. Technosci. 2020, 13, 83–94. [Google Scholar]
- Senduk, T.W.; Montolalu, L.A.D.Y.; Dotulong, V. The Rendement of Boiled Water Extract of Mature Leaves of Mangrove Sonneratia alba. J. Perikan. DAN Kelaut. Trop. 2020, 11, 9–15. [Google Scholar] [CrossRef]
- Do, E.; Lee, H.G.; Park, M.; Cho, Y.-J.; Kim, D.H.; Park, S.-H.; Eun, D.; Park, T.; An, S.; Jung, W.H. Anti-fungal Mechanism of Action of Lauryl Betaine Against Skin-Associated Fungus Malassezia restricta. Mycobiology 2019, 47, 242–249. [Google Scholar] [CrossRef]
- Hasenoehrl, A.; Galić, T.; Ergović, G.; Maršić, N.; Skerlev, M.; Mittendorf, J.; Geschke, U.; Schmidt, A.; Schoenfeld, W. In Vitro Activity and in Vivo Efficacy of Icofungipen (PLD-118), a Novel Oral Anti-fungal Agent, against the Pathogenic Yeast Candida albicans. Antimicrob. Agents Chemother. 2006, 50, 3011–3018. [Google Scholar] [CrossRef]
- Konechnyi, Y.T.; Lozynskyi, A.V.; Horishny, V.Y.; Konechna, R.T.; Vynnytska, R.B.; Korniychuk, O.P.; Lesyk, R.B. Synthesis of Indoline-Thiazolidinone Hybrids with Antibacterial and Antifungal Activities. Biopolym. Cell 2020, 36, 381–391. [Google Scholar] [CrossRef]
- Antypenko, L.; Meyer, F.; Sadykova, Z.; Garbe, L.A.; Steffens, K. Monomethyl Suberate Screening for Antifungal Activity, Molecular Docking and Drug-like Properties. Acta Chim. Slov. 2018, 65, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E. Antifungal Activity of Lactic Acid Bacteria of Genus Lactobacillus Sp. in the Presence of Polyols. Acta Aliment. 2007, 36, 495–499. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic Acid: Its Core Biological Properties and Dermatological Applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Leeming, J.P.; Holland, K.T.; Bojar, R.A. The in Vitro Antimicrobial Effect of Azelaic Acid. Br. J. Dermatol. 1986, 115, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The Versatility of Azelaic Acid in Dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- De Muynck, C.; Leroy, A.I.J.; De Maeseneire, S.; Arnaut, F.; Soetaert, W.; Vandamme, E.J. Potential of Selected Lactic Acid Bacteria to Produce Food Compatible Anti-fungal Metabolites. Microbiol. Res. 2004, 159, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Peng, Q.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Xiao, Y.; Zhao, J.; et al. Isolation and Identification of Antibacterial Bioactive Compounds from Bacillus megaterium L2. Front. Microbiol. 2021, 12, 645484. [Google Scholar] [CrossRef]
- Sitepu, I.S.B.; Suada, I.K.; Susrama, I.G.K. The Antimicrobial Activity Test of Some Kitchen Seasoning Extracts on Growth of Fungus Curvularia lunata (Wakk.) Boed. and Aspergillus flavus LINK. J. Trop. Agroecotechnol. 2012, 1, 107–114. [Google Scholar]
- Witasari, L.D.; Wahyu, K.W.; Anugrahani, B.J.; Kurniawan, D.C.; Haryanto, A.; Nandika, D.; Karlinasari, L.; Arinana, A.; Batubara, I.; Santoso, D.; et al. Antimicrobial Activities of Fungus Comb Extracts Isolated from Indomalayan Termite (Macrotermes gilvus Hagen) Mound. AMB Express 2022, 12, 14. [Google Scholar] [CrossRef]
- Goh, Y.Q.; Cheam, G.; Wang, Y. Understanding Choline Bioavailability and Utilization: First Step Toward Personalizing Choline Nutrition. J. Agric. Food Chem. 2021, 69, 10774–10789. [Google Scholar] [CrossRef]
- Rachmayanti, Y.; Firmansyah, D.; Umma, R.R.; Hertanto, D.M.; Sudiana, I.K.; Santoso, D.; Nandika, D.; Karlinasari, L.; Arinana, A.; Batubara, I.; et al. Antioxidant Activity of Fungus Comb Extracts Isolated from Indo-Malayan Termite Macrotermes gilvus Hagen (Isoptera: Termitidae). Indones. J. Chem. 2022, 22, 1693. [Google Scholar] [CrossRef]
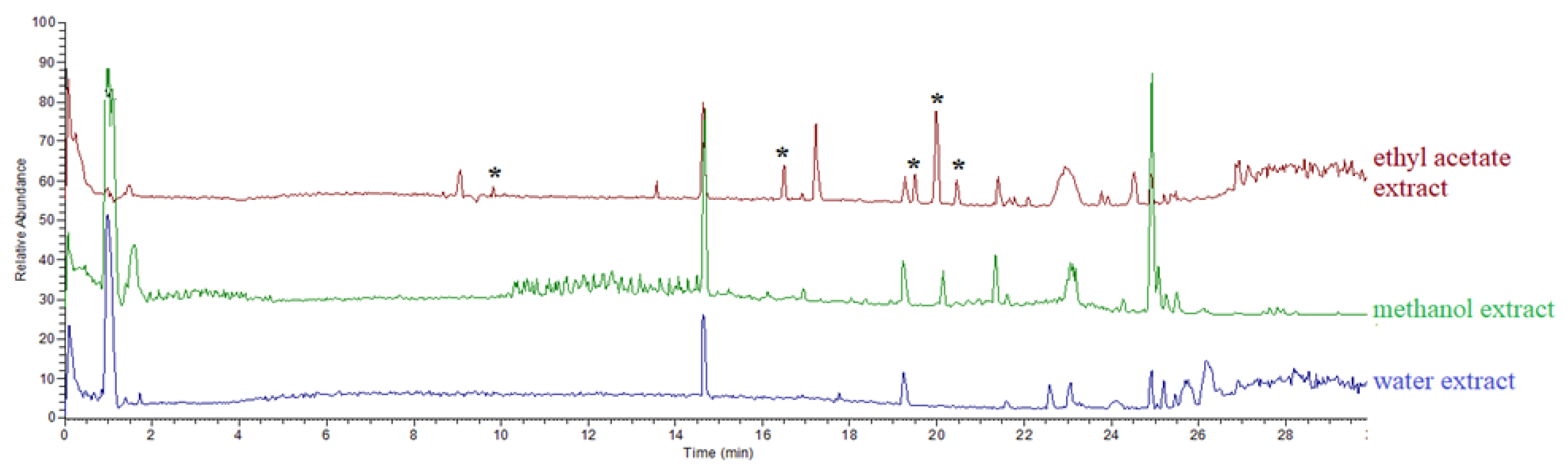
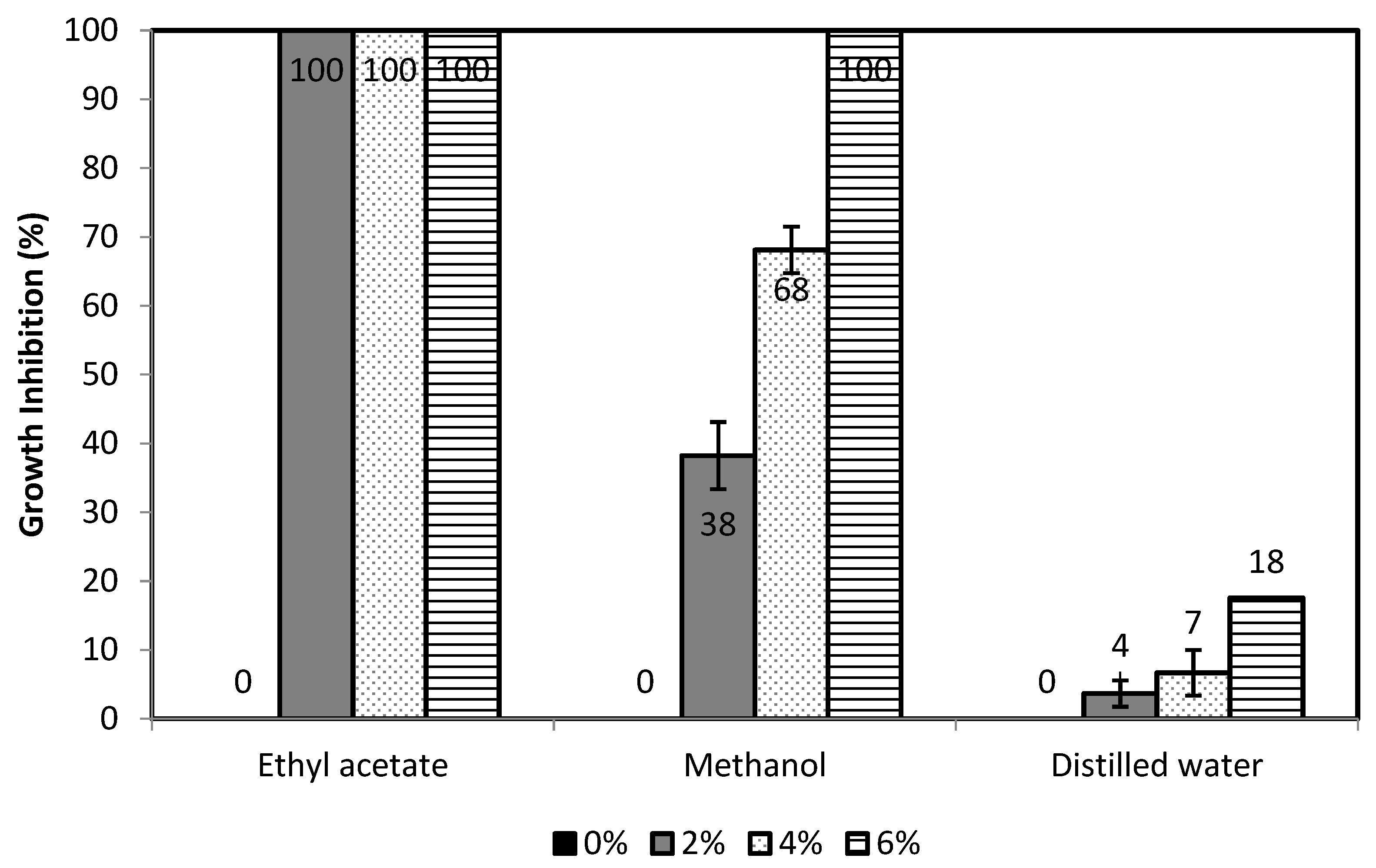
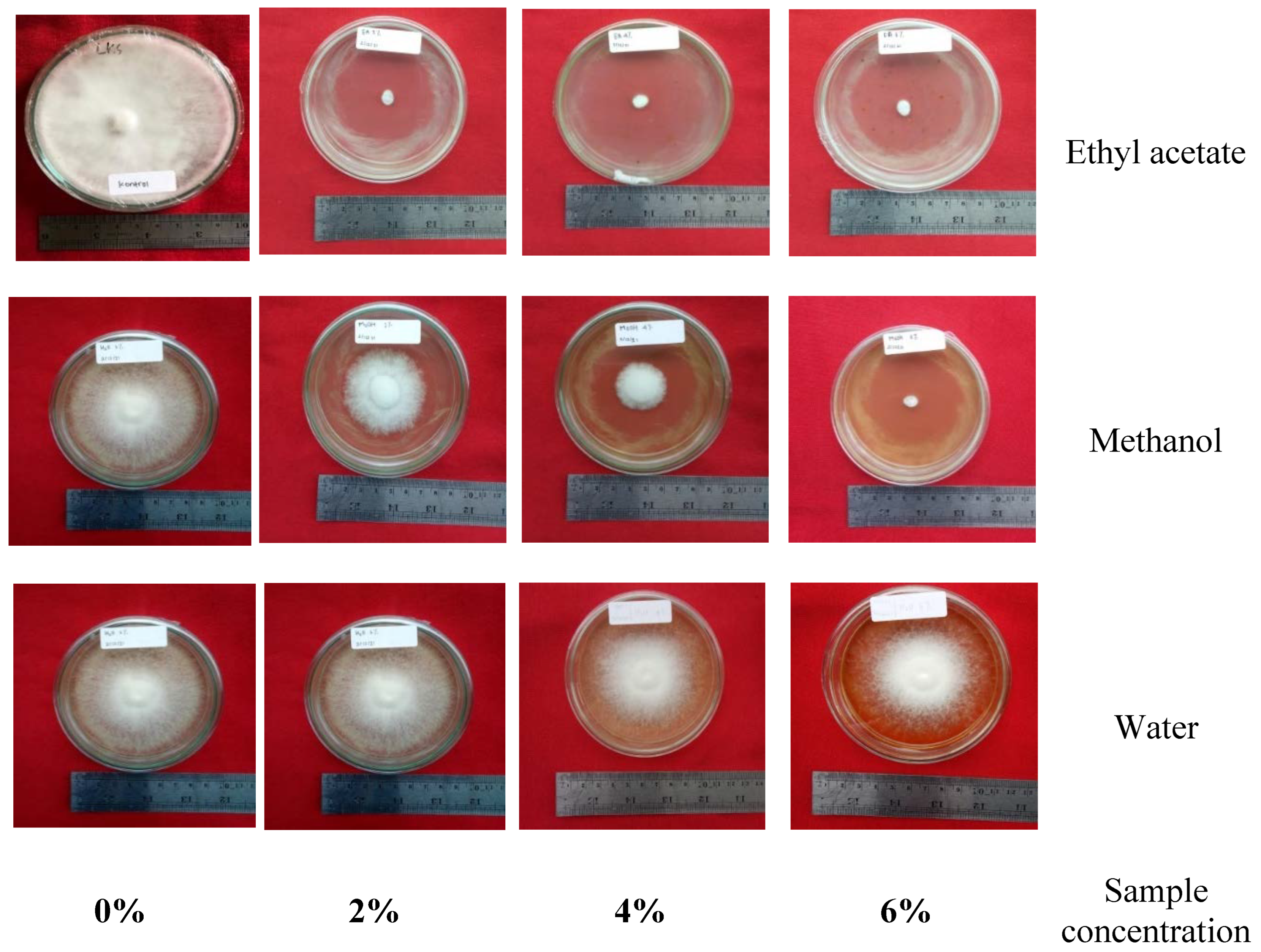

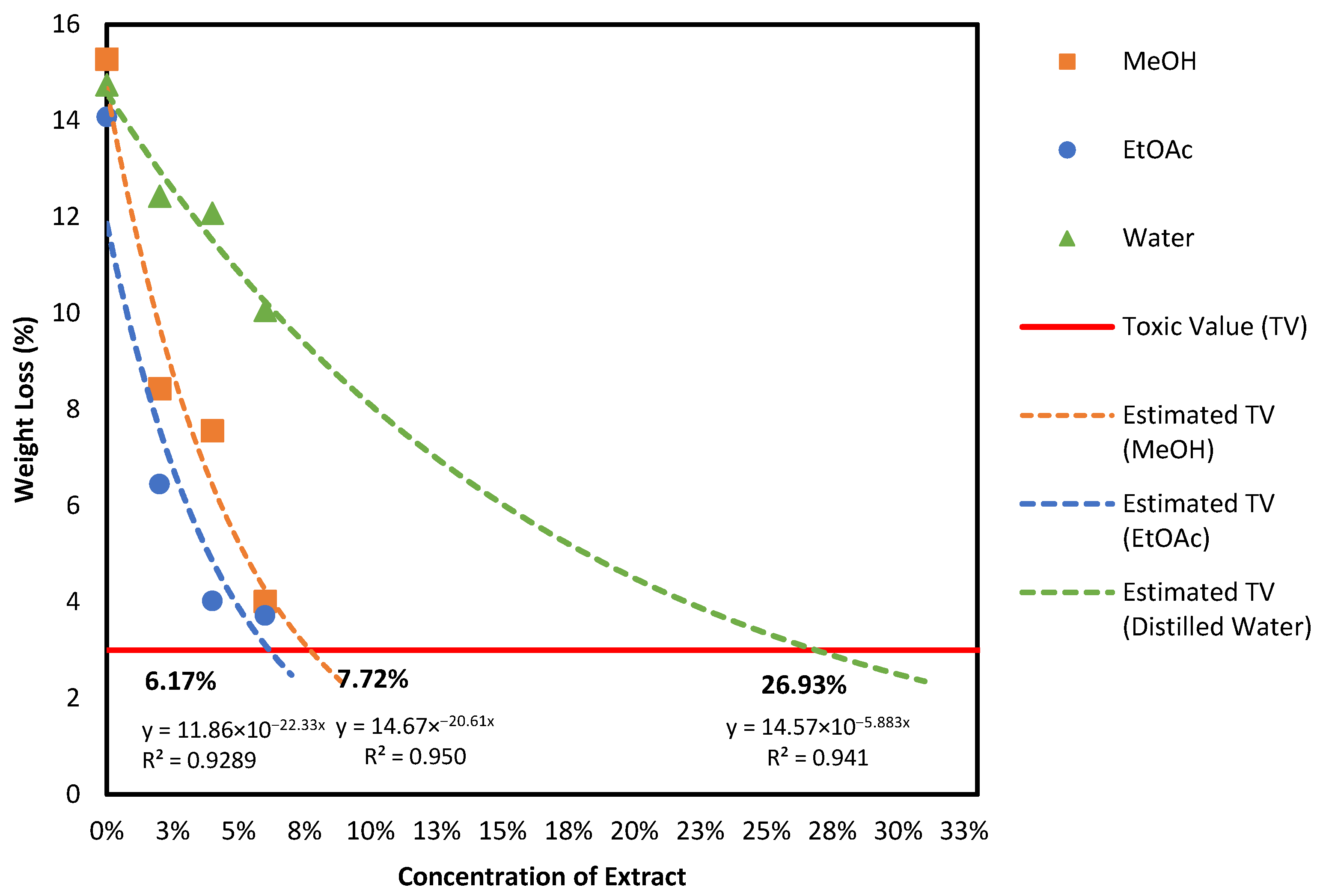

| No | Calc. MW | Formula | Predicted Compound | Detected in Extract of (% Area) | ||
|---|---|---|---|---|---|---|
| Water | MeOH | EtOAc | ||||
| 1 | 103.09988 | C5H13NO | Choline | 11.57 | 21.35 | 0.50 |
| 2 | 117.07912 | C5H11NO2 | Betaine | 5.48 | - | - |
| 3 | 131.0945 | C6H13NO2 | L-Isoleucine | 1.61 | 5.93 | - |
| 4 | 145.05209 | C9H7NO | 4-Indolecarbaldehyde | - | - | 2.17 |
| 5 | 174.08826 | C8H14O4 | Suberic acid | - | - | 1.89 |
| 6 | 182.07829 | C6H14O6 | D-(-)-Mannitol | 5.84 | 4.31 | - |
| 7 | 188.10395 | C9H16O4 | Azelaic acid | 1.11 | 1.01 | 8.98 |
| 8 | 296.23495 | C18H34O4 | NP-008993 | - | - | 1.67 |
| 9 | 300.19326 | C15H26O3 | NP-004917 | - | - | 2.33 |
| 10 | 322.17485 | C16H28O5 | (6E)-10-Heptyl-5,8,9-trihydroxy-3,4,5,8,9,10-hexahydro-2H-oxecin-2-one | - | - | 0.55 |
| 11 | 326.19126 | C18H30O3S | 4-Dodecylbenzenesulfonic acid | 14.59 | 2.69 | 3.02 |
| 12 | 337.33266 | C22H43NO | Erucamide | - | - | 6.52 |
| 13 | 470.33739 | C30H46O4 | 18-β-Glycyrrhetinic acid | - | - | 3.60 |
| Detected Compounds | Targeted Microbe | Reported Activity | References |
|---|---|---|---|
| Betaine | Malassezia restricta | Anti-fungal | [24] |
| L-Isoleucine | Candida albicans | Antifungal (Icofungipen) | [25] |
| 4-Indolecarbaldehyde | Candida albicans, Candida dubliniensis | Antifungal | [26] |
| Suberic acid | Altenaria Alternata, Phytophtora infestans, Fusarium Oxysporum, F. equiseti | Antifungal | [27,28] |
| D-(-)-Mannitol | Fusarium latenicum, Mucor hiemalis, Aspergillus niger, Aspergillus ochraceus, Candida mycoderma, Alternaria alternata, Geotrichum candidum | Antifungal | [29] |
| Azelaic acid | Staphylococcus epidermidis, Propioni bacterium, S. capitis, S. hominis, P. granulosum, P. aviduni | Antifungal | [30,31,32] |
| Erucamide | Agrobacterium tumefaciens, Erwinia carotovora, Ralstonia solanacearum | Antibacterial | [33] |
| 18-β-Glycyrrhetinic acid | Staphylococcus aureus | Antibacterial | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandika, D.; Arinana, A.; Karlinasari, L.; Batubara, I.; Santoso, D.; Witasari, L.D.; Rachmayanti, Y.; Firmansyah, D.; Sudiana, I.K.; Hertanto, D.M.; et al. Efficacy of Fungus Comb Extracts Isolated from Indo-Malayan Termite Mounds in Controlling Wood-Decaying Fungi. Forests 2023, 14, 1115. https://doi.org/10.3390/f14061115
Nandika D, Arinana A, Karlinasari L, Batubara I, Santoso D, Witasari LD, Rachmayanti Y, Firmansyah D, Sudiana IK, Hertanto DM, et al. Efficacy of Fungus Comb Extracts Isolated from Indo-Malayan Termite Mounds in Controlling Wood-Decaying Fungi. Forests. 2023; 14(6):1115. https://doi.org/10.3390/f14061115
Chicago/Turabian StyleNandika, Dodi, Arinana Arinana, Lina Karlinasari, Irmanida Batubara, Djoko Santoso, Lucia Dhiantika Witasari, Yanti Rachmayanti, Dikhi Firmansyah, I Ketut Sudiana, Decsa Medika Hertanto, and et al. 2023. "Efficacy of Fungus Comb Extracts Isolated from Indo-Malayan Termite Mounds in Controlling Wood-Decaying Fungi" Forests 14, no. 6: 1115. https://doi.org/10.3390/f14061115
APA StyleNandika, D., Arinana, A., Karlinasari, L., Batubara, I., Santoso, D., Witasari, L. D., Rachmayanti, Y., Firmansyah, D., Sudiana, I. K., Hertanto, D. M., Hadi, Y. S., & Rahman, M. M. (2023). Efficacy of Fungus Comb Extracts Isolated from Indo-Malayan Termite Mounds in Controlling Wood-Decaying Fungi. Forests, 14(6), 1115. https://doi.org/10.3390/f14061115








