Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Species and Treatments
2.2. Biometric Analyses
2.3. Physiological Analyses
2.4. Metabolic Analyses
2.5. Statistical Analyses
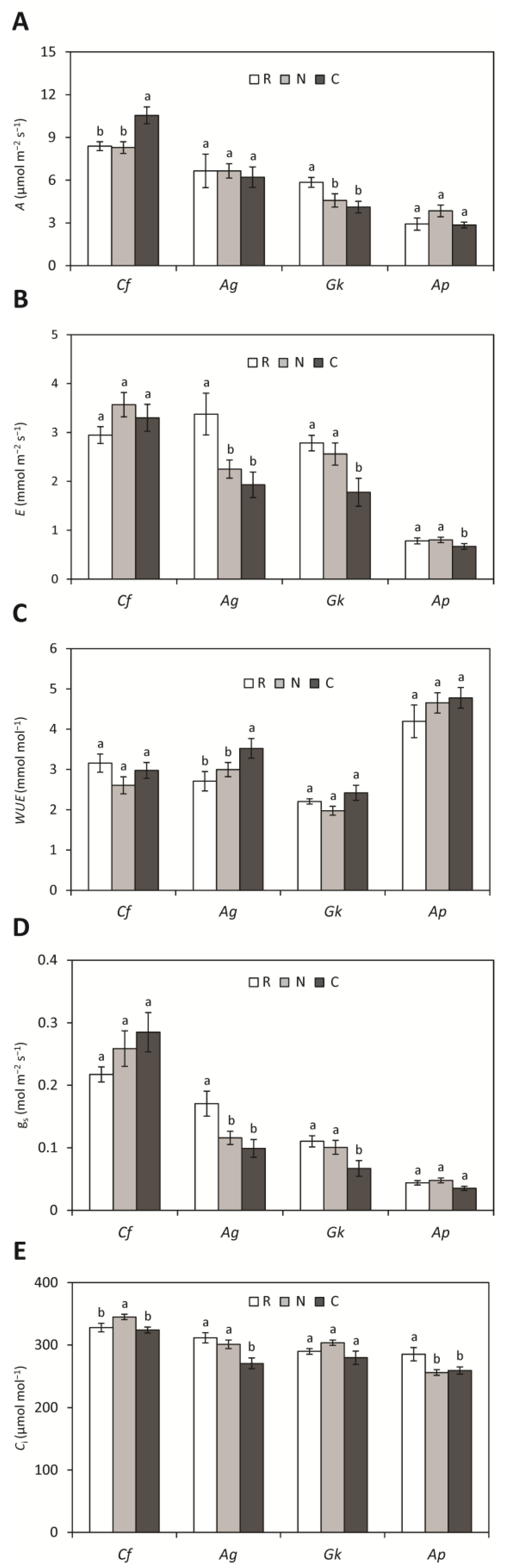
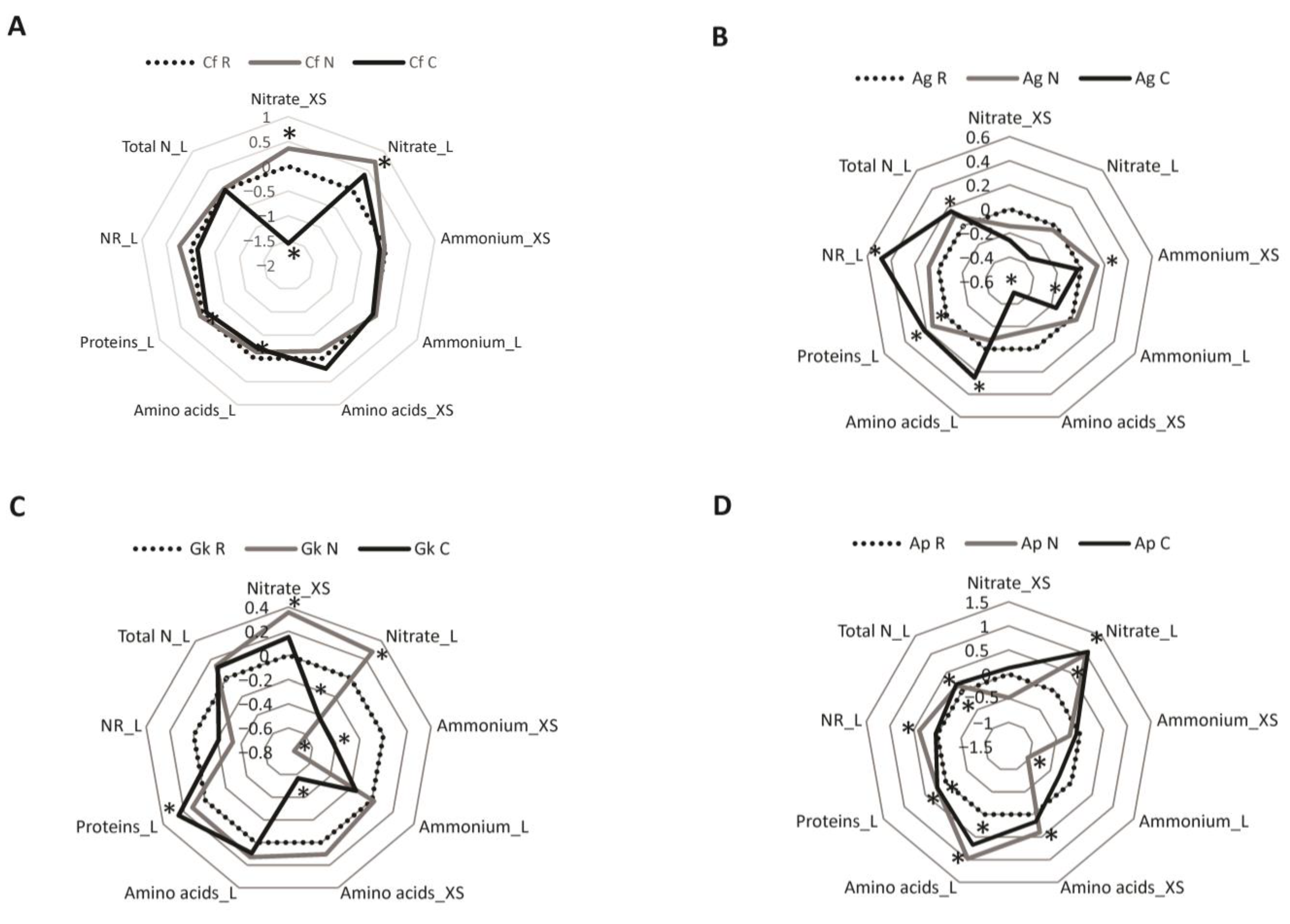
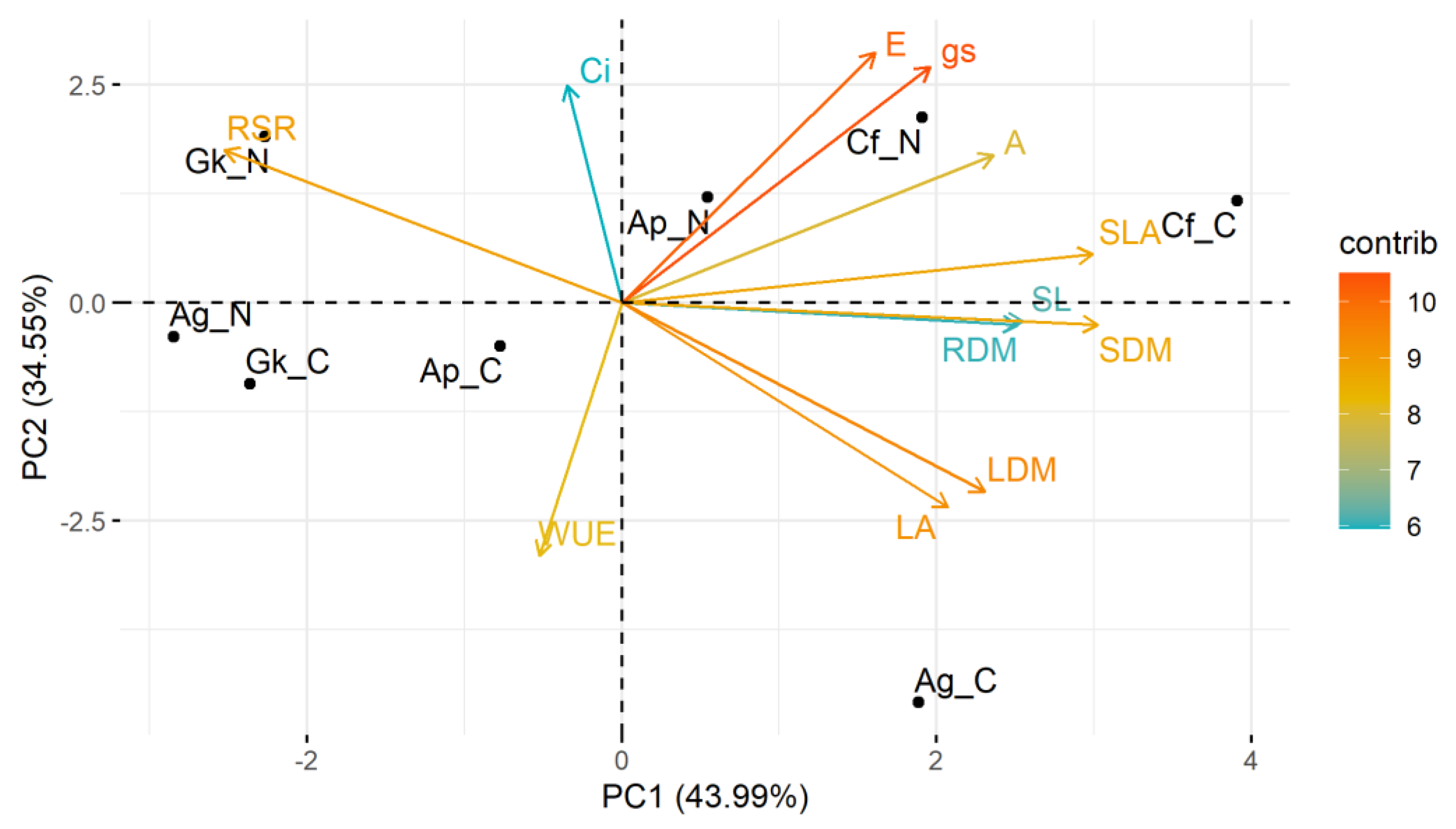
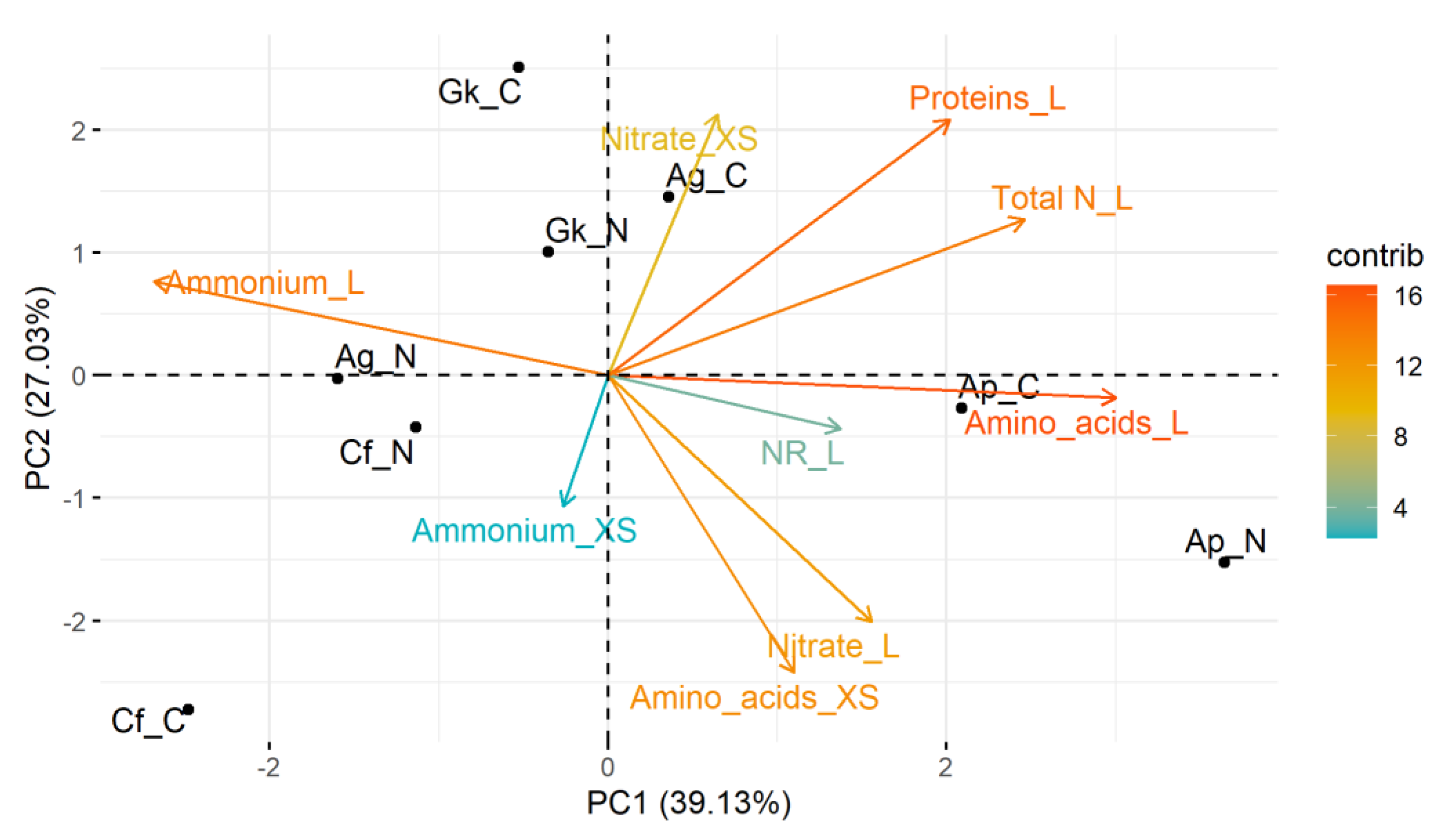
3. Results
3.1. Biometric and Physiological Parameters
3.2. Metabolic Parameters
3.3. Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P. The gap between atmospheric nitrogen deposition experiments and reality. Sci. Total Environ. 2021, 801, 149774. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.A.; McDowell, W.H.; Townsend, A.R.; Vitousek, P.M. The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry 1999, 46, 67–83. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D. Nitrogen and phosphorus losses by surface runoff and soil microbial communities in a paddy field with different irrigation and fertilization managements. PLoS ONE 2021, 16, e0254227. [Google Scholar] [CrossRef]
- Liu, D.; Song, C.; Fang, C.; Xin, Z.; Xi, J.; Lu, Y. A recommended nitrogen application strategy for high crop yield and low environmental pollution at a basin scale. Sci. Total Environ. 2021, 792, 148464. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Cukier, C.; Coleto, I.; González-Murua, C.; Limami, A.M.; González-Moro, M.B.; Marino, D. Isotopic labelling reveals the efficient adaptation of wheat root TCA cycle flux modes to match carbon demand under ammonium nutrition. Sci. Rep. 2019, 9, 8925. [Google Scholar] [CrossRef]
- Gilliam, F.S. Responses of Forest Ecosystems to Nitrogen Deposition. Forests 2021, 12, 1190. [Google Scholar] [CrossRef]
- Gundale, M.J. The impact of anthropogenic nitrogen deposition on global forests: Negative impacts far exceed the carbon benefits. Glob. Chang. Biol. 2022, 28, 690–692. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Huang, G.; Liu, R.; Wu, H.; Zhao, C.; McDowell, N.G. Effects of nitrogen enrichment on tree carbon allocation: A global synthesis. Glob. Ecol. Biogeogr. 2020, 29, 573–589. [Google Scholar] [CrossRef]
- Lu, X.K.; Mo, J.M.; Gilliam, F.S.; Zhou, G.Y.; Fang, Y.T. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.; De Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Uebbing, L.F.; Ros, G.H.; de Vries, W. Experimental evidence shows minor contribution of nitrogen deposition to global forest carbon sequestration. Glob. Chang. Biol. 2021, 28, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tian, D.; Ma, S.; Zhou, X.; Xu, L.; Zhu, J.; Jing, X.; Zheng, C.; Shen, H.; Zhou, Z.; et al. The response of tree growth to nitrogen and phosphorus additions in a tropical montane rainforest. Sci. Total Environ. 2018, 618, 1064–1070. [Google Scholar] [CrossRef]
- Santiago, L.S.; Wright, S.J.; Harms, K.E.; Yavitt, J.B.; Korine, C.; Garcia, M.N.; Turner, B.L. Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J. Ecol. 2012, 100, 309–316. [Google Scholar] [CrossRef]
- Cárate-Tandalla, D.; Leuschner, C.; Homeier, J. Performance of Seedlings of a Shade-Tolerant Tropical Tree Species after Moderate Addition of N and P. Front. Earth Sci. 2015, 3, 75. [Google Scholar] [CrossRef]
- Pasquini, S.C.; Santiago, L.S. Nutrients limit photosynthesis in seedlings of a lowland tropical forest tree species. Oecologia 2012, 168, 311–319. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Wang, C.; Zhou, K.; Mo, J. Responses of understory plant physiological traits to a decade of nitrogen addition in a tropical reforested ecosystem. For. Ecol. Manag. 2017, 401, 65–74. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Mo, H.; Gundersen, P.; Mo, J. Effects of simulated N deposition on foliar nutrient status, N metabolism and photosynthetic capacity of three dominant understory plant species in a mature tropical forest. Sci. Total Environ. 2018, 610–611, 555–562. [Google Scholar] [CrossRef]
- Mo, J.; Li, D.; Gundersen, P. Seedling growth response of two tropical tree species to nitrogen deposition in southern China. Eur. J. For. Res. 2008, 127, 275–283. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Raghubanshi, A.S. Seedling growth of five tropical dry forest tree species in relation to light and nitrogen gradients. J. Plant Ecol. 2014, 7, 250–263. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Wright, S.J.; Sardans, J.; Pérez-Trujillo, M.; Oravec, M.; Večeřová, K.; Urban, O.; Fernández-Martínez, M.; Parella, T.; Peñuelas, J. Long-term fertilization determines different metabolomic profiles and responses in saplings of three rainforest tree species with different adult canopy position. PLoS ONE 2017, 12, e0177030. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, S.; Guo, Q.; Wang, J.; Cao, C.; Wang, J. Leaf nitrogen assimilation and partitioning differ among subtropical forest plants in response to canopy addition of nitrogen treatments. Sci. Total Environ. 2018, 637–638, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Aidar, M.P.M.; Schmidt, S.; Moss, G.; Stewart, G.R.; Joly, C.A. Nitrogen use strategies of neotropical rainforest trees in threatened Atlantic Forest. Plant Cell Environ. 2003, 26, 389–399. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2003, 117, 164–170. [Google Scholar] [CrossRef]
- Oliveira, H.C.; da Silva, L.M.I.; de Freitas, L.D.; Debiasi, T.V.; Marchiori, N.M.; Aidar, M.P.M.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R. Nitrogen use strategies of seedlings from neotropical tree species of distinct successional groups. Plant Physiol. Biochem. 2017, 114, 119–127. [Google Scholar] [CrossRef]
- Debiasi, T.V.; Calzavara, A.K.; da Silva, L.M.I.; da Silva, J.G.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Aidar, M.P.M.; Sodek, L.; Oliveira, H.C. Nitrogen metabolism of Neotropical tree seedlings with contrasting ecological characteristics. Acta Physiol. Plant. 2019, 41, 131. [Google Scholar] [CrossRef]
- Debiasi, T.V.; Calzavara, A.K.; Sodek, L.; Oliveira, H.C. Nitrogen use plasticity in response to light intensity in neotropical tree species of distinct functional groups. Physiol. Plant. 2021, 172, 2226–2237. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; Lindig-Cisneros, R.; de la Barrera, E. Biomonitors of atmospheric nitrogen deposition: Potential uses and limitations. Conserv. Physiol. 2018, 6, coy011. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Soil Map of the World; FAO-UNESCO: Rome, Italy, 1994. [Google Scholar]
- Oliveira, H.C.; Sodek, L. Effect of oxygen deficiency on nitrogen assimilation and amino acid metabolism of soybean root segments. Amino Acids 2013, 44, 743–755. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- McCullough, H. The determination of ammonia in whole blood by a direct colorimetric method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.R.; Popp, M.; Holzapfel, I.; Stewart, J.A.; Dickie-Eskew, A. Localization of nitrate reduction in ferns and its relationship to environment and physiological characteristics. New Phytol. 1986, 104, 373–384. [Google Scholar] [CrossRef]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved Method for Manual, Colorimetric Determination of Total Kjeldahl Nitrogen Using Salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
- Santos, J.B.; Procópio, S.O.; Silva, A.A.; Costa, L.C. Captação e aproveitamento da radiação solar pelas culturas de soja e do feijão e por plantas daninhas. Bragantia 2003, 62, 147–153. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Korpelainen, H.; Zhang, H.; Wang, J.; Huang, H.; Yi, L. Nitrogen addition affects eco-physiological interactions between two tree species dominating in subtropical forests. Plant Physiol. Biochem. 2021, 162, 150–160. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Mott, K.A.; Franks, P.J. The role of epidermal turgor in stomatal interactions following a local perturbation in humidity. Plant Cell Environ. 2001, 24, 657–662. [Google Scholar] [CrossRef]
- Wang, J.; Hui, D.; Ren, H.; Liu, N.; Sun, Z.; Yang, L.; Lu, H. Short-term canopy and understory nitrogen addition differ in their effects on seedlings of dominant woody species in a subtropical evergreen broadleaved forest. Glob. Ecol. Conserv. 2021, 31, e01855. [Google Scholar] [CrossRef]
- Kitajima, K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 1994, 98, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Rahikainen, M.; Kangasjärvi, S. Plant Light Stress; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Huber, S.C. Post-translational regulation of nitrate reductase: Mechanism, physiological relevance and environmental triggers. J. Exp. Bot. 2001, 52, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Nitrate assimilation pathway in higher plants: Critical role in nitrogen signalling and utilization. Plant Sci. Today 2020, 7, 182–192. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Chaves-Filho, J.T.; Stacciarini-Seraphin, E. Alteração no potencial osmótico e teor de carboidratos solúveis em plantas jovens de lobeira (Solanum lycocarpum St.-Hil) em resposta ao estresse hídrico. Braz. J. Bot. 2001, 24, 199–204. [Google Scholar] [CrossRef]
- Ma, L.; Lian, J.; Lin, G.; Cao, H.; Huang, Z.; Guan, D. Forest dynamics and its driving forces of sub-tropical forest in South China. Sci. Rep. 2016, 6, 22561. [Google Scholar] [CrossRef]
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; Embrapa Informação Tecnológica: Brasília, Brazil, 2003; Volume 1, Available online: https://www.embrapa.br/florestas/publicacoes/especies-arboreas-brasileiras (accessed on 8 January 2022).
- Roth, M.; Günther, K.; Michiels, H.-G.; Puhlmann, H.; Sucker, C.; Hauck, M. Nitrogen deposition is positively correlated to foliar nitrogen content in Vaccinium myrtillus and other understory species in temperate forests on acidic soil. Acta Oecologica 2021, 110, 103696. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Khan, A.; Yan, L.; Hasan, M.M.; Wang, W.; Zou, G.; Liu, X.D.; Fang, X.W. Leaf traits and leaf nitrogen shift photosynthesis adaptive strategies among functional groups and diverse biomes. Ecol. Indic. 2022, 141, 109098. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [PubMed]
| Species | Cf | Ag | Gk | Ap | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | R | N | C | R | N | C | R | N | C | R | N | C |
| RDM (g) | 0.306 ± 0.025 a | 0.329 ± 0.020 a | 0.345 ± 0.029 a | 0.045 ± 0.004 a | 0.042 ± 0.005 a | 0.052 ± 0.006 a | 0.393 ± 0.030 a | 0.343 ± 0.028 a | 0.372 ± 0.026 a | 0.120 ± 0.009 a | 0.156 ± 0.011 a | 0.141 ± 0.008 a |
| SDM (g) | 0.258 ± 0.019 b | 0.343 ± 0.021 b | 0.548 ± 0.043 a | 0.033 ± 0.003 a | 0.024 ± 0.002 a | 0.088 ± 0.032 a | 0.260 ± 0.017 a | 0.223 ± 0.011 b | 0.258 ± 0.018 a | 0.092 ± 0.008 a | 0.076 ± 0.007 a | 0.076 ± 0.004 a |
| LDM (g) | 0.361 ± 0.039 c | 0.492 ± 0.036 b | 0.601 ± 0.036 a | 0.027 ± 0.003 b | 0.028 ± 0.002 b | 0.102 ± 0.013 a | 0.300 ± 0.033 a | 0.241 ± 0.033 a | 0.300 ± 0.046 a | 0.056 ± 0.015 a | 0.054 ± 0.008 a | 0.072 ± 0.014 a |
| RSR (g g−1) | 0.504 ± 0.019 a | 0.405 ± 0.030 b | 0.302 ± 0.018 c | 0.945 ± 0.239 a | 0.894 ± 0.129 a | 0.324 ± 0.043 b | 0.748 ± 0.084 a | 0.769 ± 0.070 a | 0.731 ± 0.095 a | 0.778 ± 0.090 a | 0.957 ± 0.104 a | 0.907 ± 0.984 a |
| SL (cm) | 16.32 ± 0.674 b | 18.3 ± 0.957 b | 23.53 ± 1.123 a | 9.717 ± 1.045 b | 5.164 ± 0.377 c | 12.52 ± 0.767 a | 11.64 ± 0.483 a | 11.76 ± 0.476 a | 12.81 ± 0.426 a | 9.68 ± 0.409 a | 10.8 ± 0.423 a | 10.96 ± 0.296 a |
| LA (cm2) | 137.4 ± 15.71 c | 193.4 ± 16.25 b | 262.0 ± 13.42 a | 7.576 ± 0.799 b | 8.466 ± 0.668 b | 33.24 ± 4.589 a | 58.92 ± 6.317 a | 48.84 ± 7.546 a | 61.12 ± 10.73 a | 25.97 ± 2.38 a | 20.18 ± 2.13 a | 26.41 ± 2.035 a |
| SLA (cm2 g−1) | 378.4 ± 10.29 c | 400.3 ± 7.889 b | 446.3 ± 6.680 a | 283.9 ± 15.51 a | 281.1 ± 11.71 a | 308.8 ± 10.34 a | 195.3 ± 4.509 a | 195.6 ± 6.093 a | 194.3 ± 4.862 a | 615.2 ± 283.9 a | 407.9 ± 34.73 a | 343.1 ± 55.66 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardy, L.R.; Debiasi, T.V.; Sanada, K.; Rondina, A.B.L.; Torezan, J.M.D.; Stolf-Moreira, R.; Bianchini, E.; Pimenta, J.A.; Oliveira, H.C. Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings. Forests 2023, 14, 1111. https://doi.org/10.3390/f14061111
Bardy LR, Debiasi TV, Sanada K, Rondina ABL, Torezan JMD, Stolf-Moreira R, Bianchini E, Pimenta JA, Oliveira HC. Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings. Forests. 2023; 14(6):1111. https://doi.org/10.3390/f14061111
Chicago/Turabian StyleBardy, Lara Raposo, Tatiane Viegas Debiasi, Karina Sanada, Artur Berbel Lirio Rondina, José Marcelo Domingues Torezan, Renata Stolf-Moreira, Edmilson Bianchini, José Antonio Pimenta, and Halley Caixeta Oliveira. 2023. "Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings" Forests 14, no. 6: 1111. https://doi.org/10.3390/f14061111
APA StyleBardy, L. R., Debiasi, T. V., Sanada, K., Rondina, A. B. L., Torezan, J. M. D., Stolf-Moreira, R., Bianchini, E., Pimenta, J. A., & Oliveira, H. C. (2023). Effect of Nitrogen Addition to the Soil on Atlantic Forest Tree Seedlings. Forests, 14(6), 1111. https://doi.org/10.3390/f14061111







