Abstract
The vascular cambium is an extensive and permanent secondary meristem with wood cells products of periclinal divisions commonly contributed to two directions and arranged in radial files of trees. Cambium activity is the origin of timber production. Taxodium ascendens Brongn is an exotic species in China, and its apical meristem and cambial activity are still elusive, resulting in a lack of understanding about its wood formation and improvement. We thus addressed this knowledge gap by studying Cambium activity. For studying, twigs from five 30-year-old healthy trees were collected between February-2017 and March-2018. Anatomy deciphered its apical meristem with a Cryptomeria–Abies type. The procambium appeared after leaf primordium and initially presented five lobes as observed transversely from a one-year-old shoot. The procambium under the apical differentiated into protophloem first and then protoxylem toward the inside. It means that protoxylem differentiated later than protophloem did. After dormancy, the vascular cambium began to be active, starting in early April 2017, which was later than shoot differentiation. On 25 July 2017, the cambial zone had 9–10 immature xylem cell layers. Both initiation and cessation of the xylem preceded that of the phloem. Until 10 October 2017, few immature elements were found, indicating the translation of cells from activity to dormancy. On 15 November 2017, the cambium contained 3–4 cells in radial rows, which demonstrated the dormancy of the cambium until next spring. Furthermore, immature xylem elements increased as cell layers in the cambium zone and cell fission increased. The growth pattern of T. ascendens revealed that cambial activity is highly seasonal and dependent on changes in abiotic conditions. Thus, the wood formation in the species will be significantly altered in a changing climatic pattern. These enhance our understanding of tree growth science, wood formation, wood structure, wood properties variation and wood improvement in tree breeding.
1. Introduction
The vascular cambium of a tree is an extensive and permanent secondary meristem, which generally begins its development between the primary xylem and phloem and is finally endowed with a cylindrical shape with one-cell thickness [1]. Cambium typically locates between the differentiating secondary xylem and phloem, where a multiseriate zone composed of periclinally divided cells can be formed. These periclinal cells are distinct in cell periclinal and anticlinal divisions [2]. Once the cambium differentiates as a continuous layer in the most distal regions of the stem and lateral branches, the secondary xylem toward the inside and the secondary phloem toward the outside can be produced. The activity of vascular cambium varies among plants and environmental factors [3,4]. New growth layers, namely the secondary xylem and secondary phloem, have been investigated during the growing season, which has revealed the difference among various tree species [5,6,7]. The mechanism of such different cambium activity could lie in the physiological and biochemical changes during cambium activity.
Cambium activity is the origin of timber production. The development and seasonal behavior of the vascular cambium of trees in temperate zone have been extensively studied [8,9]. Attention was paid to the relationship of the teak tree ring width to cambial activity in India [10]. Its tree rings produced by cambial activities are highly promising indicators of ecological and environmental processes. However, such studies for the trees in the subtropical and tropical regions were still elusive and need further study. The development of vascular cambium was significantly associated with the wet or dry season, but it’s difficult to distinguish the period of activity and dormancy [11]. Dendrochronological studies are developed mainly in temperate and arid zones, where strong climate seasonality induces vascular cambial dormancy and annual growth ring formation in most woody species, but are scarce in the tropics and subtropics, where climate seasonality and, therefore, yearly growth ring formation is not so apparent [12]. The growth of cambium in Azadirachta under local climatic conditions in different forests located in both moist-deciduous areas and deserts has been reported [13]. The developmental anatomy of the cambium, secondary vascular tissue, and periderm of Botrypus virginianus was studied along with Ophioglossaceae, with the progymnosperms and seed plants in these features compared, particularly concerning their phylogenetics [14]. For example, the cambial activity of the broadleaf tree Koelreuteria paniculata began at the end of February, and cambium cells significantly increased in size, with their walls gradually thinning in early March. In June, the cambium cell zone reached a maximum of 5–7 cell layers and gradually decreased to 2–3 layers of thick-walled cells. Cinnamomum camphora, Populus deltoides, and Populus tomentosa have also been studied before [15,16].
The typical cambium comprises a single continuous cylinder of cambial meristematic cells. The primary growth and formation of conductive tissues are achieved due to the activity of a primary vascular meristem, i.e., the procambium. The developmental transition to the second type of growth is accomplished by the emergence of a continuous cambium cylinder [4,17]. To establish a continuous cambium cylinder, parenchyma cells that locate between vascular bundles transdifferentiate and form the interfascicular cambium. The continuous cambium cylinder will form a permanent structure to produce secondary vascular tissues throughout a plant’s life [18]. The study proved that the vascular meristem is a developmental continuum from the procambial stage throughout the formation of successive cambia. At the same time, the outermost pro-cambial cells preserve their undifferentiated status to divide during the maturation of the protophloem elements and further develop to form vascular bundles [19].
Generally, three major types of gymnosperms can be distinguished from shoot apex meristerm: Cycas type, Ginkgo type, and Cryptomeria—Abies type [5]. The Cycas type lacks a central mother cell and cambium transition zone. In contrast, the Ginkgo type contains these two types of tissues as well as rib meristem, flank meristem, and surface meristem, and the cambium-like transitional zone intersperses between the central mother cell and the flank and rib meristems; as compared with the Ginkgo type, the Cryptomeria-Abies type does not have cambium-like transition zone, but their other parts are consistent [5]. The impacts of genetic and environmental factors on shoot growth and xylem formation for West African tropical tree Terminalia superba Engl. and Diels (Combretaceae) have been studied [20]. The correlation between cambial growth and rainfall has been assessed in the lowland dipterocarp forest of Peninsular Malaysia [21]. Recently, more attention has been paid to annual growth rings in tropical and subtropical trees and the distinct environmental triggers of seasonal growth [22,23]. The seasonal anatomical changes associated with the cambium in teak growing in moist deciduous and dry deciduous forests of Gujarat State in the western part of India were reported [24]. Studies on the cambial activity, mainly the xylem formation, revealed the age of trees and possible factors controlling tree growth. Such a study has enhanced our understanding of dendrochronological studies, timber prediction, biomass yield, and forest dynamics.
In conifers, most newly formed cells differentiate into axial tracheids in response to water conduction and mechanical support [25,26]. Environmental factors can physically impact the growth and development of trees, which will thus influence tree xylogenesis. Much research has been done on the occurrence and development of some species [5,8,27]. However, little was known about the shoot apex type and the activity patterns in subtropical trees, including the initiation and cessation of growth [11]. Although some studies have been reported on vascular cambium development and dynamic changes between the active and dormant states, only a few have considered every aspect of seasonal changes in conifers in the subtropical region. In recent years, many articles have reported the transcriptional regulatory factors in cambium [28,29], but basic microscopic observation is still needed.
This study focused on Taxodium ascendens Brongn, which belongs to Taxodiaceae, Taxodium Rich. This tree originates from the marshland located in the southeast of North America and has been widely planted along the Yangtze River in the subtropical region of China. T. ascendens planted in this area is a deciduous tree with pneumatophores to respire in wet sites and grow well in the alluvial plain and estuary. However, the apical meristem and cambial activity of T. ascendens have not been reported yet. In this study, we thus investigated the apical meristem of T. ascenden in the shoots, its vascular cambium formation, and the seasonal activities of the vascular cambium. This mechanistic study will reveal the science underlying the growth of such a tree, especially the seasonal activity of cambium and its dividing rate, which directly influence the wood structures, properties, and production. Factors such as the cell division quantity and rate and cell differentiation degree were critical to controlling tree growth and, thus, the yield and quality of wood [30]. This study, therefore, can deliver information on wood formation to guide the planting and breeding of Taxodium ascenden, control variation in wood properties and promote the production and quality improvement of wood.
2. Materials and Methods
2.1. Plant Materials and Chemicals
T. ascendes was harvested from the field in the Forest Farm of the South Lake in Wuhan City, a subtropical area in China. The study was conducted from February 2017 to March 2018. Five healthy 30-year-old trees of T. ascendes in the field were selected. For each tree, 2–3 specimens of the bud, one-year branch, two-year branch, and three-year branch were collected weekly from the point 3~4 m above the ground. After that, the sample taken was 0.5~1 cm long and was immediately preserved in a formaldehyde-ethanol-acetic acid (5:90:5, v/v/v) solution before use [31]. All chemicals used in this study were purchased from Sinopharm Chemical Reagent Co., LTD (Shanghai, China).
2.2. Microscopy
Sample preparation was as we reported before [30]. In brief, after dehydration with ethyl alcohol and clarity with dimethyl benzene, the samples were embedded in paraffin. For observation under light microscopy (Olympus Fv1000, Tokyo, Japan), the samples were cut into 10 μm thickness using a rotary microtome. The observation was conducted using both 40× and 100× objective lenses. The sections were dewaxed and strained with a combination of hematoxylin and safranine-fast green, and the staining for different samples was adapted to their different lignification to get the best quality images. After dehydration with a series of ethyl alcohol and clarity with dimethyl benzene, the slices were sealed with Canada gum. To quantify each cell, the cell layers were counted under the microscope when the clear-quality sections were chosen for picture-taking.
2.3. Terminology
Cambial cells or cambial zone are radial rows of fusiform and ray initials, including phloem and xylem mother cells. In other words, the fusiform cambium initials and part of the phloem and xylem, of which the cells are enlarging but still capable of dividing, as described by Wilson et al. [32].
Cambial inactivity defines the suspension of cell division activity in the cambial zone [13], which was determined by counting lying cells between the xylem and phloem in the transverse section. As proposed by Esau [8], immature xylem and phloem were defined as the secondary xylem and phloem that are undergoing differentiation, respectively.
Conductive and nonconductive phloem were determined based on anatomical characteristics considering the conductive potential of the sieve cells, in which the conductive phloem is formed of non-collapsed and living sieve cells of vertical parenchyma and the non-conductive phloem is located outside and contains only dead and collapsed sieve cells [33], while in Taxodium ascendens the sieve cells generally functionalize for conduction.
2.4. Cellular Measurements in Longitudinal Sections of the Shoot Apex
The pictures of longitudinal sections of the shoot apex were taken under light microscopy (Olympus Fv1000, Japan). Ten cells with clear structures were selected from the figure and marked with letters using Adobe Photoshop CS6. The areas of nuclei and protoplasts were measured using ImageJ software. SPSS 17.0 was used to test the data and for statistics.
3. Results
3.1. Occurrence and Development of Procambium
The shoot apex of T. ascendes was determined as the Cryptomeria—Abies type because the cambium–like transitional zones were not found, and the outermost layer had divided in an anticlinal direction toward the surface of the meristem (Figure 1). From Figure 1A,B, four meristematic zones, namely superficial meristem (SM), central mother cells (CMC), peripheral meristem (PM) and rib meristem (RM), can be seen on the longitudinal sections of the shoot apex of T. ascendes. Cells in SM were divided anticlinally and periclinally to induce both the epidermis and apical meristematic zones. The cells in this meristematic zone were polyhedral, and frequent cell divisions were observed. The nucleus comprises eukaryotic cells, the control center of genetics and cell metabolism. The main components of the protoplasm are carbohydrates, proteins, nucleic acids, lipids, etc. The areas of this nucleolus calculated from Figure 1A were from 29.86 μm2 to 56.94 μm2, and the mean was 42.68 μm2. Cytoplasm was from 175.87 μm2 to 599.13 μm2, and the mean was 417.04 μm2. The ratio of the nucleolus to the cytoplasm was 0.102. These reflected that cytoplasm accounted for a large proportion of cells and contained rich nutrients, which supplied rich nutrition materials for nuclear division. CMC, apart from the shoot apex, contains numerous vacuoles and large light-staining nuclei. Its cell divisions were more infrequent in the central mother cell zone. The PM was mitotically active [5], and the cells had grumous, deep-staining protoplasm. Owning to this mitotical activity, the cells induced leaf primordium, leading to the elongation of the stem (anticlinal division) and augmentation of the bulk (periclinal division). RM was under the central mother cells [5], which can develop into the pith of the stem. In this area, vacuolated cells have divided transversely.
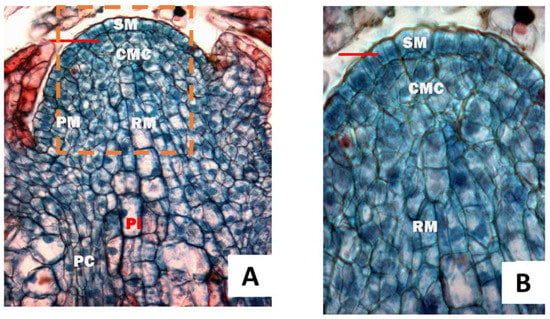
Figure 1.
Longitudinal sections of the shoot apex. (A), the subarea of the bud sampled on 30 April 2017; (B) magnification of the dashed area of A. SM, superficial meristem; CMC, central mother cells; PM, peripheral meristem; RM, rib meristem; Pi, pith, PC, procambial cell. The bars were 40 μm. (The terminologies were seen as in Fahn [5]).
Figure 2 shows the longitudinal anatomic section of a one-year tender stem. Different sites were selected to make a series of sections, marked A, B, C, D, E, and F. The continuous transverse sections of apical meristems were translated into the primary structure (A–F), as shown in Figure 3. All apical meristem cells were of a similar size and shape in the beginning stage, with some cells then vacuolated, suggesting differentiation in the cortex and the pith (Figure 3A). Approximately 0.1 mm from the apex, the cortex and pith cells were vacuolated even more than the meristem cells. Residual meristem was derived from the apical differentiation in one cycle, which had thick cytoplasm, small vacuole and deep-staining protoplasm (Figure 3B). Most cells in residual meristem were polyhedral, similar to that in apical meristem but different from the developing cortex and pith.
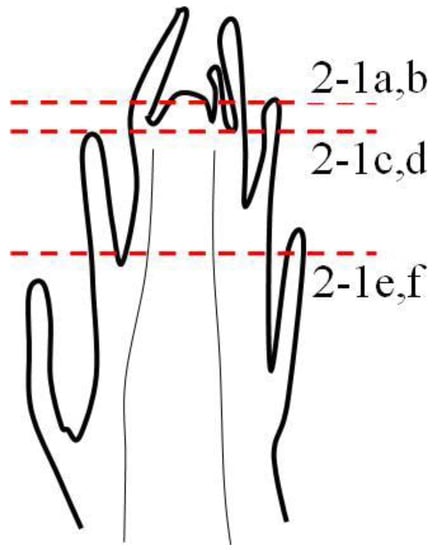
Figure 2.
The longitudinal section sites of the one-year tender stem.
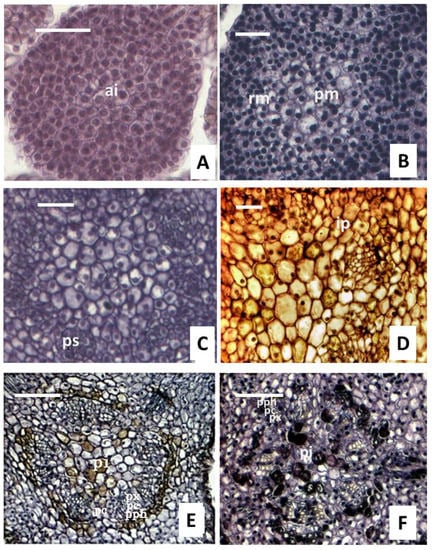
Figure 3.
Transverse sections of apical meristems translated into the primary structure. (A), apical, the differentiation of the vascular tissue did not observe. (B), some cells were vacuolated, the cortex and pith began to differentiate, and residual meristem was observed. (C), the residual meristem transformed into procambial strand. (D,E), the developing procambial strand at different regions. (F), the procambium had a circular outline except for the interfascicular area. Ai, apical; rm, residual meristem; ps, procambium strand; pm, pith meristems; ip, interfascicular parenchyma; pph, protophloem; pc, procambium; px, protoxylem; pi, pith. Bars in a-d are 40 μm, and e and f’s are 100 μm.
Along with the development of the stem, the residual meristem located below the leaf primordium (0.26 mm far from the apical) transformed into the procambial strand, which suggested the start of the procambium (Figure 3C). The strands were basic meristem, 1–2 cells, and light-staining protoplasm, which can develop into the interstrand parenchyma cells and the interfascicular cambium. About 0.35 mm away from the apex, the procambium differentiated into the protophloem with 1–3 layers put forth first and then the protoxylem toward the inside (Figure 3C). Between the protophloem and protoxylem, the shape of the cells divided was irregular in Figure 3D. As the development continued, the procambial cells had irregular long transverse sections, _vacuoles, and light-staining protoplasm in the mature vascular bundle (Figure 3D). In the late growth stage, approximately 0.56 mm of procambial cells underwent a periclinal division to give birth to platode cells in a radial direction, producing 1–3 layers in the early days and up to 3–5 layers in the latter days (Figure 3E). In addition, the procambial cells differentiated into both the metaphloem outside and the metaxylem inside. In the meantime, new interfascicular parenchyma and vascular bundles were formed between the bundles (Figure 3F).
Moreover, vascular bundles can be observed in the developing procambial strand (Figure 3D,E), indicating the differentiation of the procambial cells. The procambium initially presented five lobes, as displayed in the transverse view of a one-year-old shoot (Figure 3F). The procambium cells first differentiated into the primary cambium in the corner, the border of the lobes remained immature, and a circular outline appeared on the procambium as the shoot developed. Resulted of this process, the primary structure of the stem was formed, which consisted of the epidermis, cortex, and vascular cylinder, and the cortex was under the epidermis (Figure 3). In the tree stems, the primary phloem developed from the outer cells of the procambium, while the primary xylem developed from the inner cells and pith located in the center (Figure 3E).
Lastly, continuous longitudinal sections of many vegetative buds revealed that the procambium appeared after the leaf primordium (Figure 4). The cells were not augmented but prolonged and were the earliest procambial cells. After developing the shoot, the cells are arranged longitudinally. The procambium cells looked similar to a short rectangle in shape and were short in the longitudinal section, with no significant difference in cell length and width, respectively. Most procambium cell terminals had transverse and small wedge shapes (Figure 4A). With the development of the cells, their terminal became wedge-shaped (Figure 4B). The protoplasm was deeply stained in the cross-section view. The procambium cells had not yet differentiated into long or short cells, and the procambium cell nucleus extended axially in an oblong shape (Figure 4A). Above the procambium, the cells had increased cell numbers. Approximately 0.75 mm from the apex, the procambium cells divided transversely into long and short cells (Figure 4A) due to the unequal frequency division. The procambium cells had gabled ends, as shown in the radial view, since they were deeply stained, whereas the cambium cells had flat endings in this view, and their protoplasm was slightly stained (Figure 3A). The procambium was not differentiated into long and short cells, while long fusiform initials and short ray cells can be distinguished in the cambium [34].
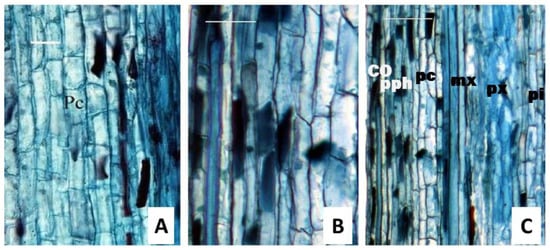
Figure 4.
The longitudinal section of the one-year tender stem indicates the transformation in the cell terminal. (A), procambium cells were similar, and most cell terminals were transverse-shaped and small wedge-shaped. (B), cells had differentiated into long or short cells, and the long cells had diagonal or even walls and were wedge-shaped. (C), cortex parenchyma cells with a length 5-fold higher than its width and pith cells with similar length and width. Pc, procambial cell; co, cortex; pph, protophloem; mx, metaxylem; px, protoxylem; pi, pith. Bars in (A,B) were 40 μm, and those in (C) were 100 μm.
Additionally, the procambium consisted of similar cells in both morphology and structure (Figure 4A), but these were not continuous in the vertical section (Figure 4B). In Figure 4C, the length of parenchyma cells in the cortex varied from 62.96 μm to 118.52 μm, with a mean of 83.21 μm. Their width is from 12.35 μm to 24.69 μm, with a mean of 16.29 μm. It was observed that parenchyma cells in the cortex had a length that was 5-fold higher than their width, while the length of the pith cells was similar to their width (Figure 4C).
3.2. Occurrence of the Vascular Cambium
3.2.1. Occurrence of the Fascicular Cambium
The cambial zone was initiated in a procambial ring of the stem before the differentiation of the primary vascular tissue was completed. When the cambium began to differentiate, the cells in the fascicular cambium developed earlier than those in the interfascicular cambium. On the transverse section of the one-year-old shoot, several cambial bundles were separated by the primary rays (Figure 5). It can be seen in Figure 3D–F, that the cambial bundles were produced by periclinal divisions and that the primary cambial bundle was between the protoxylem and the protophloem. These cambial bundles were thin-walled cells with 3–4 layers, a large nucleus, and abundant cytoplasm, while the cambium cells were not yet developed at this stage. Larson [16] named these cambial bundles procambial traces. Cambial initials were flanked along their two tangential walls, phloem mother cells (phloem initials) toward the outside and xylem mother cells (xylem initials) toward the inside. These cambial initials also remained as one-layer cells, so the procambial cells in this phase were called genetic horizon. The cells in the procambial zone had different sizes and were developed into fusiform and ray initials. When the metaphloem began to create, the parenchyma cells appeared between the phloem mother cells and the xylem mother cells, which were flat in the radial direction and arranged in a line. These characteristics were typical for cambial cells; therefore, fascicular cambium has been developed.
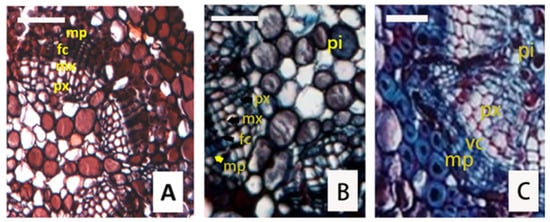
Figure 5.
Transverse sections of one-year stem display the fascicular cambium and interfascicular cambium. (A,B), cambial bundles separated by the primary rays from the wood harvested on 20 May 2017 and 27 May 2017, respectively. (C), the cambium with circular outlines was from the wood harvested on 11 November 2017. pi, pith; mp, metaphloem; fc, fascicular cambium; vc, vascular cambium; mx, metaxylem; px, protoxylem. Bars are 40 μm.
3.2.2. Occurrence of the Interfascicular Cambium
The interfascicular cambium was derived from the dedifferentiation of mature interfascicular parenchyma [5,34]. The cambial activity started from the vascular bundles. Even when the fascicular cambium was well developed, the parenchyma cells of the interfascicular region just began their differentiations to produce interfascicular cambium. The interfascicular cambium differentiated as panels extended slowly from the edges of the fascicular cambium. Subsequently, the two panels of adjacent bundles matured and formed the cambium, which further matured and formed the entire cambium.
In the transverse section of the one-year branch in Figure 5, the interfascicular cambium can be found in the parenchyma of the medullary rays. With the extension toward the base, single small vascular bundles successively merged into big bundles, causing the primary vascular bundles to become irregular. When the interfascicular cambium appeared in the parenchyma cells of the leaf gap, the xylem and the phloem split from the edge of the leaf gap first and then from the center (Figure 5C), this process resulted in the circular cambium. However, in the one-year-old and two-year-old branches, we did not find significant interfascicular cambium. Overall, cambium formed first and then its cell division resulted in the thickening of the secondary xylem and phloem.
3.3. Seasonal Activity of the Vascular Cambium
It is well known that plant growth is seasonal and is generally related to temperatures in temperate regions and rainfall in subtropical and tropical regions [9]. Cambium activity is the only source of timber production, and understanding the seasonal activity of cambium is thus crucial to elucidate tree growth and the properties of the formed wood. The width of the cambial zone reflected the growth rate of cambial derivatives and their differentiation rate into mature cells of the xylem and phloem. If the cambial zone was broad at the beginning of growth, the division of cells exceeded their differentiation; otherwise, the narrow cambial zone indicated that the differentiation rate exceeded that of cell division. Therefore, we studied the seasonal activity of the cambium of Taxodium ascendens and the cell layers of the cambium zone, immature xylem, immature phloem, and mature tracheids produced in all its active processes. Such a study could enhance our understanding of tree growth, wood formation and wood properties.
The cambium of Taxodium ascendens was non-storied, and the cambial rays were uniseriate. In the subtropical zone of Wuhan, the bud break of T. ascendens in this region generally occurred in the last ten days of March, and its cambium activity began at the beginning of April. That said, the vascular cambium became active after dormancy and was later than the bud break of the shoot. When cambium activity started, 1–2 layers of cambium cells in the cambial zone expanded a little earlier than cell division, and the cambial zone also became a little wider; in other words, cell expansion in the cambial zone began before cell division. Most cambial cells were densely cytoplasmic, with numerous small vacuoles and abundant lipid droplets found in them, suggesting that many cambial cells were probably still dormant [35].
In our study, the cambium activity of T. ascendes was first observed at the beginning of April 2017. There were 2–4 layers of cells found between the xylem and the phloem (Figure 6A), and 1–2 layers of cells located close to the phloem presented periclinal divisions, which indicated the beginning of cambium activity. Furthermore, a layer of phloem mother cells thickened in the radial direction but became thin in tangential directions. The xylem mother cells next to the xylem differentiated afterwards. Between the phloem and xylem mother cells, another 1–3 layers of flat cells were found. At 19 days after the observation of cambium activity (19 April), we did not find any further noticeable change in the cambium. In this period, the cells were translating from dormancy to activity. During periods of active growth, the cambial initials were difficult, often impossible, to distinguish from their recently formed derivatives. During cell division, the tangential walls of fusiform initials were very thin, but they became thicker during cell dormancy. The radial walls also become thicker during this cell dormancy, with bead-like shapes observed, attributed to numerous primary pit fields that plasmodesmsa can pass through in their thin areas.
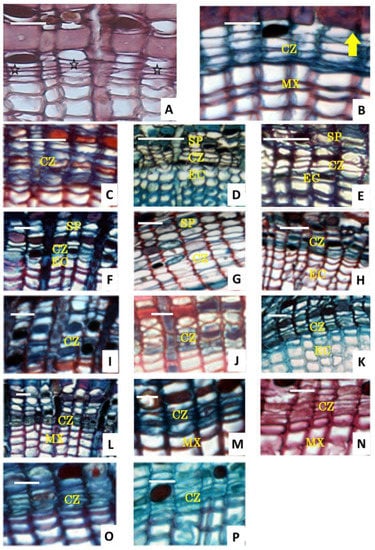
Figure 6.
Seasonal activity of vascular cambium on transverse sections of three-year stem harvested from different times in 2017. (A), sample harvested on 5 April 2017 displayed the starting cambium (indicated by  ) and the first division of phloem mother cells. (B), sample harvested on 19 April 2017, when cell division and first periclinal division began (indicated by
) and the first division of phloem mother cells. (B), sample harvested on 19 April 2017, when cell division and first periclinal division began (indicated by  ). (C), sample harvested on 30 April 2017, when transverse sections of the three-year stem had an early stage. (D), sample harvested on 7 May 2017, which displayed cambium division. (E,F), samples harvested on 26 May 2017 and 3 July 2017, respectively, which show fast periclinal division. (G), samples harvested from 25 July 2017, which showed the fastigium cambium. (H–J), the activity was attenuation. (K), The sample harvested on 12 September 2017 showed the transformation of cells from activity to dormancy. In panel (K), the periclinal division was observed. (L), sample harvested on 10 August 2017, showing flat and deeply stained dormant cambium cells and thick radial cell walls. (M), samples harvest on 25 November. (N), samples harvested on 24 January 2018. (O,P), samples harvested on 5 and 15 March 2018, respectively, which contain dominated dormant cambium cells. cz: cambium zone; mx: mature xylem; sp: second phloem; ec: enlarged cell. Bars are 20 μm, except in panels (C) (100 μm) and panels (D,H) (40 μm).
). (C), sample harvested on 30 April 2017, when transverse sections of the three-year stem had an early stage. (D), sample harvested on 7 May 2017, which displayed cambium division. (E,F), samples harvested on 26 May 2017 and 3 July 2017, respectively, which show fast periclinal division. (G), samples harvested from 25 July 2017, which showed the fastigium cambium. (H–J), the activity was attenuation. (K), The sample harvested on 12 September 2017 showed the transformation of cells from activity to dormancy. In panel (K), the periclinal division was observed. (L), sample harvested on 10 August 2017, showing flat and deeply stained dormant cambium cells and thick radial cell walls. (M), samples harvest on 25 November. (N), samples harvested on 24 January 2018. (O,P), samples harvested on 5 and 15 March 2018, respectively, which contain dominated dormant cambium cells. cz: cambium zone; mx: mature xylem; sp: second phloem; ec: enlarged cell. Bars are 20 μm, except in panels (C) (100 μm) and panels (D,H) (40 μm).
 ) and the first division of phloem mother cells. (B), sample harvested on 19 April 2017, when cell division and first periclinal division began (indicated by
) and the first division of phloem mother cells. (B), sample harvested on 19 April 2017, when cell division and first periclinal division began (indicated by  ). (C), sample harvested on 30 April 2017, when transverse sections of the three-year stem had an early stage. (D), sample harvested on 7 May 2017, which displayed cambium division. (E,F), samples harvested on 26 May 2017 and 3 July 2017, respectively, which show fast periclinal division. (G), samples harvested from 25 July 2017, which showed the fastigium cambium. (H–J), the activity was attenuation. (K), The sample harvested on 12 September 2017 showed the transformation of cells from activity to dormancy. In panel (K), the periclinal division was observed. (L), sample harvested on 10 August 2017, showing flat and deeply stained dormant cambium cells and thick radial cell walls. (M), samples harvest on 25 November. (N), samples harvested on 24 January 2018. (O,P), samples harvested on 5 and 15 March 2018, respectively, which contain dominated dormant cambium cells. cz: cambium zone; mx: mature xylem; sp: second phloem; ec: enlarged cell. Bars are 20 μm, except in panels (C) (100 μm) and panels (D,H) (40 μm).
). (C), sample harvested on 30 April 2017, when transverse sections of the three-year stem had an early stage. (D), sample harvested on 7 May 2017, which displayed cambium division. (E,F), samples harvested on 26 May 2017 and 3 July 2017, respectively, which show fast periclinal division. (G), samples harvested from 25 July 2017, which showed the fastigium cambium. (H–J), the activity was attenuation. (K), The sample harvested on 12 September 2017 showed the transformation of cells from activity to dormancy. In panel (K), the periclinal division was observed. (L), sample harvested on 10 August 2017, showing flat and deeply stained dormant cambium cells and thick radial cell walls. (M), samples harvest on 25 November. (N), samples harvested on 24 January 2018. (O,P), samples harvested on 5 and 15 March 2018, respectively, which contain dominated dormant cambium cells. cz: cambium zone; mx: mature xylem; sp: second phloem; ec: enlarged cell. Bars are 20 μm, except in panels (C) (100 μm) and panels (D,H) (40 μm).
The differentiating phloem was another indication of cambial activity because that xylem production was initiated simultaneously with or after the phloem. The cambial cells in the sample harvested on 19 April 2017 began to undergo periclinal division, but the cell layers did not visibly increase (Figure 6B). On 30 April 2017, the layers added up to 4–5 layers, and the immature phloem increased to 2–3 layers, while the immature xylem, namely the daughter cells divided from mother cell division and cells that are expanding in radial direction before lignification occurs, was not observed (Figure 6C). On 7 May 2017, the immature xylem was 3–4 layers, and the immature phloem was 4–6 (Figure 6D). All these results highlighted that the phloem split more frequently than the xylem at the start of cell activity, which might be due to the transportation of nutriment. Cells in the phloem and xylem with six layers appeared simultaneously in the samples harvested in the middle of May. On 26 May 2017, the cambial zone consisted of 5–6 cell layers, and immature xylem cells had 6–7 layers, indicating high cambial activity. On 3 July 2017, the cambium had seven layers of fusiform cells, and the immature xylem cells were 8–9 layers (Figure 6E). On 25 July 2017, the cambial zone had 7–8 layers, and the immature xylem cell had 11–12 layers (Figure 6G), indicating that fastigium cambium had high activities. The immature xylem began to reduce in the last ten days of July (Figure 6H–J), and the cambium from the sample harvested in the last ten-day of August had 4–5 layers, which is fewer than that of the cambium. During the growth season, both initiation and cessation of the xylem production precede that of phloem. Up to 10 October 2017, only a few immature elements were found (Figure 6L), indicating the translation from activity to dormancy. On 15 November 2017, the cambium contained 3–4 cells in the radial rows (Figure 6M), clearly displaying that the cambial came into dormancy until next spring, as shown in Figure 6N–P. Subsequently, the cell layers remained unchanged, the cambial cells remained flat and deeply stained in dormant phases, and the radial cell wall became thicker.
As shown in Figure 7, the layers of the mature tracheids increased over the entire time scale from 16 April to 12 November, while the immature xylem, cambium zone, and immature phloem increased from 16 April to 23 July and then decreased slowly until 12 November. When the cambial cells increased their layers and further split, the layers and volumes of immature xylem also largely increased (Figure 7). When the plant materials were taken at the end of May or the beginning of June, the bark was easily separated from the wood. The reason was that when the cambial cells expanded, their radial walls became thinner and weaker; as a result, the bark (all tissues outside the vascular cambium) was easily separated from or peeled off the tree. The mature tracheids thickened quickly from May to August, while this thickening occurred more slowly in September and October. From November to the January and February of the following year, the number of mature tracheids remained unchanged.
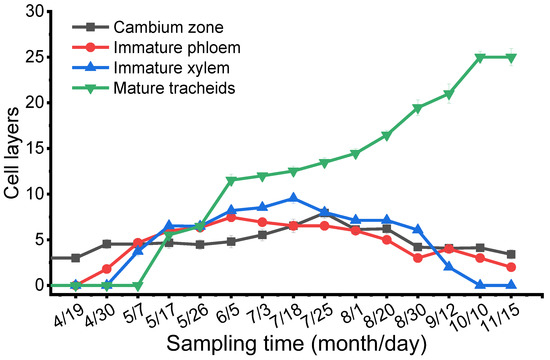
Figure 7.
Seasonal changes in the cell layers of the cambium zone, immature xylem, immature phloem, and mature tracheids were counted under microscopy observation.
4. Discussion
4.1. Discontinuity and Continuity in the Cambium Occurrence
Philipson et al. [3] reported that the differences between primary growth and secondary growth of trees in subtropical regions were not clear enough because primary tissues were derived from cell divisions, and all lateral growth was a “continuous process, unbroken from the apex to the mature trunk”. However, two speculations have been reported for the continuity of meristems: a sudden transition from the procambium to the vascular cambium or simply a gradual change [5]. Similarly, the metaxylem, secondary xylem, and the metaphloem and secondary phloem also had a slow or abrupt transition. Larson [17] expatiated that the apex meristems were formed before the procambium, while the procambium was formed earlier than the vascular cambium. The same phenomenon has been observed in our study. Meanwhile, the cytological characteristics in every abovementioned development phase were continuous along the stem toward the tip, and some remaining apical meristems were vacuoled to form procambium in the bundles.
4.2. The Leaf Trace and the Occurrence of the Vascular Cambium
The procambial strands of the stem appeared after the apical meristem. Procambial strands were under the developing leaf primordia or future leaf primordia even before the procambial strands began to develop. Therefore, the vascular cambium occurrence generally had a phylogenetic relationship with the leaf trace. The occurrence of the vascular cambium usually had two different theories regarding stem origin and leaf origin. The first theory expatiates that the vascular bundle occurred when the stem end formed, which was inherent in the stem, while the other theory was that the vascular bundle was formed by the prolongation of the leaf trace. In this study, as shown in the transverse view of the one-year-old stem, the procambium initially presented five lobes, which were related to the leaf trace (Figure 3E,F). The procambium in the leaf trace differentiated first, while the procambium in other parts retained its primary state. Therefore, the occurrence of the vascular cambium was predicted to have a phylogenetic relationship with the leaf trace.
4.3. Activity Period of the Vascular Cambium
Plants exhibited successive active and dormant phases during a calendar year. This cambium behavior was believed to be regulated by several internal and external factors, including heredity constitution, physiological phenomenon, and environmental conditions of the habitat [3,10].
The activity of vascular cambium varied from different tree species. As our study has shown, most cypress and diffuse-porous wood had two various activities. One is the cambium resuming activity from the basal of the buds after bud break, while the other one, in most ring porous wood, started its activity before the sprouting. For some cypress and diffuse-porous wood such as Broussonetia papyrifera (L.) Vent. and Pinus Sylvestris L., the cambium started developing before sprouting, while in Taxodium ascendens, the cambium began to develop after sprouting. The start of the cambium activity in 2017 was discovered first in late April. This may be because the content of procambial strands IAA was low, so the development was slow. After the development of the buds, the cambium had high activity, indicating that the IAA compositive newly transports to the base in the cambial zone, making the initial cells split more quickly to maintain the state of the initial cells.
Meanwhile, the abundant IAA resulted in the xylem differentiation. The cell layers of the cambial cells are related to the cambium activity, which means that the cell layers were more dormant when the cambium was active. However, our study was based on wood anatomy, which still needs future study regarding wood ultrastructure, including (a) the ultrastructure of the procambium cells, (b) the ultrastructure of the cambial cells consisting of the fusiform initials and short ray cells, (c) the ultrastructure of the tracheids and sieve tube developing, (d) the relationships between climate and seasonal activity of the vascular cambium. Such study in the future will help us to holistically understand the development of the vascular cambium of Taxodium ascendens, its seasonal activity, its wood ultrastructure and wood properties variation.
5. Conclusions
In this work, we have elucidated the development of the vascular cambium of Taxodium ascendens, especially its seasonal activity, by studying five healthy trees harvested between February 2017 and March 2018 from a subtropic area in Wuhan City, China. This study revealed that its apical meristem belongs to a Cryptomeria–Abies type. The differentiation of the protoxylem was found to be later than that of the protophloem. When the interfascicular cambium appeared in the parenchyma cells of the leaf gap, the xylem and phloem split from the edge of the leaf gap and then to the center. The occurrence of the procambium determined the seasonal activity of the vascular cambium of T. ascendens from dormancy to action.
In the processes of trees’ cambial activities, the cell division quantity and rate and cell differentiation degree were the key controlling tree growth and, thus, the yield and quality of wood. After the dormancy of T. ascendens, its vascular cambium began to be active, which was later than shoot differentiation, and the vascular cambium started in early April-2017. Cells in the phloem and xylem appeared simultaneously (6-layer) in the middle of May-2017. On 25 July 2017, the cambial zone had 9–10 immature xylem cell layers. Both initiation and cessation of the xylem preceded that of the phloem. Until 10 October 2017, few immature elements were found, indicating the translation of cells from activity to dormancy. On 15 November 2017, the cambium contained 3–4 cells in radial rows, which demonstrated the dormancy of the cambium until next spring. Furthermore, immature xylem elements increased as cell layers in the cambium zone and cell fission increased.
This study reinforces our understanding of the growth process of T. ascendens, and its wood formation mechanism. In addition, it lays the foundations of cell development of this tree planted as an exotic species in China. These are also helpful in guiding the planting and breeding of T. ascendens, controlling wood properties variation and promoting wood production and quality improvement.
Author Contributions
Conceptualization, Y.X.; methodology, validation, and formal analysis, Y.X. and C.L.; investigation, C.L. and K.W.; resources, H.L. and Z.H.; data curation, Y.X. and H L; writing—original draft preparation, C.L. and Y.X; writing—editing, supervision and funding acquisition, Y.X.; project administration and visualization, Y.X. and H.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC (31971584, 31570551).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Hong-Yan Zhang and Yan-Ni Feng for their guidance in using facilities in our experimental works. We also thank Fa-En Liu and Xiao-Dong Li for their sistences in collecting samples weekly. Finally, Qiang Li from the USA made a substantial contribution to correcting this manuscript in English, and we would express our sincere thanks to him.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zimmermann, M.H.; Brown, C.L. Trees: Structure and Function; Springer: New York, NY, USA, 1971; p. 348. [Google Scholar]
- Butterfield, B.G. Terminology used for describing the cambium. IAWA Bull. 1975, 1, 13–14. [Google Scholar]
- Philipson, W.R.; Word, J.; Butterfield, B.G. The Vascular Cambium, Its Development and Activity; Chapmen & Hall: London, UK, 1971; p. 182. [Google Scholar]
- Iqbal, M.; Ghouse, A.K.M. Cambial concept and organisation. In The Vascular Cambium; Iqbal, M., Ed.; Wiley: New York, NY, USA, 1990; pp. 1–36. [Google Scholar]
- Fahn, A. Plant Anatomy, 3rd ed.; Pergamon: Oxford, UK, 1982; pp. 51–56. [Google Scholar]
- Cui, K.M.; Wei, L.B. Periodicity of cambial activity and changes of starch content in Broussnetia papyrifera. J. Int. Plant Biol. 1995, 37, 53–57. [Google Scholar]
- Gričar, J.; Zupančič, M.; Čufar, K.; Oven, P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Technol. 2007, 41, 463–475. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy, 2nd ed.; Wiley: New York, NY, USA, 1967; p. 624. [Google Scholar]
- Fahn, A.; Werker, E. Seasonal cambial activity. In The Vascular Cambium; Iqbal, M., Ed.; John Wiely and Sons Ind.: New York, NY, USA, 1990; pp. 138–157. [Google Scholar]
- Upadhyay, K.K.; Shah, S.K.; Roy, A.; Tripathi, S.K. Dendroclimatology of teak indicates prevailing climatic conditions of tropical moist forests in India. Ecol. Indic. 2021, 129, 107888. [Google Scholar] [CrossRef]
- Calixto, L.; Arcadio, M.A. Seasonality cambial activity of four Linas from a Mexican lowland tropical rainforest. IAWA J. 2005, 26, 111–120. [Google Scholar]
- Worbes, M.; Staschel, S.; Roloff, A.; Junkc, W.J. Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For. Ecol. Manag. 2003, 173, 105–123. [Google Scholar] [CrossRef]
- Rao, K.S.; Rajput, K.S. Relationship between seasonal cambial activity, development of xylem and phenology in Azadirachta indica growing in different forests of Gujarat State. Ann. For. Sci. 2001, 58, 691–698. [Google Scholar] [CrossRef]
- Akira, T.; Masahiro, K. Developmental anatomy of vascular cambium and periderm of Botrypus virginianus and its bearing on the systematic position of Ophioglossaceae. Mag. Tokyo 1998, 101, 373–385. [Google Scholar]
- Yin, Z.F.; Fan, R.; Gan, X.; Huang, G.B. The anatomical observation on the occurrence and development of vascular cambium in Populus deltoides. J. Nanjing For. Univ. 1999, 6, 56–60. [Google Scholar]
- Peng, Y.; Fan, R. Anatomical observation on development of cambium in Cunninghamia lanceolata. J. Nanjing For. Univ. 1999, 5, 13–17. [Google Scholar]
- Larson, P.R. Procambium vs. cambium and protoxylem vs. Metaxylem in Populus deltoids seedlings. Am. J. Bot. 1976, 63, 1332–1348. [Google Scholar] [CrossRef]
- Spicer, R.; Groover, A. Evolution of development of vascular cambia and secondary growth. New Phytol. 2010, 186, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Myśkow, E.; Gola, E.M.; Tulik, M.T. Continuity of procambium and anomalous cambium during formation of successive cambia in Celosia argentea. J. Plant Growth Reg. 2019, 38, 1458–1466. [Google Scholar] [CrossRef]
- Longman, K.A.; Leakey, R.R.B.; Denne, M.P. Genetic and environmental effects on shoot growth and xylem formation in a tropical tree. Ann. Bot. 1979, 44, 377–380. [Google Scholar] [CrossRef]
- Killmann, W.; Thong, H.L. The periodicity of growth in tropical trees with special reference to dipterocarpaceae—A review. IAWA J. 1995, 16, 329–335. [Google Scholar] [CrossRef]
- Jacoby, G.C. Overview of tree-ring analysis in tropical regions. IAWA J. 1989, 10, 99–108. [Google Scholar] [CrossRef]
- Worbes, M. Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J. Ecol. 1999, 87, 391–403. [Google Scholar] [CrossRef]
- Rao, K.S.; Rajput, K.S. Seasonal behaviour of vascular cambiun in Teak (Tectona grandls) growing in moist deciduous and dry deciduous forests. IAWA J. 1999, 20, 85–93. [Google Scholar] [CrossRef]
- Larson, P.R. The Vascular Cambium: Development and Structure; Springer: Berlin, Germany, 1994; p. 725. [Google Scholar]
- Chaffey, N.J. Wood Formation in Trees: Cell and Molecular Biology Techniques; Taylor and Francis: London, UK, 2022; p. 364. [Google Scholar]
- Butterfield, B.G. Developmental changes in the vascular cambium of Aeschynomene hispida Willd. N. Zeal. J. Bot. 1971, 10, 373–386. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, J.S.; Jeon, H.W.; Sangsawang, K.; Do, S.; Choi, Y.; Park, E.J.; Lee, H.; Ko, J.H. Wood transcriptome profiling identifies critical pathway genes of secondary wall biosynthesis and novel regulators for vascular cambium development in Populus. Genes 2019, 10, 690. [Google Scholar] [CrossRef]
- Jing, Z.; Gugan, E.; Juan, A.S. Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots. Nat. Plants 2019, 5, 1033–1042. [Google Scholar]
- Dong, M.; Xu, Y.; Lin, H.; Li, X.; Xia, Q. Seasonal dynamics in cambial activity and the formation of xylem and phloem in the branches of Cinnamomum camphora. Dendrobiology 2016, 75, 13–21. [Google Scholar] [CrossRef]
- Sass, J.E. Botanical Microtechnique, 3rd ed.; The Iowa State College Press: Ames, IA, USA, 1958; p. 228. [Google Scholar]
- Wilson, B.F.; Wodzicki, T.J.; Zahner, R. Differentiation of cambial derivates: Proposed terminology. For. Sci. 1966, 12, 438–440. [Google Scholar]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.P.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. IAWA list of microscopic bark features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Pashin, A.J.; Zeeuw, C. Textbook of Wood Technology, 4th ed.; McGraw-Hill Book Company: New York, NY, USA, 1980; pp. 55–84. [Google Scholar]
- Farrar, J.J.; Evert, R.F. Ultrastructure of cell division in fusiform cells of the vascular cambium of Robinia pseudoacacia. Trees 1997, 11, 203–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).