Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Aggregates and Measurements
2.4. Statistical Analysis
3. Results
3.1. Distribution of Soil Aggregates and Nutrients
3.1.1. Distribution of Soil Aggregates
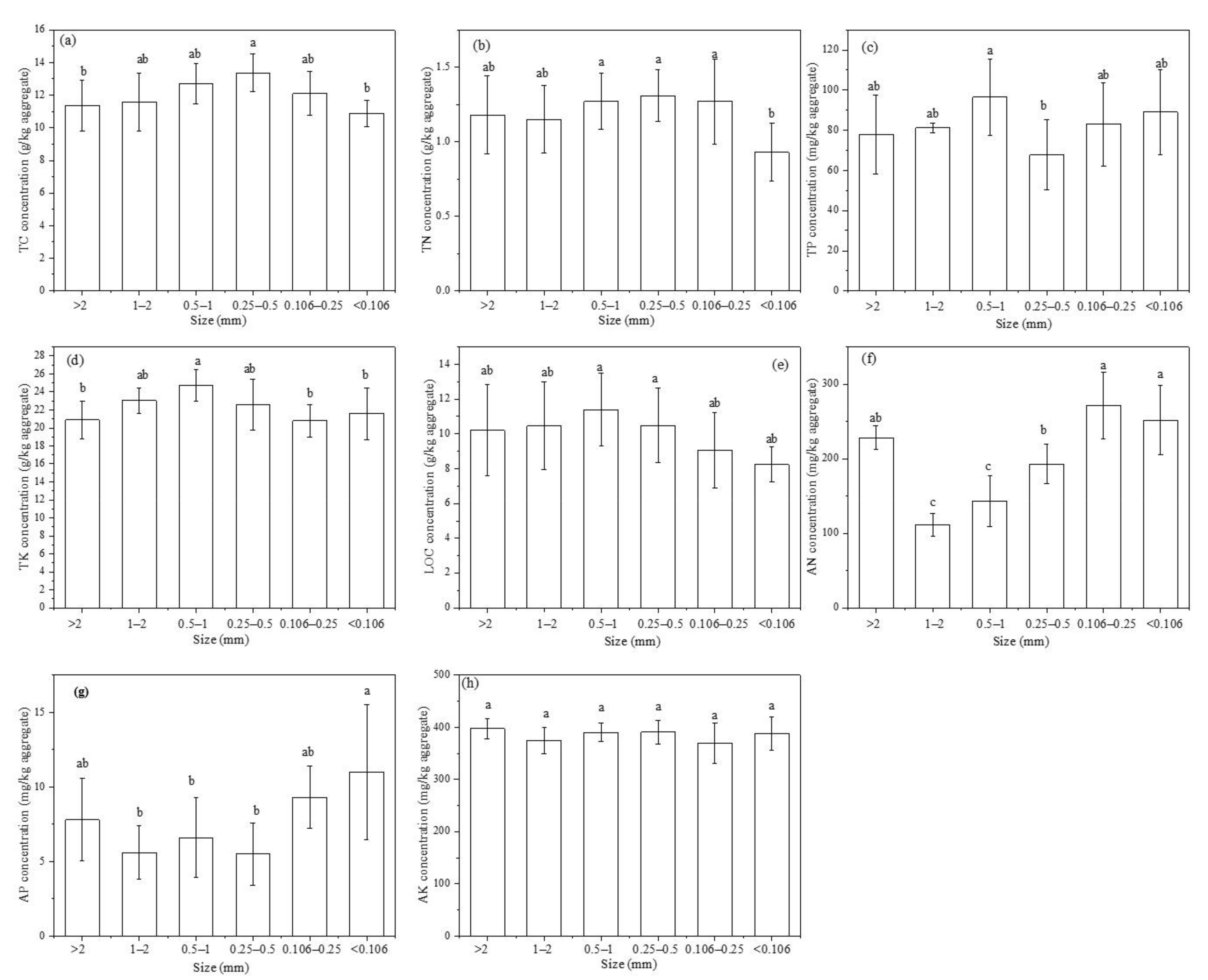
3.1.2. Nutrient Concentration Distribution in Soil Aggregates
3.1.3. Contribution of Soil Aggregates to Soil Nutrients
3.1.4. Nutrient Availability in Aggregates
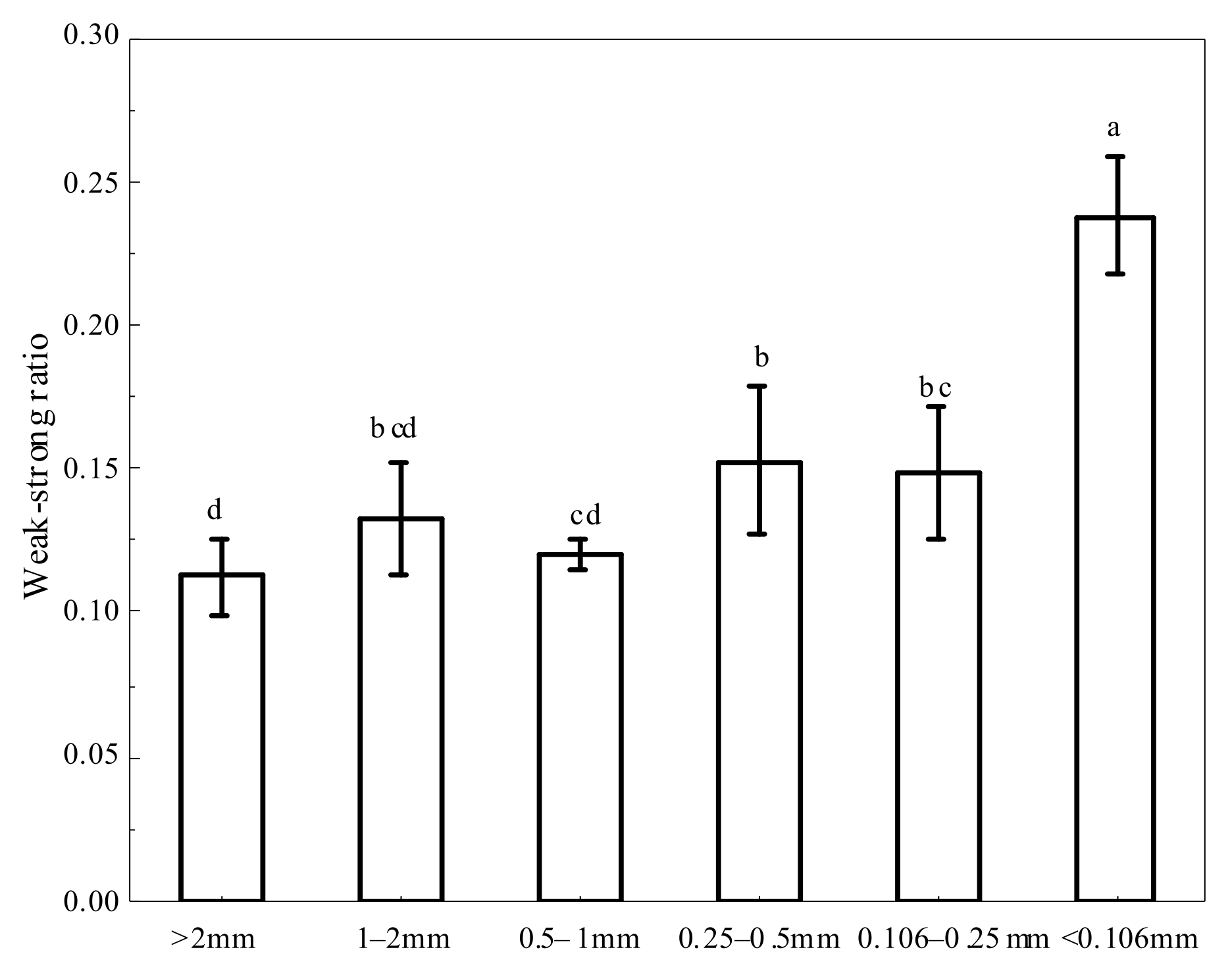
3.2. Organo-Mineral Bonds in Soil Aggregates
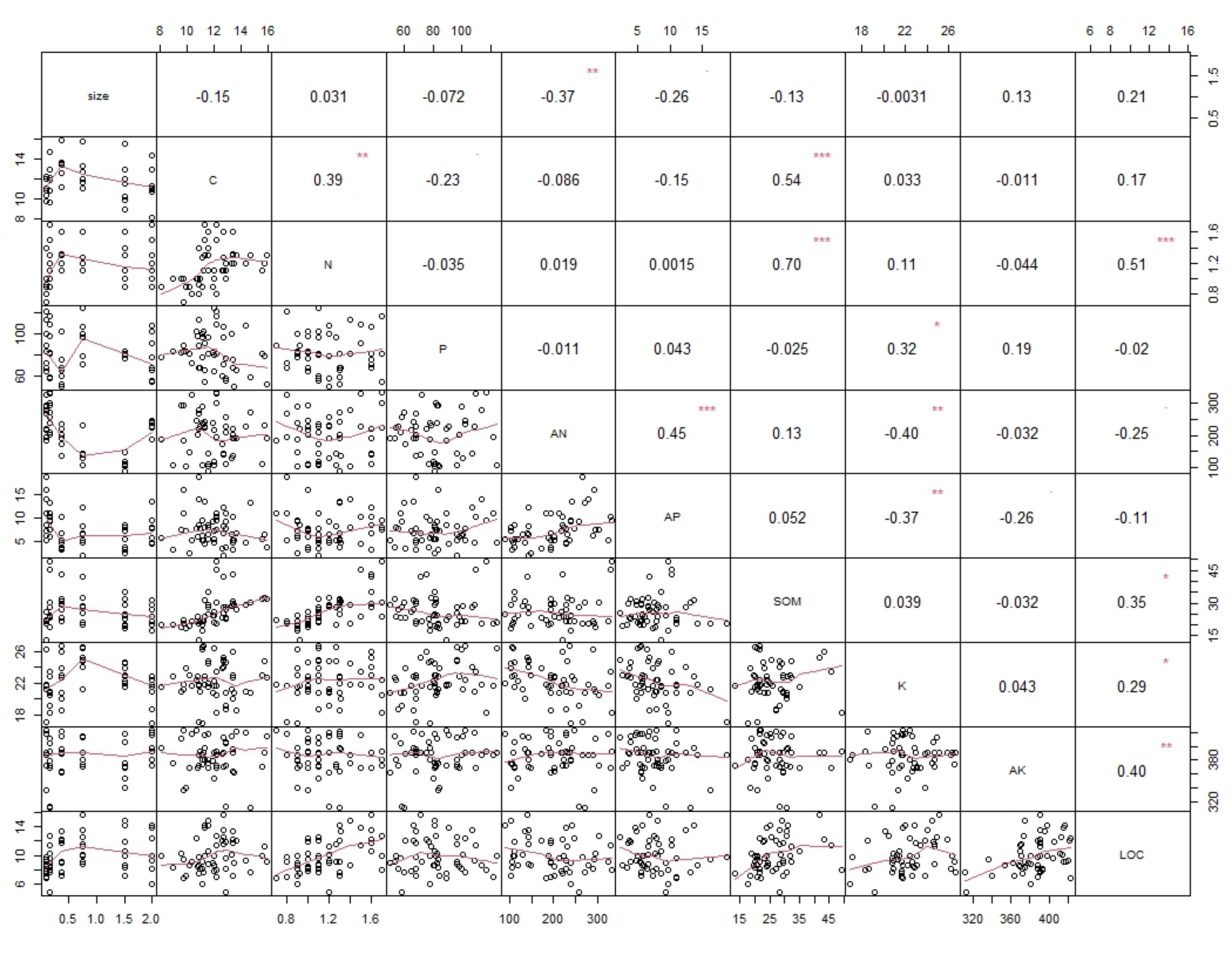
3.3. Correlation of Soil Aggregates’ Nutrients and Size
4. Discussion
4.1. Nutrient Availability in Soil Water-Stable Aggregates
4.2. Organo-Mineral Bonds for Nutrient Availability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.-H.; Xu, L.-L.; Tong, Z.-K.; Lin, E.-P.; Liu, Q.-P.; Cheng, L.-J.; Zhu, M.-Y. De novo characterization of the Chinese fir (Cunninghamia lanceolata) transcriptome and analysis of candidate genes involved in cellulose and lignin biosynthesis. BMC Genom. 2012, 13, 648. [Google Scholar] [CrossRef]
- Lin, E.; Zhuang, H.; Yu, J.; Liu, X.; Huang, H.; Zhu, M.; Tong, Z. Genome survey of Chinese fir (Cunninghamia lanceolata): Identification of genomic SSRs and demonstration of their utility in genetic diversity analysis. Sci. Rep. 2020, 10, 4698. [Google Scholar] [CrossRef]
- Lu, N.; Xu, X.; Wang, P.; Zhang, P.; Ji, B.; Wang, X. Succession in arbuscular mycorrhizal fungi can be attributed to a chronosequence of Cunninghamia lanceolata. Sci. Rep. 2019, 9, 18057. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, J.; Zheng, J.; Liu, J.; Liu, S.; Lin, W.; Wu, C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 6691. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Hayashi-Tsugane, M.; Kawaguchi, M. Spatial regulation of resource allocation in response to nutritional availability. J. Theor. Biol. 2020, 486, 110078. [Google Scholar] [CrossRef]
- Ju, W.; Jin, X.; Liu, L.; Shen, G.; Zhao, W.; Duan, C.; Fang, L. Rhizobacteria inoculation benefits nutrient availability for phytostabilization in copper contaminated soil: Drivers from bacterial community structures in rhizosphere. Appl. Soil Ecol. 2020, 150, 103450. [Google Scholar] [CrossRef]
- Thormann, M.N.; Bayley, S.E. Aboveground plant production and nutrient content of the vegetation in six peatlands in Alberta, Canada. Plant Ecol. 1997, 131, 1–16. [Google Scholar] [CrossRef]
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community- and global-scale comparisons. New Phytol. 2007, 176, 635–643. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Sharma, J.; Pal, R. Novel potassium solubilizing bio-formulation improves nutrient availability, fruit yield and quality of pomegranate (Punica granatum L.) in semi-arid ecosystem. Sci. Hortic. 2019, 255, 14–20. [Google Scholar] [CrossRef]
- Merino, A.; Jiménez, E.; Fernández, C.; Fontúrbel, M.T.; Campo, J.; Vega, J.A. Soil organic matter and phosphorus dynamics after low intensity prescribed burning in forests and shrubland. J. Environ. Manag. 2019, 234, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.; Alamgir, M.; Marschner, P. Impact of heating and rewetting on soil respiration and nutrient availability is enhanced by prior growth of plants. J. Soil Sci. Plant Nutr. 2020, 20, 925–932. [Google Scholar] [CrossRef]
- Warren, C.R. Response of osmolytes in soil to drying and rewetting. Soil Biol. Biochem. 2014, 70, 22–32. [Google Scholar] [CrossRef]
- Jha, P.; Garg, N.; Lakaria, B.L.; Biswas, A.; Rao, A.S. Soil and residue carbon mineralization as affected by soil aggregate size. Soil Tillage Res. 2012, 121, 57–62. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Change 2018, 8, 1104–1108. [Google Scholar] [CrossRef]
- Possinger, A.R.; Zachman, M.J.; Enders, A.; Levin, B.D.A.; Muller, D.A.; Kourkoutis, L.F.; Lehmann, J. Organo–organic and organo–mineral interfaces in soil at the nanometer scale. Nat. Commun. 2020, 11, 6103. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Qu, Z.; Jiang, R.; Wang, K.; Li, M. Soil organic carbon, aggregates, and fractions under different land uses in the loess plateau, China. Pol. J. Environ. Stud. 2019, 28, 1877–1885. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.; Zuo, Q.; Du, B.; Chen, W.; Wei, D.; Huang, Q. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of northeastern China. Sci. Total Environ. 2018, 635, 784–792. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Gao, J.; Zhang, X. Dynamics of soil aggregate-associated organic carbon along an afforestation chronosequence. Plant Soil 2015, 391, 237–251. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Sheng, M.; Han, X.; Zhang, Y.; Long, J.; Li, N. 31-year contrasting agricultural managements affect the distribution of organic carbon in aggregate-sized fractions of a Mollisol. Sci. Rep. 2020, 10, 9041. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, M.; Gale, W.J.; Zhang, X.; Li, L. Dynamics of aggregate-associated organic carbon following conversion of forest to cropland. Soil Biol. Biochem. 2013, 57, 876–883. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M. Qualitative and quantitative differences in particulate organic matter fractions in organic and conventional farming systems. Soil Biol. Biochem. 2006, 38, 1527–1536. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Peñuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Change Biol. 2020, 27, 454–466. [Google Scholar] [CrossRef]
- Oades, J.; Waters, A. Aggregate hierarchy in soils. Soil Res. 1991, 29, 815–828. [Google Scholar] [CrossRef]
- Edwards, A.P.; Bremner, J.M. Microaggregates in soils. J. Soil Sci. 1967, 18, 64–73. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Li, F.-B.; Liu, C.-S.; Huang, W.; Liu, T.-X.; Yu, W.-M. Chapter five-iron redox cycling coupled to transformation and immobilization of heavy metals: Implications for paddy rice safety in the red soil of south china. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 279–317. [Google Scholar]
- Duan, H.; Cao, S.; Zheng, H.; Hu, D.; Lin, J.; Cui, B.; Lin, H.; Hu, R.; Wu, B.; Sun, Y.; et al. Genetic characterization of Chinese fir from six provinces in southern China and construction of a core collection. Sci. Rep. 2017, 7, 13814. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, H.; Yu, M.; Cheng, X. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Cui, L.; Feng, W.; Wang, Y.; Zhang, J. Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems. Sci. Total Environ. 2019, 673, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Gai, X.; Zhong, Z.; Zhang, X.; Bian, F.; Yang, C. Effects of chicken farming on soil organic carbon fractions and fungal communities in a Lei bamboo (Phyllostachys praecox) forest in subtropical China. For. Ecol. Manag. 2021, 479, 118603. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons: Hoboken, NJ, USA, 1983; Volume 9, pp. 643–698. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Jackson, M. Soil Chemical Analysis; Prentice Hall, Inc.: Hoboken, NJ, USA, 1958. [Google Scholar]
- Jones, J.B. Soil testing in the united states. Commun. Soil Sci. Plant Anal. 1973, 4, 307–322. [Google Scholar] [CrossRef]
- Juo, A.S.R.; Kamprath, E.J. Copper Chloride as an Extractant for Estimating the Potentially Reactive Aluminum Pool in Acid Soils. Soil Sci. Soc. Am. J. 1979, 43, 35–38. [Google Scholar] [CrossRef]
- Reeuwijk, L.P. Procedure for soil analysis. Technical Paper 9. 1995. Available online: https://www.isric.org/documents/document-type/technical-paper-09-procedures-soil-analysis-6th-edition (accessed on 1 January 2002).
- Gallardo-Carrera, A.; Léonard, J.; Duval, Y.; Dürr, C. Effects of seedbed structure and water content at sowing on the development of soil surface crusting under rainfall. Soil Tillage Res. 2007, 95, 207–217. [Google Scholar] [CrossRef]
- Sun, L.; Feng, Y.; Dyck, M.F.; Puurveen, D.; Chang, S.X. Tillage reversal did not reverse N fertilization enhanced C storage in a Black Chernozem and a Gray Luvisol. Geoderma 2020, 370, 114355. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, X.; Lu, Q.; Li, J.; Chen, J. Changes in soil organic carbon and aggregate stability following a chronosequence of Liriodendron chinense plantations. J. For. Res. 2021a, 32, 355–362. [Google Scholar] [CrossRef]
- Algayer, B.; Le Bissonnais, Y.; Darboux, F. Short-term dynamics of soil aggregate stability in the field. Soil Sci. Soc. Am. J. 2014, 78, 1168–1176. [Google Scholar] [CrossRef]
- Elhaja, M.E.; Ibrahim, I.S.; Adam, H.E.; Csaplovics, E. Soil aggregate stability and wind erodible fraction in a semi-arid environment of white nile state, Sudan. In Proceedings of the SPIE—The International Society for Optical Engineering, Beijing, China, 8 November 2014; Volume 9260, p. 926017. [Google Scholar] [CrossRef]
- Polakowski, C.; Sochan, A.; Ryżak, M.; Beczek, M.; Mazur, R.; Majewska, K.; Turski, M.; Bieganowski, A. Measurement of soil dry aggregate size distribution using the laser diffraction method. Soil Tillage Res. 2021, 211, 105023. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Shangguan, Z.-P.; Deng, L. Variations in soil aggregate stability due to land use changes from agricultural land on the Loess Plateau, China. Catena 2021, 200, 105181. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of Soil Aggregate Stability on Soil Organic Carbon and Nitrogen under Land Use Change in an Erodible Region in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3809. [Google Scholar] [CrossRef]
- Liang, A.; McLaughlin, N.B.; Zhang, X.; Shen, Y.; Shi, X.; Fan, R. Short-term effects of tillage practices on soil aggregate fractions in a Chinese Mollisol. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 535–542. [Google Scholar] [CrossRef]
- He, Y.; Yang, M.; Huang, R.; Wang, Y.; Ali, W. Soil organic matter and clay zeta potential influence aggregation of a clayey red soil (Ultisol) under long-term fertilization. Sci. Rep. 2021, 11, 20498. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yan, X.; Zhou, H.; Zhang, Y.; Sun, H. Assessing the contributions of sesquioxides and soil organic matter to aggregation in an Ultisol under long-term fertilization. Soil Tillage Res. 2015, 146, 89–98. [Google Scholar] [CrossRef]
- Zhang, G.; Kou, X.; Zhang, X.; Bai, W.; Liang, W. Effect of row spacings on soil nematode communities and ecosystem multifunctionality at an aggregate scale. Sci. Rep. 2020, 10, 4779. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, W.; Ye, Y.; Zhao, J.; Wang, K. Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem. Sci. Rep. 2017, 7, 41402. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Dai, L.; Xie, L.; Zhao, S.; Liu, Y.; Zhang, Z. Hydrological connectivity improves soil nutrients and root architecture at the soil profile scale in a wetland ecosystem. Sci. Total Environ. 2021, 762, 143162. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Dey, D.; Mavi, M.S. Co-application of biochar with non-pyrolyzed organic material accelerates carbon accrual and nutrient availability in soil. Environ. Technol. Innov. 2021, 25, 102128. [Google Scholar] [CrossRef]
- Tavares, R.L.M.; de Oliveira, C.L.; de Assis, R.L.; Filho, S.V.D.P.; Ferreira, C.D.S.; Boldrin, P.F. Humic substances with mineral fertilization on nutrient availability in irrigated soil. J. Agric. Sci. 2021, 13, 104. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Zhai, L.; Zhang, B.; Peng, X.; Liu, X.; Zhang, J. Effects of Mineral-Solubilizing Microorganisms on Root Growth, Soil Nutrient Content, and Enzyme Activities in the Rhizosphere Soil of Robinia pseudoacacia. Forests 2021, 12, 60. [Google Scholar] [CrossRef]
- Liu, D.; Ju, W.; Jin, X.; Li, M.; Shen, G.; Duan, C.; Guo, L.; Liu, Y.; Zhao, W.; Fang, L. Associated soil aggregate nutrients and controlling factors on aggregate stability in semiarid grassland under different grazing prohibition timeframes. Sci. Total. Environ. 2021, 777, 146104. [Google Scholar] [CrossRef]
- Fan, R.; Du, J.; Liang, A.; Lou, J.; Li, J. Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry. Biol. Fertil. Soils 2020, 56, 1109–1120. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zheng, T.; Zhao, X.; Liu, H.; Zhang, Y. Nutrient and stoichiometric characteristics of aggregates in a sloping farmland area under different tillage practices. Sustainability 2021, 13, 890. [Google Scholar] [CrossRef]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiluweit, M.; Lawrence, C.R.; Berhe, A.A.; Blankinship, J.C.; Crow, S.E.; Druhan, J.L.; Pries, C.E.H.; et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter One—Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D.; Kinyangi, J.; Dathe, L.; Wirick, S.; Jacobsen, C. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 2008, 1, 238–242. [Google Scholar] [CrossRef]
- Vogel, C.; Mueller, C.W.; Höschen, C.; Buegger, F.; Heister, K.; Schulz, S.; Schloter, M.; Kögel-Knabner, I. Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat. Commun. 2014, 5, 2947. [Google Scholar] [CrossRef]
- Amelung, W.; Meyer, N.; Rodionov, A.; Knief, C.; Aehnelt, M.; Bauke, S.L.; Biesgen, D.; Dultz, S.; Guggenberger, G.; Jaber, M.; et al. Process sequence of soil aggregate formation disentangled through multi-isotope labelling. Geoderma 2022, 429, 116226. [Google Scholar] [CrossRef]
- Pihlap, E.; Steffens, M.; Kögel-Knabner, I. Initial soil aggregate formation and stabilisation in soils developed from calcareous loess. Geoderma 2021, 385, 114854. [Google Scholar] [CrossRef]




| No | Coordinate | Elevation/m | Mean Height/m | Mean DBH/cm | Density (*/ha) | Basal Area (m2/ha) |
|---|---|---|---|---|---|---|
| 1 | 27°14′20.81″ N, 115°34′23.68″ E | 118 | 7.45 | 9.90 | 3175 | 25.67 |
| 2 | 27°14′21.51″ N, 115°34′22.57″ E | 116 | 6.53 | 9.27 | 3475 | 24.56 |
| 3 | 27°14′21.94″ N, 115°34′21.94″ E | 111 | 5.68 | 9.17 | 2575 | 17.83 |
| 4 | 27°14′22.44″ N, 115°34′21.57″ E | 111 | 7.10 | 9.29 | 3125 | 22.03 |
| 5 | 27°14′22.98″ N, 115°34′20.95″ E | 112 | 7.16 | 10.43 | 2525 | 22.61 |
| 6 | 27°14′23.50″ N, 115°34′20.53″ E | 112 | 6.63 | 9.26 | 3025 | 21.13 |
| 7 | 27°14′25.30″ N, 115°34′18.84″ E | 113 | 5.67 | 8.77 | 2700 | 17.30 |
| 8 | 27°14′25.57″ N, 115°34′18.24″ E | 111 | 6.73 | 9.65 | 2500 | 19.22 |
| 9 | 27°14′33.28″ N, 115°34′17.72″ E | 113 | 6.08 | 9.71 | 2175 | 16.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Xiang, J.; Ji, X.; Jiang, J. Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China. Forests 2023, 14, 975. https://doi.org/10.3390/f14050975
Feng M, Xiang J, Ji X, Jiang J. Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China. Forests. 2023; 14(5):975. https://doi.org/10.3390/f14050975
Chicago/Turabian StyleFeng, Ming, Jian Xiang, Xiaofang Ji, and Jiang Jiang. 2023. "Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China" Forests 14, no. 5: 975. https://doi.org/10.3390/f14050975
APA StyleFeng, M., Xiang, J., Ji, X., & Jiang, J. (2023). Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China. Forests, 14(5), 975. https://doi.org/10.3390/f14050975






