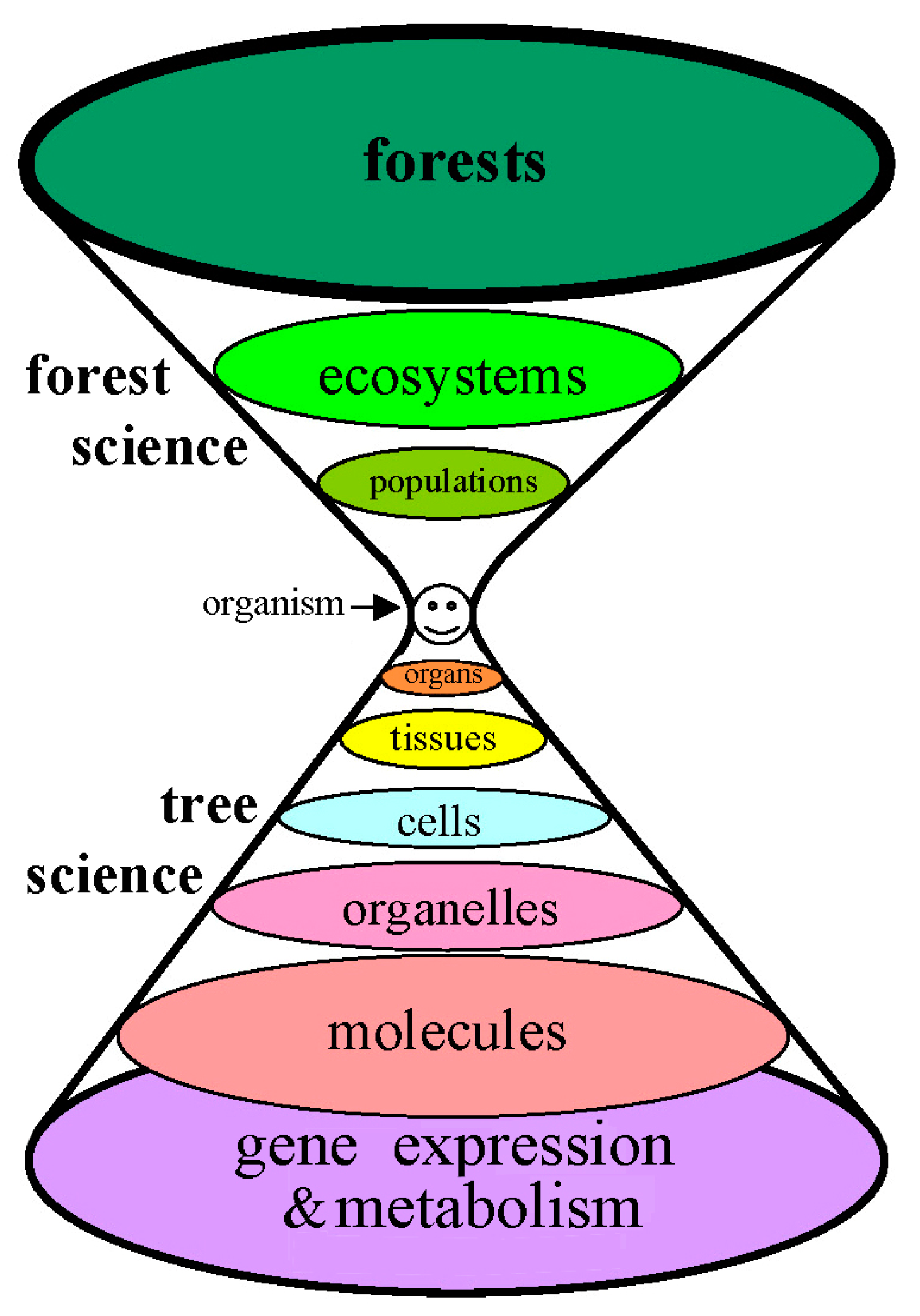
The defining feature of each forest is the organism that humanity generically designates as ‘tree’. More than 70,000 tree species are known [
1], each having a genome for the formation of similar but, nevertheless, distinguishable organs. As indicated by the changing areas of the ellipses in
Figure 1, the number of physiological considerations increases from organism to forest, and the discernible complexity increases from organism to genome. Numerous levels of investigation are needed.
The primary focus of this Special Issue of Forests is on secondary growth, emphasizing intrinsic regulation of cell division, expansion, and differentiation. Additionally considered are abiotic and biotic tolerances and survival fitness in relation to meristems (vascular cambium and phellogen), their daughter cells and finer considerations, and those of cytosol and cellular organelles within cells within the tissues that contribute to secondary growth. Papers in this Special Issue address several conifer and hardwood species, each presenting examples for ongoing investigation [
2,
3,
4,
5,
6,
7,
8,
9]. All consider the regulation of gišogenesis (secondary-xylem development), and several also address phloegenesis (phloem formation) and its vital role in enabling secondary growth and overall survival of both conifers and eudicots. One paper [
3] addresses explicitly, and others [
2,
8,
9] indirectly acknowledge, the ongoing need to understand the role of phytohormones and their specific regulatory effects. Additional aspects of intrinsic regulation are addressed in terms of photosynthate allocation [
2], biophysical phenomena [
3,
4,
8], phenology [
9], and the most vital element of life, i.e., water [
3,
4,
8].
Any attempt to relate molecular or cell biology data to the formation of multi-cellular tissues, such as wood and bark, might be described as a ‘quantum leap.’ Another leap is needed to place such data into the context of whole-tree growth and development, and yet another leap is required to make them relevant to forest communities (
Figure 1). Moreover, as is apparent in the depth and breadth of topics addressed in this Special Issue, an immense research effort is needed to explain all the ways that secondary growth can occur and how it contributes to overall tree survival. As knowledge increases, the biological complexity at the level of molecules and genes keeps growing. Nevertheless, each research contribution is a step forward.
The anatomical, physical, and chemical properties of wood and bark vary not only between species, but also within individual trees. That diversity enhances their importance to the biosphere and challenges industries concerned with utilization of wood and bark. Knowledge remains inadequate to explain how internal variation arises, and it is even more challenging to produce explanations for infra- and inter-specific variation. For example, wood hardness spans two orders of magnitude among tree species, ranging from >22,000 N for lignum vitae (
Guaiacum officinale) to <400 N for balsa (
Ochroma pyramidale) and quipo (
Cavanillesia platanifolia) woods [
10]. Although hardness and wood density are directly correlated, at the subcellular formative level, neither the physical nor the chemical basis, and much less the biological basis, for such a range is yet well understood in terms of cell wall structure, biochemistry, and molecular bonding.
The most basic principle of tree science is embodied in the expression P = G × E, where P is the phenotype, G is the genome (in particular its expressible genes encoding metabolic processes and regulators of gene expression), and E is the intrinsic environment that exerts a direct effect on gene expression and resulting metabolism.
Phenotypic levels span a range of dimensions from whole trees to individual cells, and within cells from organelles to the 340-nanometre length of a gene comprising 1000 base pairs to the sub-nanometre sizes of organic stereoisomers, such as glucose and galactose. All these factors, including the living and dead elements in wood and bark, are phenotypes that arise through gene expression in response to internal environmental signals.
G and E interact to produce the phenotype, but both are complex variables. Thus, research to explain G × E interactions and their individual contributions requires, ideally, that G or E be held constant while investigating effects of manipulation of the other. Somatic G can, in principle, be controlled and limited to particular genotypes, for example, by use of propagated clones or cell cultures, and the extrinsic environment of seedlings or cell cultures can be somewhat controlled within environmental chambers.
However, setting extrinsic parameters, such as temperature, humidity, light, and air compositional levels within a controlled-environment chamber, does not permit the assumption that intrinsic E of a tree or cell culture is the same as or even similar to that within the chamber. In fact, it is well established that intrinsic E varies from one organ to another, from one tissue to another, within the same extrinsic environment. That reality, attributable to variable transduction and transmission, makes tree science research especially challenging. In a forest, radiant energy, water, air, soil nutrients, atmospheric pressure, and gravity are some of the extrinsic phenomena utilized, or reacted to, in variable amounts as tree cells work to modulate their intrinsic environments, thereby influencing regulatory aspects of growth, development, functioning, and survival.
Many forest science investigations have provided data on changes in tree growth, such as height and diameter, and provided correlative data on extrinsic environmental factors and/or on overt organ numbers and sizes. However, in the final analysis, conclusions drawn from such correlations are inescapably attended by major uncertainty about internal control and survival mechanisms. For example, trees having similar crown sizes, and, when growing side by side in the same extrinsic environment, may nevertheless perform quite differently. In recognition of this, selection programs aimed at improving commercial productivity have, for more than a century, focused on exploiting the intrinsic nature of more desirable genotypes, but, sadly, this has resulted in minimal support to explain the basis for it.
In other words, if humanity is ever to understand how a forest actually materializes, functions, and survives, it is necessary to know the invisible biochemical and biophysical processes within the tree and how they change hourly, diurnally, seasonally, and from juvenility to maturity to old age. Regarding the present state of knowledge, no matter which tree species is considered, a satisfactory explanation for overall secondary growth and its regulation is lacking. Moreover, it is nowhere written in stone that the identical regulatory mechanisms must be operative across the diversity of tree species.
All of tree science is beneath the overarching roof of physiology, supported by many sub-disciplines, all founded on physics and chemistry (
Figure 2). The overall aim is to integrate research inferences into a holistic perspective of growth, development, and survival for intelligent management of our terrestrial biosphere.
Acknowledging the foregoing complexity, some attempts at generalization and some scrutiny of the current research emphasis are presented in the following comments.
Metabolism, both primary and secondary, catabolic and anabolic, is facilitated by transcription and translation to produce RNA and proteins, some of which catalyze biochemical transformations, while others fulfil various biophysical roles, and still others accumulate as reserves awaiting utilization. Intrinsic E is generated and sustained through metabolism, yet intrinsic E supported by ongoing gene expression also regulates both the rate and the nature of metabolism (
Figure 3). It is a two-way interactive network and a delicate balancing act between homeostasis and overbalanced cascading metabolic changes operating throughout every living cell and permeating all living tissues within a tree.
Water, once inside a tree, becomes the most vital intrinsic regulator. Adequate water is necessary for ion exchange, endocytosis, exocytosis, generation of osmotic turgor pressure, buffering, dissolution, hydrolysis, cyclosis, protein folding, gene expression, transpiration, evaporative cooling, translocation, cell expansion, cell wall elasticity, mitosis, cell plate formation, cytoskeletal dynamics, plastid and mitochondrial divisions, and many additional processes. Whenever water is internally limiting, those processes and the resulting phenotypic properties—all of which are essential for sustaining the living state—become compromised, such that growth and development slow or halt.
Given sufficient water, in general, the rate of metabolism is a function of intrinsic heat content. However, both the rate and the nature of metabolism vary depending on the chemical transformation and the catalyst under consideration. For example, starch hydrolysis during the cooler nighttime increases osmotica, hence encouraging uptake of soil water to recharge the storage water reservoir in the trunk in support of daytime photosynthesis and transpiration. The reverse process of starch biosynthesis and accumulation of starch granules occurs in association with photosynthesis and evapotranspirative water loss during daylight hours. Many distinct enzymes are involved in this slow diurnal ‘heartbeat’. It is facilitated not merely through variable gene expression, but also because some enzymes have three-dimensional structures and catalytic capability enabling them to function more efficiently at lower temperatures, and some function better at higher temperatures [
11,
12].
Internal thermogenesis is an important consideration, at least in some plant species [
13], but, with the present knowledge, radiant heat from the sun and absorbed heat in air and in soil water are the primary sources contributing to the enthalpy within trees. However, intrinsic heat absorption, retention, and transfer by macromolecules of cell walls and cellular constituents are neglected research realms within tree science research, although they are undoubtedly thermodynamic considerations relevant to growth, development, and survival. The specific heat capacities of wood and bark tissues are known to vary, and it can be predicted that heat capacities of the several types of lignin, also of cellulose and hemicelluloses, of cell walls, will also be found to vary. Internal temperature data are needed to understand intrinsic heat accumulation, retention, and transfer into metabolically active cells.
Given the needed aqueous and thermal environments and competence for cellular metabolism within heterotrophic stem and root tissues, superimposed on this dynamic is internal perturbation of intrinsic E from a distance. Perturbation occurs through production, export, and movement of molecules and physical signals within and through tree tissues and cells, eliciting reactions that affect secondary growth. In addition to photosynthates and nutriments exported from leaves and roots, endogenous transmissibles include phytohormones, microRNAs, small RNAs, peptides, enzyme cofactors, and other regulators. Effects of these ‘endogenous regulators’ have been discovered using plant or cell culture bioassays in laboratories under well defined conditions. Many endogenous regulators have been discovered, and new ones will almost certainly be found.
The formation of wavy, spiraled, and interlocked grains during gišogenesis is one of the more obvious manifestations of both long-distance and cell-to-cell signal transmission (
Figure 4).
During natural spiral–grain formation, microdomains consisting of adjoining fusiform cells change their orientations as the vanguard, followed by reorientation of the general cellular population [
14]. There evidently are preferred flow channels, though only of quite narrow width, through the population of cells within the cambial zone or its sandwiching tissues. Mechanistically, orientation changes of fusiform cells within the vascular cambium are preceded and facilitated by changes in both anticlinal and periclinal cell division planes and by constraints on the direction of intrusive tip elongation growth [
14]. A change in the directional transmission of a regulator is a hypothetical, though somewhat nebulous, explanation for changes in the plane of cytokinesis and preferred direction of tip elongation. However, the existence of support for that hypothesis leaves unanswered how and why the transmission direction should acquire different angles of flow at different locations within tree trunks and branches during the same growing season or, in the case of interlocked grain, how reorientation reversals to the opposing flow direction are triggered during successive periods of gišogenesis. Not until these dynamically changing fundamental polarity phenomena are explainable at the level of biophysics, biochemistry, and gene expression can there be any confidence that tree science has begun to achieve an adequate level of understanding of secondary growth.
In principle, transmission pathways include the transpiration and translocatory streams, as well as specialized longitudinal and radial structures, such as resin canals, and the ray cell radial transport system that interfaces with the several longitudinal transport systems. Cell-to-cell transmission occurs through cambia and other living cells, and it evidently can occur in any direction. Apoplastic movement via diffusion or active transport through aqueous fluids within cell walls and external to them is also possible.
In addition to transmissible molecules and ions, there are regulatory proteins and gene sequences within cells and between adjoining cell walls. They evidently are not cell-to-cell transmissible either across plasma membranes or through plasmodesmata channels. It remains uncertain if these non-transmissibles are needed to initiate production of transmissibles or vice versa, or if it may be even more complex. For example, do growing leaves biosynthesize and export the phytohormone auxin to the vascular cambium because they are genetically determined by non-transmissibles to do so, or is the underlying cause for production or export of auxin a signal conveyed to the leaves by another endogenous regulator, or is it in response to some extrinsic factor impinging on the leaves? There are countless questions of this nature remaining to be answered.
Tree scientists recognize that physical transmission may be equally or more important than that of molecular transmission, but progress in the transduction field awaits the talents of adequately supported biophysicists. In addition to photosynthetic light harvesting and evapotranspiration, physical processes influencing development within trees are at present mainly understood in terms of photomorphogenetic, photoperiodic, and gravitational responses. Some other physical phenomena that undoubtedly also influence secondary growth include heat absorption and retention, light piping through stems, proton pumping to create pH differences between adjoining tissues (e.g., phloem slightly alkaline, developing xylem slightly acidic), tensile stretching of capillary water, wind-sway effects on piezoelectric charging in cell walls, and responses within stems to changing telluric currents and voltage potentials. The contributions made to secondary growth by those phenomena remain within a vast realm of obscurity awaiting frontier discovery research. Detection of transduction into stems of physical phenomena requires use of appropriately sensitive equipment, and achieving the needed micro-resolution and accounting for invasive wound effects are complicated considerations.
Based on the foregoing, it is reasonable to assume that, in the natural wild or even managed forests, intrinsic regulation involves more than just the endogenous transmissibles that laboratory physiologists refer to as regulatory or signalling molecules, or as phytohormones. For example, if cells of vascular cambium lack adequate water because of drought, or have an internal temperature too low, their metabolism is likely to be compromised, regardless of what phytohormone or other endogenous regulator may be present as a promoter of cambial growth. In this light, seasonally and diurnally variable substances assimilated or transduced into trees from the extrinsic environment constitute ‘assimilated regulators’, which are no less important than endogenous ones.
Research to elucidate the roles of the genome in plant growth and development so far has focused on only a few vascular plants, notably Populus spp. and Arabidopsis thaliana. Populus research has favored potted trees in their first year of development. Arabidopsis is an annual, and its stems do not accumulate layers (‘annual rings’) of secondary growth. Nevertheless, those eudicots are portrayed as ‘models’ relevant to all physiological aspects of all extant vascular plants. Plausibly, common genetic factors do underlie xylogenesis in all species, e.g., creation of bordered-pit and or perforation-plate water-flow channels between adjoining cells and lignification of both primary and secondary walls, as relatively permanent genetic innovations throughout evolution. In this vein, tracheophyte evolution diversified morphologically and histologically, and the diversity of extant woody plants manifests variations on a central theme, which originated about 400 million years ago and then persisted. Within such reasoning, it is tempting to suggest that competency for vascular cambium formation and diameter growth also is monophyletic, perpetuated through atomistic inheritance. It deserves emphasis, however, that these concepts of gene conservation remain hypothetical. Plausibly, more than one genetic program for xylogenesis, cambium formation, or others facets of secondary growth arose during plant evolution.
Extrapolation of genomic and other physiological interpretations from annuals and juvenile tree species to long-lived organisms presently contains the implicit assumption that epigenetic changes do not occur during aging. This assumption finds support in the concept of recurrent embryogenesis, whereby the apical meristems of perennials annually produce youthful tissues of primary growth [
15]. On the other hand, it remains uncertain if annuals or young perennials can provide comprehensive insight into physiological processes of secondary growth, which function throughout the longevity of trees.
For example, within woody perennials, there is a ‘corewood’ produced in association with the early years of the vascular cambium’s productivity [
16,
17]. Thereafter, corewood abruptly changes into a distinguishable ‘outerwood’ (
Figure 5).
“Juvenile wood” has been extensively investigated, and some investigators have considered juvenile wood and corewood to be synonymous terms (e.g., [
16]). On the other hand, the transition from juvenile to mature wood during secondary growth has frequently been described as a gradual, rather than well delineated, change in various wood properties, making a transition point difficult to identify clearly [
16,
18,
19,
20]. In contrast, corewood terminates abruptly at a latewood-earlywood boundary and physically is clearly delineated from the surrounding outerwood, particularly after wood has dried. The clean separation that arises between corewood and outerwood after wood drying (
Figure 5C,D) presumably is due to chemical differences in molecular bond strengths, which originate precisely at that interface. It follows that the ambiguity within the earlier literature should be eliminated and, thus, corewood and juvenile wood are not equated here.
Corewood within branches appears to be identical and continuous with that of the main-trunk axis, as is apparent in
Figure 5C. In most species, corewood is darker than the surrounding outerwood. The contrasting colours are enhanced by drying and air/light oxidation of the wood (
Figure 5C–E), or by treating the wood with H
2O
2 solution, pointing to differences in wood chemistry (e.g., see [
21]). Pigmentation of corewood may involve the same biochemistry that contributes to heartwood formation, but heartwood continues to propagate radially outward during the life of a tree, whereas corewood terminates abruptly and becomes surrounded by the outerwood (
Figure 5C–E).
When trunk wood is sawn into lumber, branch corewood appears as the central darker region of knots surrounded by annual layers of less-dark branch outerwood (
Figure 6). Again, chemical and biochemical differences are the probable explanation for the colour differences. In trunk wood of tree species where corewood is little or not at all evident, well delineated visible knots in sawn lumber nevertheless indicate that corewood was formed.
Needed research remains to be performed, but it seems probable that the ratio of the diameter of corewood to total trunk or branch diameter varies among trees, even within the same species. Longitudinally split trunk segments generally display corewood diameters of a few centimetres, the largest seen by the writer being 6 cm in diameter (in Acer rubrum). The number of annual xylem layers occupied by corewood evidently is also a variable. In other words, although the abrupt change from corewood to outerwood has an attending physical boundary that can be clearly distinguished visually, the existence of the boundary separation between the two does not appear to be explainable in terms of either physical stresses arising during girth increase or the precise number of years of juvenile growth.
Corewood in young stems when examined by microscopy is sound and shows no evidence for microbial presence. However, as a trunk-height position increases in years, corewood, not uncommonly, becomes subject to heart rot, indicating that corewood is more susceptible to microbial decay than its surrounding outerwood. Thus, it may be that some earlier researchers interpreted the corewood–outerwood boundary as the radial position of decay compartmentalization.
The existence of a physically abrupt corewood–outerwood transition challenges the assumption that research into the vascular cambium of young stems is sufficient to provide comprehensive information on how gišogenesis occurs and is regulated throughout the life of a long-lived tree. The properties of outerwood have been more appreciated than those of juvenile wood by commerce. On the other hand, the pigmentation of heartwood has generally attracted greater economic interest. It can be suggested, therefore, that there is a strong commercial case to be made for discovery of how both corewood and outerwood are produced. In other words, research into the intrinsic regulation of secondary growth in both young trees and those beyond the sapling stage is needed.
The corewood-outerwood transition may involve epigenetic change within fusiform or ray cells of the cambial zone, or within their derivatives. Plausibly, the abruptness of the change results from a metabolic switch activated by changing chemistry of one or more transmissible regulators reaching vascular cambium from either the foliage or the root system, or both, with the signal fading as the transmission distance increases. Another possibility is that the transition is initiated by a change in metabolism or gene expression within living ray parenchyma of secondary xylem. The role of living parenchyma in regulation of secondary growth remains very poorly understood.
A recent investigation [
22] provides an example of a research approach that could be used to discover physiological changes during the transformation from corewood to outerwood production. However, because the corewood–outerwood boundary in freshly cut trunks can be difficult to detect, to ensure that outerwood is being investigated, it would be advisable to look in lower trunks of trees more than several decades old. Both corewood and outerwood are obviously important for tree survival.
Most everyone appreciates that forests are vitally important for survival of both humanity and the entire biosphere. Nevertheless, in addition to losses due to harvesting, fire and deliberate deforestation, inexplicable forest mortality events are becoming increasingly common, evidently in response to the many survival challenges posed by climate change [
23]. Acknowledging how little is yet truly understood about the physiology of any tree species, it seems obvious that all nations should be concerned and strongly supporting tree science research. There is a global responsibility to sustain the terrestrial biosphere.
It is instructive to recall some comments made long ago, following repetitive observations on diameter growth over several years in a variety of tree species growing on the same site [
24]:
“Why should growth be distributed over the months so variously in different species, some effecting the largest part of their increase in a single month, while in others it is pretty equally distributed over two, three, or even four months? Why should the maximum growth in different species occur at such various periods as June, July, and August? Why is it that some species complete by far the greater part of their growth by the end of June, before the real heat of summer has begun?” Those questions, and countless others about how secondary growth in woody plants occurs and is regulated, remain unanswered after a century of tree science research. Phenological variation can be attributed to physiological variation, but the basis for it in terms of gene expression and metabolism remains unknown.
To summarize, within the small number of tree scientists who have been provided their meagre support to research secondary growth in woody plants, the constraints have been conducive to an inductive wishfulness to explain the formation of bark-clad stems of wood in simple terms. However, as knowledge has increased, it has become all too apparent that diameter growth in woody plants involves innumerable phenomena of cell biology, biochemistry, biophysics, and gene expression, far more complex and variable than can presently be explained. In other words, tree science knowledge remains rudimentary. It is difficult research requiring excellent infrastructure and strong financial support for progress.
The day is long past when the world, as a show of appreciation for the immense commercial benefits obtained from forests, should have begun strongly supporting tree science research. However, to provide all the many research capabilities and enable researchers to come together in the needed collaborative efforts, support for an international ‘big science’ research facility is clearly needed. Considering the number of species and the phenomenological magnitude each embodies, the facility should be at least comparable to that provided the various space agencies focused on space exploration. For most earthlings, it is entirely obvious that the top scientific priority should be to sustain our terrestrial biosphere, as well as that the future of Earth, including humanity, depends on our understanding of how trees grow, develop, and survive.