Abstract
Stumps are a significant component of coarse woody debris in plantations, but their effect on microsite soil organic carbon (C) and enzyme activities remains understudied. Soil (Alfisol) samples were collected at varying distances from larch (Larix olgensis Henry) stumps and at different soil depths (0–20 cm and 20–40 cm) to analyze soil total organic C (TOC), particulate organic C (POC), easily oxidizable C (EOC), microbial biomass C (MBC), and enzyme activities. Results indicated that stumps significantly affected TOC and POC contents, with the greatest horizontal range of impact reaching up to 15 cm in both the topsoil and subsoil layers. Stumps also significantly affected MBC content, with the greatest horizontal range of impact reaching up to 55 cm in the subsoil layer. EOC content was the most affected, with the stumps’ impact extending to 55 cm in both soil layers. Additionally, the study showed that stumps had a significant impact on the activities of β-glucosidase and β-cellobiohydrolase, with the greatest horizontal range of impact reaching up to 15 cm for glucosidase and 35 cm for cellobiohydrolase in the topsoil layer. Stumps also significantly affected the activities of phenol oxidase and peroxidase, with the maximum horizontal range of stump impact extending up to 35 cm for phenol oxidase and 55 cm for peroxidase in the topsoil layer. This study enhances our understanding of the role of stumps in plantation ecosystems and offers valuable insights for future management strategies to maintain soil fertility and improve site productivity.
1. Introduction
In forest ecosystems, coarse woody debris (CWD) is a vital component that serves important functions. CWD not only provides habitat for forest biodiversity but also acts as a significant nutrient reservoir, contributing greatly to maintaining the integrity and stability of forest ecosystems [1,2,3]. In intensively managed forest ecosystems, stumps are often left behind at sites after thinning or clear-cutting. As an essential component of CWD, stumps play a critical role in the material cycle, energy flow, and soil processes within forest ecosystems. Moreover, stumps are a major source of soil organic matter and nutrients, playing a crucial role in maintaining soil fertility. Stumps typically represent 10%–25% of the total biomass in live trees and store 15%–20% of essential nutrients such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) [4]. Therefore, it can be hypothesized that the decomposition of stumps from intensively managed forest ecosystems has a positive impact on nutrient cycling and soil processes, which in turn affect the biodiversity and stability of forest ecosystems.
During the decomposition process of stumps, part of the C is released into the atmosphere through respiration, while the remaining C is stored in the soil through transformation, fragmentation, and leaching [5]. Consequently, stumps serve as one of the important sources of soil C storage and play a vital role in maintaining soil fertility. Current research on the impact of stumps on soil nutrients mainly focuses on exploring their effects on soil nitrogen and phosphorus [6,7], while studies on the impact of stumps on soil organic C and its active fractions are relatively scarce [8,9].
Soil organic C is an important indicator of soil quality, and its active fractions play a crucial role in assessing changes in regional soil quality sensitivity [10]. From a physical, chemical, and biological perspective, soil active organic C can be classified into particulate organic C (POC), easily oxidizable organic C (EOC), and microbial biomass C (MBC) [11,12]. Previous studies have shown that the closer the stumps are, the higher the soil’s total organic C (TOC) content [13]. However, it is currently unclear how stumps affect the changes in the active organic C fractions that have a fast turnover rate and is easily mineralized and decomposed [1,6]. Therefore, it is urgent to obtain this information to better understand the impact of stumps on soil and manage soil fertility and site productivity more effectively [14].
Soil organic C turnover is a typical microbial-driven process [15]. As the most active microbial component in soil, the enzymatic activity of soils plays a crucial role in the decomposition and transformation of active organic carbon. This process causes the most labile and mineralizable active carbon fractions to respond rapidly [16]. Therefore, soil enzyme activity not only reflects the intensity and direction of various biochemical reactions in soil but also is an important factor in causing changes in the active organic C fractions. Different types of soil carbon are decomposed by different extracellular enzymes. For example, cellulase is a type of soil hydrolytic enzyme that mainly decomposes monosaccharides, starch, cellulose, and hemicellulose. On the other hand, soil oxidases, such as peroxidase, mainly decompose recalcitrant materials such as lignin [17,18]. A comprehensive analysis of soil active organic C fractions and soil enzyme activity is helpful to understand the impact of the stump on microsite soil and provides valuable data on soil quality and ecosystem changes.
Larch (Larix olgensis Henry) plantations are the most long-standing and extensive type of plantation ecosystem in the temperate region of Northeast China [19]. Due to the high initial planting density, a large number of stumps are formed in the forest during management and utilization. In forest ecosystems, microorganisms play a critical role in the transformation of stumps and litter [20]. They break down complex organic compounds in dead plant material into simpler compounds that can be absorbed by plants, contributing to nutrient cycling and soil processes within the ecosystem [21,22]. Compare with other species, larch wood has a higher concentration of lignin and cellulose [23]. So, in the case of larch stumps, the decomposition process can take much longer. However, microorganisms still play a critical role in the process. The decomposition of stumps by microorganisms releases the stored nutrients back into the soil, contributing to nutrient cycling and soil processes within the ecosystem.
In this study, the soil TOC, POC, EOC, MBC, and the activities of hydrolytic enzymes and oxidase enzymes at different distances from larch stumps were determined and analyzed in the larch plantation. The objectives were (1) to explore whether stumps affect the TOC, active organic C fractions, and enzyme activities of the microsite soil; (2) to investigate the relationship between the change of soil C and enzyme activity in the context of the presence of stumps. The study can help inform the selection of microsites and C management strategies during the cultivation of plantations and will provide valuable information on the impact of stumps on microsite soil quality and ecosystem changes in plantation ecosystems.
2. Materials and Methods
2.1. Characteristics of the Study Site
The study area was located in Mengjiagang National Forest Farm, Jiamusi City, Heilongjiang Province in Northeastern China (Figure 1). The forest farm is situated on the western foothills of the Wandashan Mountains, with a continental monsoon climate that has dry and mild springs, short, warm, and wet summers, windy but dry falls, and cold and dry winters. The mean annual air temperature is 2.7 °C. The extreme maximum temperature in the study region is 35.6 °C, and the lowest temperature is −34.7 °C. The annual ≥10 °C accumulation temperature is about 2547 °C. The annual sunshine hours are 1955 h, and the frost-free period lasts approximately 120 d. The annual average precipitation is 535 mm, with most of it concentrated from June to September, accounting for 72.6% of the annual precipitation. The soil is an Alfisol [24]. The plantation primarily consists of Larix olgensis Henry, with additional species such as Fraxinus mandshurica Rupr., Betula davurica Pall., and Pinus sylvestris L. present in the area. The predominant ground cover plants include Carex tristachya Thunb., Sanguisorbae radix L., Equisetum arvense L., Corylus heterophylla Fisch., Lespedeza bicolor Turcz., Acanthopanax senticosus Rupr., Schisandra chinensis Turcz., and Aster scaber Thunb. The area also features smaller quantities of Vitis amurensis Rupr. and Aralia elata Seem.
Figure 1.
Location of the study area. The tree species classification sketch map of Mengjiagang Forest Farm is based on Jia et al. [25]. The  represents the study area’s location in China, while the
represents the study area’s location in China, while the  represents the sampling location in the Forest Farm for this study.
represents the sampling location in the Forest Farm for this study.
2.2. Soil Sampling
In October 2019, three decayed larch stumps with similar decay conditions were selected in the larch plantation in Mengjiagang Forest Farm (soil properties of larch plantation see Table 1). The decay status of the selected stumps was consistent. The criteria for selecting stumps were that the bark of it had fallen off, the wood was soft, and some could be crumpled into pieces (Figure 2). Based on the research on the classification of stumps’ decayed degree by Hunter [26] and Petrillo et al. [27], the decay degree of stumps could be divided into five classes. The higher the classes are, the higher the degree of decay. The larch stumps selected in this study could be defined as decay class IV. The stump diameter was within 20 ± 5 cm to reduce the error caused by the diameter.

Table 1.
The soil properties of larch plantation.

Figure 2.
The picture of soil sampling in larch stump.
Considering factors such as a planting spacing of 150 cm × 150 cm in the plantation and the larch’s superficial root systems [28], soil (Alfisol) samples were gathered from depths of 0–20 cm and 20–40 cm, which was describe as topsoil layer and subsoil layer, respectively. Soil sampling points of D1 to D5 were uniformly arranged horizontally on one side of the stump’s range (with the east as the consistent direction). Each sample point was evenly distributed within a 5–95 cm distance from the stump. Four soil sampling points were collected from one side of the stumps at distances of 5–15 cm, 25–35 cm, 45–55 cm, and 65–75 cm in each soil layer, labeled as D1, D2, D3, and D4, respectively. The soil located over half of the plant spacing (85–95 cm) from the stumps was less influenced by the stumps and other trees. Thus, the soil sampling point at a horizontal distance of 85–95 cm in each soil layer served as the control and was designated as D5 (Figure 3). In this study, three larch stumps were selected, and a total of 30 soil samples were collected.

Figure 3.
The depicts soil sampling design for investigating stumps. This study collected samples at two depths, 0–20 cm and 20–40 cm, and at five horizontal distances from each stump, ranging from 5–95 cm. H denotes the depth of the soil layer. D1, D2, D3, D4, and D5 denote sampling points within horizontal distances of 5–15 cm, 25–35 cm, 45–55 cm, 65–75 cm, and 85–95 cm from the stump, respectively.
Moist soil samples were transported to the laboratory in a cooler. After removing plant roots, stones, and debris, the soil was sieved through a 2 mm sieve and divided into two parts. One portion was stored after the initial collection at −80 °C and subsequently stored at 4 °C until soil enzyme activity and microbial organic C analysis. While the other portion was air-dried and kept at room temperature for soil chemical property analysis.
2.3. Laboratory Analysis
Soil total organic C content was determined by the combustion method with an elemental analyzer (Elemental Combustion System 4024, Bussero, Italy).
Separation of soil particulate organic C was measured by following the procedures described by Cambardella & Elliot [29] of the physical dispersion method. 20 g of air-dried soil passing a 2 mm sieve was dispersed in 100 mL of sodium hexametaphosphate (5 g/L) with shaking on a reciprocating shaker (100 rpm/min) for 18 h. The soil suspension was passed through a 0.053 mm sieve using a flow of distilled water to ensure particle separation. All materials remaining on the sieve were washed into a dry dish and then dried at 60 °C for 48 h, weighed, and ground to measure the C content by the elemental analyzer.
Soil easily oxidizable C content was estimated by reaction with dilute permanganate solution described by Weil et al. [30] and Blair et al. [31]. Soil samples containing approximately 15 mg C were oxidized with 25 mL 333 mM KMnO4 after shaking for 1 h. After shaking, the tubes were centrifuged for 5 min. Then the supernatant was diluted 1:500 with deionized water. The absorbance of diluted supernatants and standards were read at 565 nm. The change of KMnO4 concentration was used to estimate the amount of C oxidized, assuming that 1 mM KMnO4 is consumed in the oxidation of 0.75 mM or 9 mg of C.
Soil microbial biomass C was determined by chloroform fumigation- K2SO4 extraction method [32]. The moist soil (equivalent to 10 g air-dried soil) was extracted with 50 mL 0.5 mol/L K2SO4 for 1 h. The extracts were filtered through a 0.45-μm membrane filter and analyzed for unfumigated soil of organic C using a Multi 3100 N/C TOC analyzer (Analytik, Jena, Germany). Another equal amount of moist soil samples was fumigated with chloroform and incubated in the dark for 24 h, then extracted with 0.5 mol/L K2SO4 solution similar to the previous method to determine the organic C content of the fumigated soil. The microbial biomass C content was calculated as the difference in organic C content between the fumigated and unfumigated samples.
The activities of β-glucosidase and β-cellobiohydrolase were determined using fluorescence spectrophotometry [33]. The 4-Methylumbelliferyl (MUB) linked model substrates used were listed in Table 2. In detail, Fresh soil of 1 g was added to 125 mL of 50 mmol/L sodium acetate buffer (pH = 5), creating a suspension. 200 μL of soil sample suspension, 50 μL of buffer solution, 50 μL MUB solution, and 50 μL of MUB-linked substrate were added to a 96-well black enzyme-linked immunosorbent assay plate. The substrate plates in six replicates were incubated in the dark at 25 °C for 2 h, and 10 μL of 1 mol/L sodium hydroxide solution was added to terminate the reaction. Then the substrate plate was measured with SpectroMax M5 (Molecular Devices, Inc., San Jose, CA, USA) using 365 nm excitation and 450 nm emission filters. Negative control and MUB standard ranging from 0 to 100 μM were prepared in three replicates for each sample to compute the extracellular enzyme activities. Soil hydrolase activities were calculated in nmol·h–1·g–1.

Table 2.
Extracellular enzymes with corresponding substrate and the EC number.
Soil peroxidase and phenol oxidase activities were determined using the DOPA-UV spectrophotometry method [34,35]. L-3,4-dihydroxyphenylalanine (L-DOPA) and H2O2 were used as substrates (Table 2). In order to ensure that L-DOPA was fully dissolved in deionized water, the water was heated to 90 °C in a microwave oven and then L-DOPA was subsequently added to achieve a 5 mM solution. With the same procedure as above, soil sample suspension, buffer solution, and substrates (L-DOPA for phenol oxidase, L-DOPA and H2O2 for peroxidase) were added to a 96-well transparent enzyme-linked immunosorbent assay plate. The substrate plates were incubated at 25 °C for 4 h for peroxidase and 20 h for phenol oxidase, then absorbance was measured at 460 nm to calculate soil oxidase activities. Soil oxidase activities were calculated in μmol·h–1·g–1.
2.4. Statistical Analysis
All statistical analyses were performed in SPSS 21.0 (IBM, Chicago, IL, USA), and the LSD method of one-way ANOVA was used to test the different significance of the data (α = 0.05). Pearson correlation analysis was used to analyze the correlation of indicators.
3. Results
3.1. TOC and Active Organic C Fractions
As illustrated in Figure 4, the stumps had a significant impact on the TOC content within the soil microsite of the larch plantation. The horizontal influence range of the stumps on the TOC content of forest microsite soil could be determined based on whether the TOC content of each sampling point in the microsite soil was significantly higher than that of D5, which was outside of the microsite. In the topsoil layer, the TOC content at sampling point D1 was found to be significantly higher compared to D5 (p < 0.05). The impact of the stumps on the TOC content in the topsoil layer could reach up to 15 cm in the microsite soil of the larch plantation. Similarly, in the subsoil layer, the TOC content at the sampling point of D1 was significantly higher than that of D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on TOC content in the subsoil layer also could reach up to 15 cm.
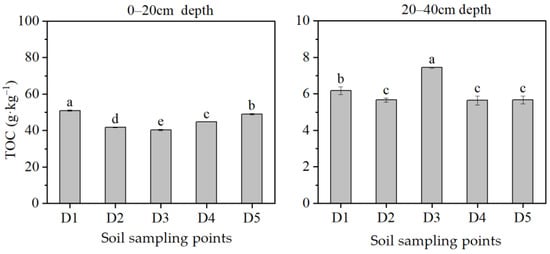
Figure 4.
Variations in total organic carbon (TOC) content of soil sampling points at different distances from larch stumps within the larch plantation. Lowercase letters indicate significant differences among different soil sampling points in the same plantation, p < 0.05. n = 3, standard error bars are shown.
As depicted in Figure 5, the stumps significantly impacted the active organic C fractions, including POC, EOC, and MBC, within the microsite soil of the larch plantation. In the topsoil layer, POC content at the soil sampling point of D1 was significantly higher than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on POC content in the topsoil layer could reach up to 15 cm in the microsite soil of the larch plantation. Similarly, in the subsoil layer, the POC content at D1 was significantly higher than that of D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on soil POC content also could be reached up to 15 cm in this layer.
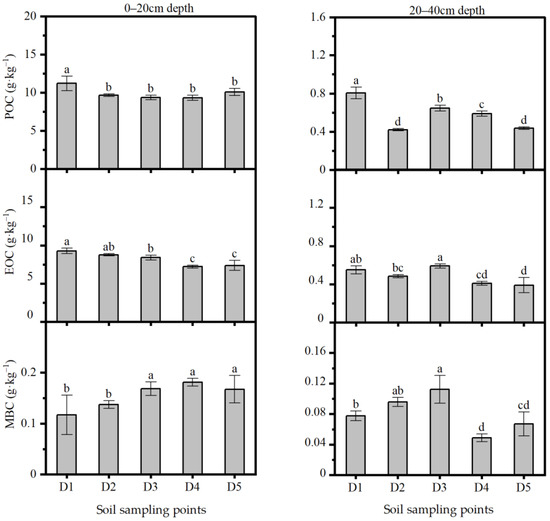
Figure 5.
Variations in active organic carbon fractions contents of soil sampling points at different distances from larch stumps within the larch plantation. The active organic carbon fractions include particulate organic carbon (POC), easily oxidizable organic carbon (EOC), and microbial biomass carbon (MBC). Lowercase letters indicate significant differences among different soil sampling points in the same plantation, p< 0.05. n = 3, standard error bars are shown.
In the topsoil layer, the stumps had no significant effect on the microsite soil of MBC content. However, in the subsoil layer, the MBC content from the soil sampling points of D1 to D3 was significantly higher than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on MBC content in the subsoil layer could extend up to 55 cm.
In both the topsoil and subsoil layers, the EOC content from soil sampling points of D1 to D3 was all significantly higher than at D5 (p < 0.05). The maximum horizontal range of EOC content influenced by the stumps extended up to 55 cm in both the topsoil and subsoil layers, which were the active organic C fractions with the largest horizontal range affected by stumps in the larch plantation.
3.2. Hydrolase Activity
As shown in Figure 6, the stumps significantly impacted the hydrolase activity which was β-glucosidase and β-cellobiohydrolase of microsite soil within the larch plantation. Furthermore, the magnitude of the stumps’ effects on soil β-cellobiohydrolase activity differed across various soil layers. In the topsoil layer, the β-cellobiohydrolase activity at soil sampling points of D1 and D2 was significantly higher than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on β-cellobiohydrolase activity in the topsoil layer could reach up to 35 cm. In the subsoil layer, β-cellobiohydrolase activity at the sampling point of D1 was significantly greater than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on β-cellobiohydrolase activity in the subsoil layer could reach up to 15 cm.

Figure 6.
Variations in hydrolase activity of soil sampling points at different distances from larch stumps within the larch plantation. Lowercase letters indicate significant differences among different soil sampling points in the same plantation, p < 0.05. n = 3, standard error bars are shown.
Table 1 was significantly higher than at D5 in the topsoil layer (p < 0.05). The maximum horizontal range of the stumps’ influence on β-glucosidase activity in the topsoil layer extended up to 15 cm. Similarly, in the subsoil layer, the β-glucosidase activity at the soil sampling point of D1 was also significantly greater than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on β-glucosidase activity in the subsoil layer also could reach up to 15 cm.
3.3. Oxidase Activity
As depicted in Figure 7, the stumps significantly influenced the oxidase activity of phenol oxidase and peroxidase in the microsite soil of the larch plantation. However, the extent of the stumps’ impact on soil peroxidase activity varied across different soil layers. In the topsoil layer, the peroxidase activity from soil sampling points of D1 to D3 was significantly higher than at D5 (p < 0.05). The maximum horizontal range of the stumps’ impact on peroxidase activity in the topsoil layer could extend up to 55 cm. In addition, the peroxidase activity at soil sampling point D1 was significantly greater than at D5 in the subsoil layer (p < 0.05). The maximum horizontal range of the stumps’ influence on peroxidase activity in the subsoil layer could reach up to 15 cm.

Figure 7.
Variations in oxidase activity of soil sampling points at different distances from larch stumps within the larch plantation Lowercase letters indicate significant differences among different soil sampling points in the same plantation, p < 0.05. n = 3, standard error bars are shown.
Table 1 and D2 were higher than at D5 for both topsoil and subsoil layers (p < 0.05). The maximum horizontal range of the stumps’ influence on phenol oxidase activity in both the topsoil and subsoil layers of the larch plantation could reach up to 35 cm.
4. Discussion
In this study, it was found that the stumps significantly impacted the microsite soil TOC content in the plantation, with an increase in content observed closer to the stumps (Figure 4). This aligns with the findings of Błońska et al. [13], which showed that the TOC content at distances of 0–10 cm from stumps was higher than that at distances of 50 cm from stumps. The primary reason for this increase is the higher accumulation of polyphenols near the stumps, which in turn enhances the soil TOC content [8].
The correlation between soil TOC and POC was greater than that of other active organic C fractions, indicating that POC is the fraction most sensitive to changes in soil TOC and most likely a sensitive indicator of TOC dynamics (Table 3). TOC also showed a highly significant positive correlation with both EOC and MBC because microorganisms are more likely to decompose soils with high C content [36]. In addition, the stumps had the most significant impact on EOC content in the microsite soil of the plantation among active C fractions. EOC, a crucial nutrient source and vital energy source for soil microbial activities, has the fastest turnover among soil organic C fractions [37]. In this study, the horizontal range of the stumps’ influence on EOC content in both the topsoil and subsoil layers reached up to 55 cm (Figure 5). This suggests that the stumps can enhance the available nutrient content in plantation microsite soil, promoting soil microbial activity and accelerating soil organic C turnover rates.

Table 3.
Pearson correlation among different soil organic carbon fractions and enzyme activity in plantation microsite.
The enzymatic activity of soils, as active organic soil components, is sensitive to external environmental changes [16] and is closely associated with soil organic matter decomposition [38]. Organic matter serves as a substrate for microbial growth and activity, which influences enzymatic activity. Soils with higher organic matter content typically have higher enzymatic activity than soils with lower organic matter content [17]. Some soil enzymes require the presence of metal ions in their active centers to catalyze chemical reactions. These metal ions can act as cofactors, stabilizing the enzyme-substrate complex or participating in chemical reactions. Examples of metal ions found in soil enzyme active centers include zinc, iron, copper, and magnesium [39,40]. Some peroxidases will contain heme as a cofactor [41]. Heme cofactors play a key role in the ability of enzymes to catalyze the oxidation of organic compounds by providing reaction centers for electron transfer. Likewise, some phenol oxidases contain copper ions in their active sites, which are required for the enzyme to function properly [42]. In the case of glucosidases and cellobiohydrolases, these enzymes are mainly involved in the breakdown of complex carbohydrates such as cellulose and generally do not require metal ions as cofactors. However, some studies have shown that these enzymes may be activated or inhibited by metal ions such as calcium or copper, thereby affecting their activity and specificity [43].
Previous studies have shown that oxidative enzymes, such as peroxidase and phenol oxidase, mainly decompose stubborn organic matter in the soil. Hydrolases such as glucosidase and cellobiohydrolase mainly decompose cellulose and hemicellulose [18]. This study discovered that larch stumps impacted the activities of glucosidase, cellobiohydrolase, peroxidase, and phenol oxidase in the microsite soil of the plantation (Figure 6 and Figure 7). This suggests that the stumps not only influenced soil oxidase activity, promoting the decomposition of recalcitrant organic matter but also increased active organic matter, such as active organic C fractions, by affecting soil hydrolytic enzyme activity. Furthermore, as shown in Table 3, plantation microsite soil POC and EOC had significant positive correlations with β-glucosidase, which is consistent with the findings of Banerjee et al. [44].
While this study investigated the effects of the stumps on soil C content and enzyme activity in plantation microsites, there is still room for improvement. (1) Enzymatic activity in soils plays a crucial role in the decomposition of organic matter and the biological cycling of nutrients, such as carbon, nitrogen, phosphorus, sulfur, and other microelements [44,45]. This study primarily focused on soil enzymes related to carbon and did not address enzymatic activity related to phosphorus, sulfur, or other nutrients in soils. (2) Enzymatic activity of soils predominantly originates from microorganisms [46], and changes in microbial biomass significantly impact enzyme activity. This study did not consider the influence of microbial indicators, which warrants further investigation.
5. Conclusions
This study discovered that the stumps significantly influence the microsite soil properties in the plantation, such as TOC, active organic C fractions, and hydrolase and oxidase enzyme activities. Higher concentrations of these properties were observed closer to the stumps. Among the various active organic C fractions, the range of EOC content was most affected by the presence of stumps. Additionally, the stumps influenced the EOC content in microsite soil by altering the activity of the enzyme β-glucosidase. These insights enhance our comprehension of the role stumps play in plantation ecosystems and can guide future management strategies to preserve soil fertility and boost site productivity.
Author Contributions
X.C. and Z.S. conceived and designed the study; Y.Y. and X.M. collected field samples, performed the laboratory trials, analyzed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, grant number 31870612).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on reasonable request.
Acknowledgments
We gratefully acknowledge the assistance of numerous staff from Meng Jia Gang Forest farm with the field investigation and sample processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kappes, H.; Catalano, C.; Topp, W. Coarse woody debris ameliorates chemical and biotic soil parameters of acidified broad-leaved forests. Appl. Soil Ecol. 2007, 36, 190–198. [Google Scholar] [CrossRef]
- Palviainen, M.; Finer, L.; Laiho, R.; Shorohova, E.; Kapitsa, E.; Vanha, M.I. Carbon and nitrogen release from decomposing Scots pine, Norway spruce and silver birch stumps. For. Ecol. Manag. 2010, 259, 390–398. [Google Scholar] [CrossRef]
- Freschet, G.T.; Weedon, J.T.; Aerts, R.; Van, H.J.R.; Cornelissen, J.H.C. Interspecific differences in wood decay rates: Insights from a new short-term method to study long-term wood decomposition. J. Ecol. 2012, 100, 161–170. [Google Scholar] [CrossRef]
- Finer, L.; Mannerkoski, H.; Piirainen, S.; Starr, M. Carbon and nitrogen pools in an old-growth, Norway spruce mixed forest in eastern Finland and changes associated with clear-cutting. For. Ecol. Manag. 2003, 174, 51–63. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Tietema, A.; Cornelissen, J.H.C.; Hefting, M.M.; Kalbitz, K. Tamm Review: Sequestration of carbon from coarse woody debris in forest soils. For. Ecol. Manag. 2016, 377, 1–15. [Google Scholar] [CrossRef]
- Hafner, S.D.; Groffman, P.M. Soil nitrogen cycling under litter and coarse woody debris in a mixed forest in New York State. Soil Biol. Biochem. 2005, 37, 2159–2162. [Google Scholar] [CrossRef]
- Yue, Y.; Men, X.; Sun, Z.H.; Chen, X.W. Effects of Larix olgensis Henry Stumps and Coarse Roots on Phosphorus Fractions and Availability in Plantation Microsite Soils. Forests 2022, 13, 2166. [Google Scholar] [CrossRef]
- Spears, J.D.H.; Lajtha, K. The imprint of coarse woody debris on soil chemistry in the western Oregon Cascades. Biogeochemistry 2004, 71, 163–175. [Google Scholar] [CrossRef]
- Zalamea, M.; Gonzalez, G.; Ping, C.L.; Michaelson, G. Soil organic matter dynamics under decaying wood in a subtropical wet forest: Effect of tree species and decay stage. Plant Soil 2007, 296, 173–185. [Google Scholar] [CrossRef]
- Gu, L.H.; Post, W.M.; King, A.W. Fast labile carbon turnover obscures sensitivity of heterotrophic respiration from soil to temperature: A model analysis. Glob. Biogeochem. Cycles 2004, 18, 1–11. [Google Scholar] [CrossRef]
- Haynes, R. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 5, 221–268. [Google Scholar] [CrossRef]
- Xu, M.G.; Lou, Y.L.; Sun, X.L.; Wang, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fert. Soils 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump Harvesting for Bioenergy—A Review of the Environmental Impacts. Forestry 2010, 83, 17–38. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Gallo, M.E.; Lauber, C.; Waldrop, M.P.; Zak, D.R. Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 2005, 75, 201–215. [Google Scholar] [CrossRef]
- Puglisi, E.; Re, D.A.A.M.; Rao, M.A.; Gianfreda, L. Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 2006, 38, 1673–1681. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef]
- Peng, W.; Li, F.; Dong, L. Individual tree diameter growth model for Larix olgensis plantation in Heilongjiang Province, China. J. Nanjing For. Univ. 2018, 42, 19–27. [Google Scholar]
- Deng, X.; Cheng, F.; Li, M.; He, P.; Shen, L.; Liu, H. Effect of different decay classes of Eucalyptus stump substrates on microbial resource limitation and carbon-use efficiency. Plant Soil 2022, 478, 651–669. [Google Scholar] [CrossRef]
- Tláskal, V.; Brabcová, V.; Větrovský, T.; Jomura, M.; López-Mondéjar, R.; Oliveira Monteiro, L.M.; Baldrian, P. Complementary roles of wood-inhabiting fungi and bacteria facilitate deadwood decomposition. Msystems 2021, 6, e01078-20. [Google Scholar] [CrossRef]
- Waring, B.G. Exploring relationships between enzyme activities and leaf litter decomposition in a wet tropical forest. Soil Biol. Biochem. 2013, 64, 89–95. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Chen, S.; Xia, D.; Yang, C.; Zhao, X. Genetic variation and superior provenances selection for wood properties of Larix olgensis at four trials. J. For. Res. 2022, 33, 1867–1879. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Chen, X. Effects of thinning intensity on carbon storage of Larix olgensis plantation ecosystem. J. Beijing For. Univ. 2016, 38, 1–13. [Google Scholar] [CrossRef]
- Jia, W.; Pang, Y. Tree species classification in an extensive forest area using airborne hyperspectral data under varying light conditions. J. For. Res. 2023. [Google Scholar] [CrossRef]
- Hunter, J.M.L. Wildlife, Forests, and Forestry. Principles of Managing Forests for Biological Diversity; Prentice Hall: Hoboken, NJ, USA, 1990; p. 370. [Google Scholar]
- Petrillo, M.; Cherubini, P.; Fravolini, G.; Marchetti, M.; Ascher-Jenull, J.; Schärer, M.; Synal, H.; Bertoldi, D.; Camin, F.; Larcher, R.; et al. Time since death and decay rate constants of Norway spruce and European larch deadwood in subalpine forests determined using dendrochronology and radiocarbon dating. Biogeosciences 2016, 13, 1537–1552. [Google Scholar] [CrossRef]
- Kajimoto, T.; Matsuura, Y.; Osawa, A.; Prokushkin, A.S.; Sofronov, M.A.; Abaimov, A.P. Root system development of Larix gmelinii trees affected by micro-scale conditions of permafrost soils in central Siberia. Plant Soil 2003, 255, 281–292. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agr. 2003, 18, 3–17. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agr. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 2002, 19, 697–702. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Kourtev, P.S.; Ehrenfeld, J.G.; Huang, W.Z. Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol. Biochem. 2002, 34, 1207–1218. [Google Scholar] [CrossRef]
- Bach, C.E.; Warnock, D.D.; Van, H.D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D. Measuring phenol oxidase and peroxidase activities with pyrogallol, L-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef]
- Xiang, H.M.; Wen, D.Z.; Zhang, L.L.; Li, J. Altitudinal changes in active and recalcitrant soil carbon pools of forests in the Dinghu Mountains. Acta Ecol. Sin. 2015, 35, 6089–6099. [Google Scholar]
- Zou, X.M.; Ruan, H.H.; Fu, Y.; Yang, X.D.; Sha, L.Q. Estimating soil labile organic carbon and potential turnover rates using a sequential fumigation-incubation procedure. Soil Biol. Biochem. 2005, 37, 1923–1928. [Google Scholar] [CrossRef]
- Rietl, A.J.; Jackson, C.R. Effects of the ecological restoration practices of prescribed burning and mechanical thinning on soil microbial enzyme activities and leaf litter decomposition. Soil Biol. Biochem. 2012, 50, 47–57. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Zámocký, M.; Hofbauer, S.; Schaffner, I.; Gasselhuber, B.; Nicolussi, A.; Soudi, M.; Obinger, C. Independent evolution of four heme peroxidase superfamilies. Arch. Biochem. Biophys. 2015, 574, 108–119. [Google Scholar] [CrossRef]
- Duran, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B Environ. 2000, 28, 83–99. [Google Scholar] [CrossRef]
- Tejirian, A.; Xu, F. Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Appl. Environ. Microb. 2010, 76, 7673–7682. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bora, S.; Thrall, P.H.; Richardson, A.E. Soil C and N as causal factors of spatial variation in extracellular enzyme activity across grassland-woodland ecotones. Appl. Soil Ecol. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W.S. Recent Developments in Using Advanced Sequencing Technologies for the Genomic Studies of Lignin and Cellulose Degrading Microorganisms. Int. J. Biol. Sci. 2016, 12, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Zimmerman, A.R.; Comerford, N.B.; Sickman, J.O.; Grunwald, S. Carbon Mineralization and Labile Organic Carbon Pools in the Sandy Soils of a North Florida Watershed. Ecosystems 2009, 12, 672–685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).