Temperature and Tree Size Explain the Mean Time to Fall of Dead Standing Trees across Large Scales
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. Literature Search and Data Extraction
- Studies based on counts of dead standing trees (DSTs) not distinguishing size classes;
- Studies based on counts of DSTs distinguished by size classes;
- Studies based on volume/dry mass of DSTs.
2.1.2. Ancillary Data
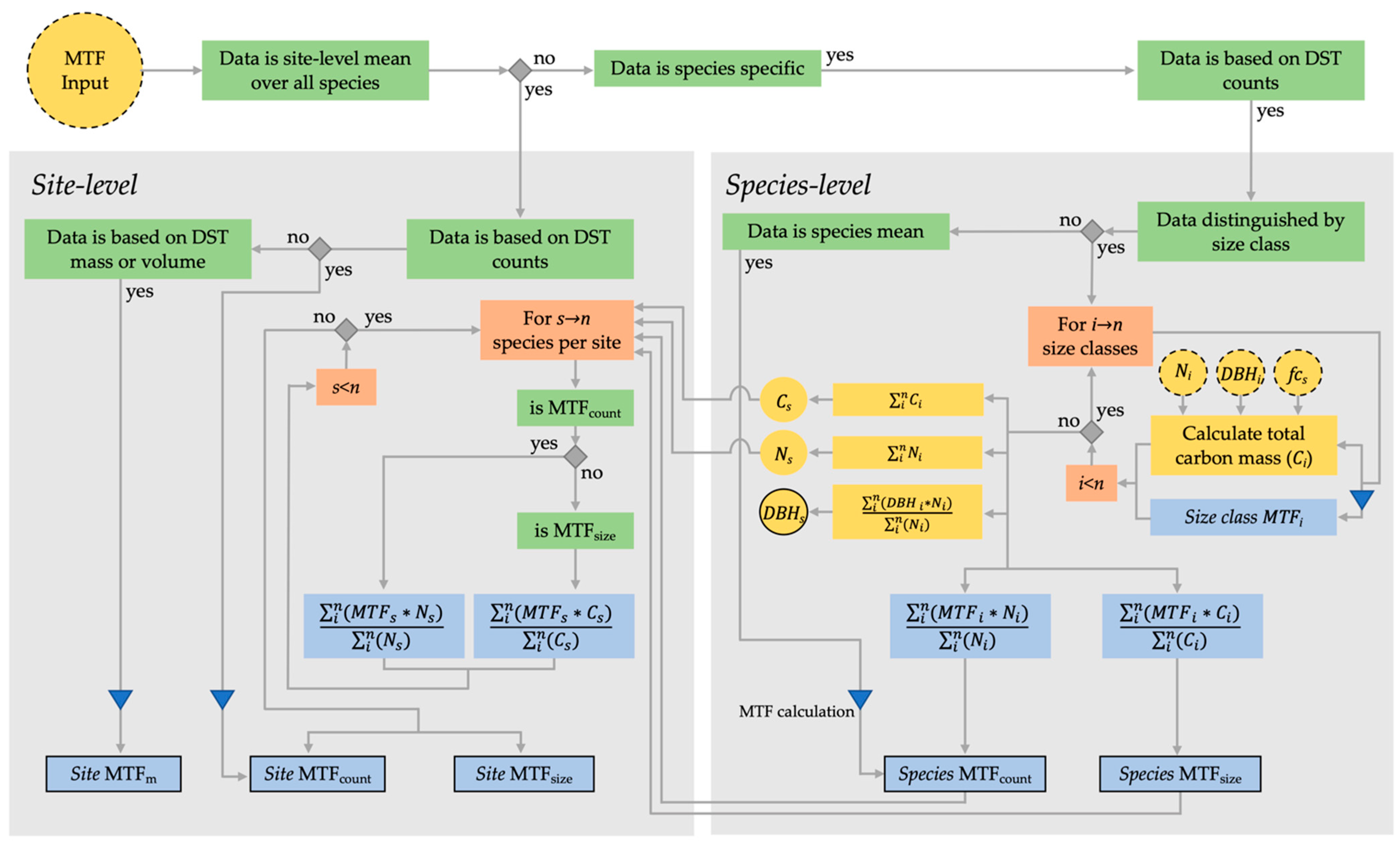
2.2. Primary MTF Calculation and Pre-Processing of Collected Data
2.3. MTF Calculation at Site and Species Level
2.3.1. Studies Based on Counts of Dead Standing Trees (MTFcount)
2.3.2. Studies Based on Counts of Dead Standing Trees Distinguished by Size Classes (MTFsize)
2.3.3. Studies Based on Volume/Dry Mass of the Dead Standing Trees (MTFm)
2.4. Relationships of MTF to Explanatory Variables
3. Results
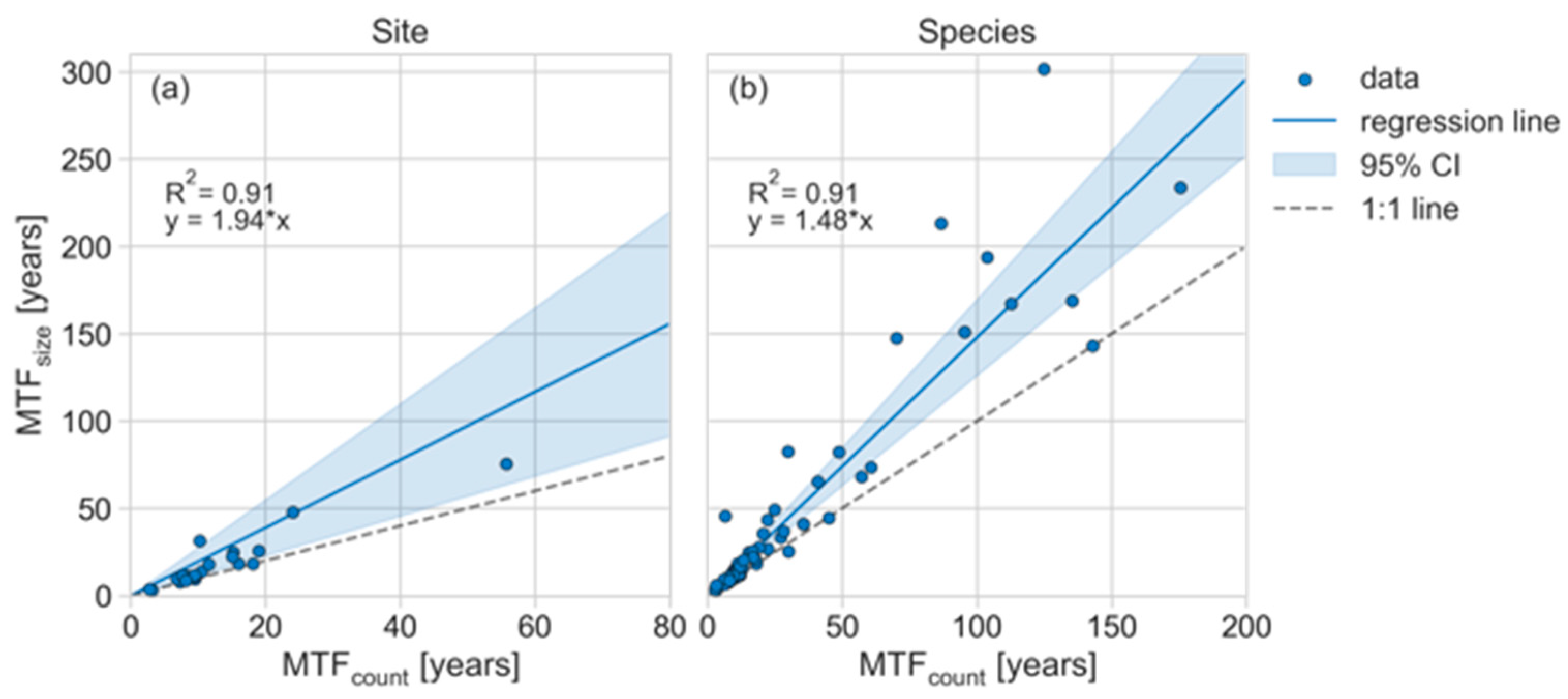
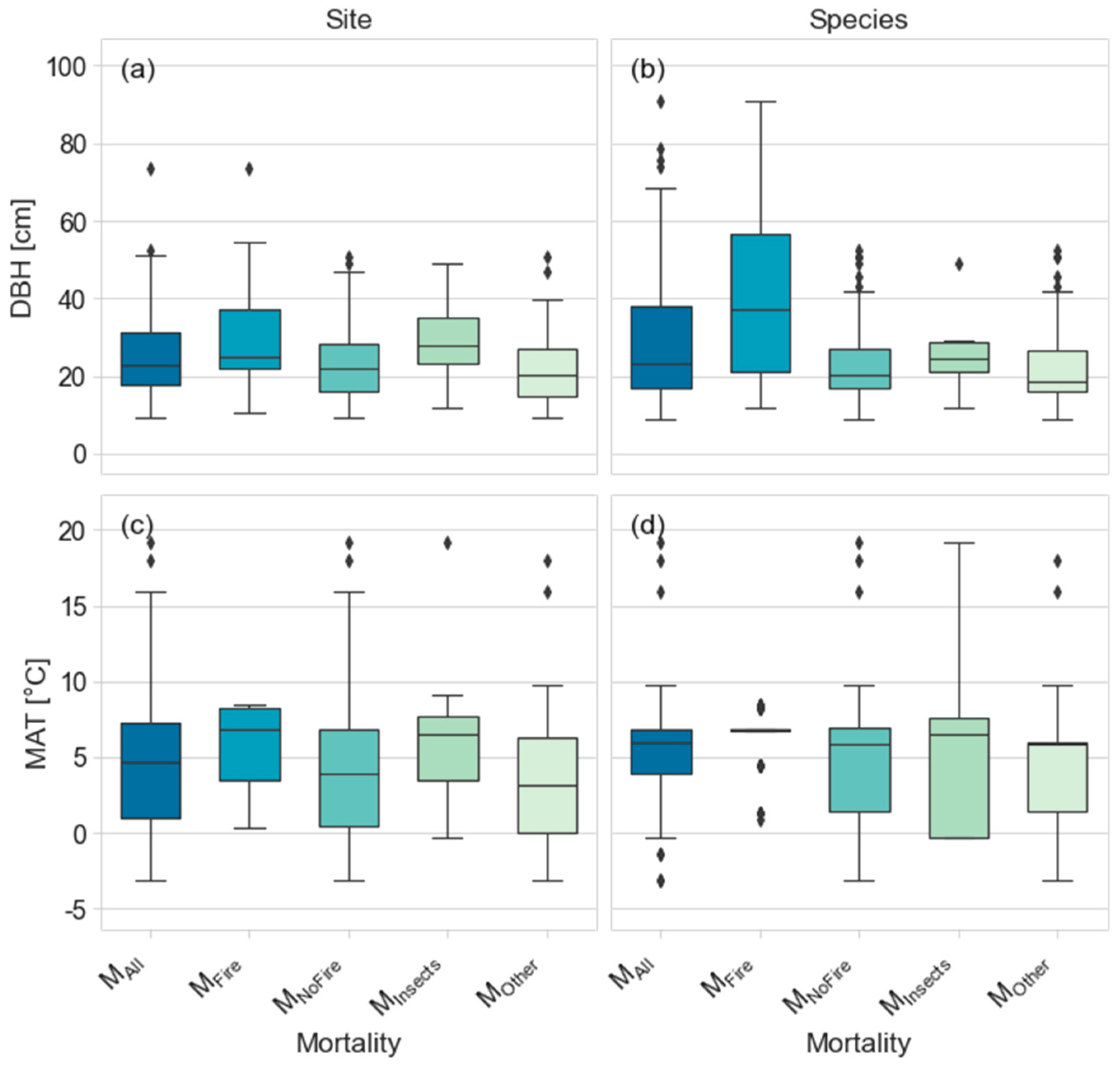
3.1. Overview the of Mean Time to Fall (MTF) for Dead Standing Trees (DST)
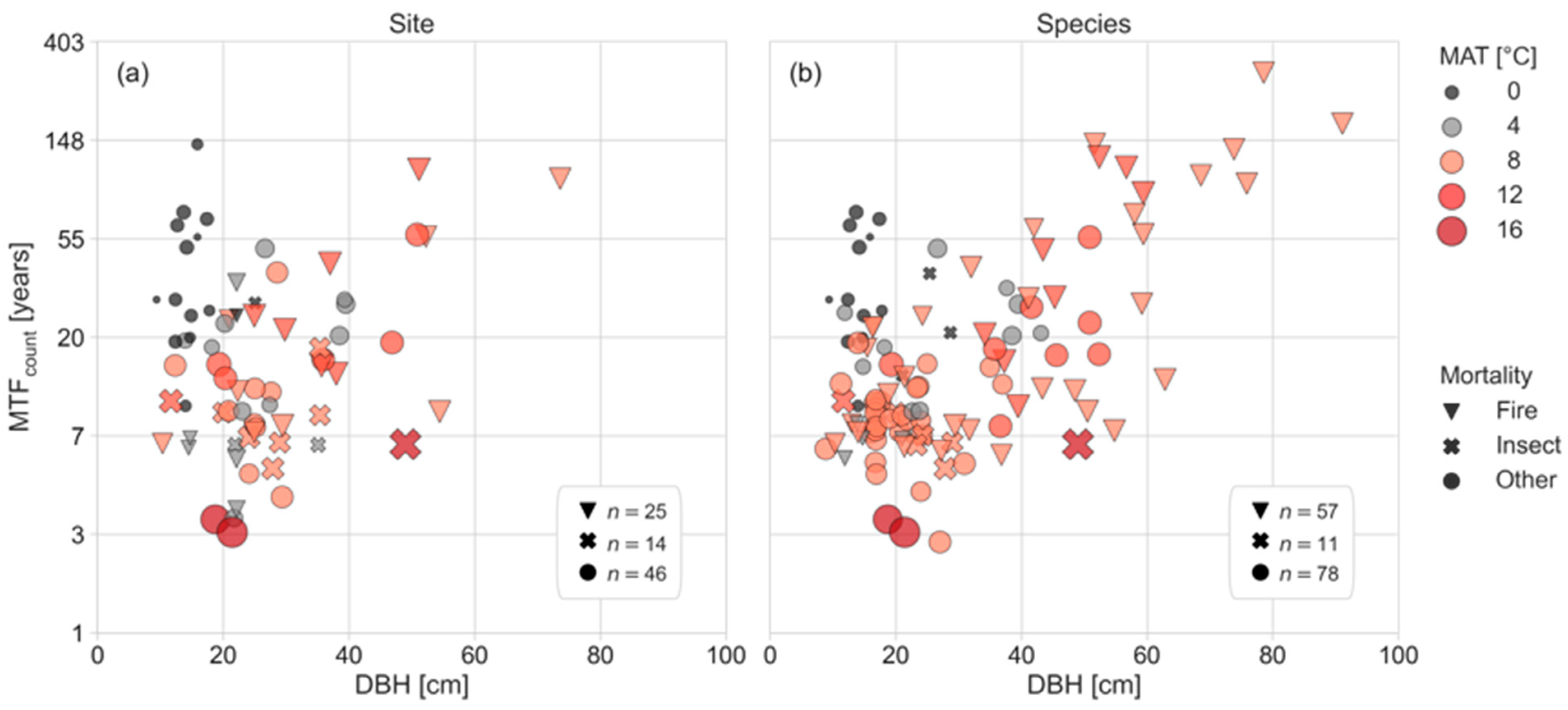
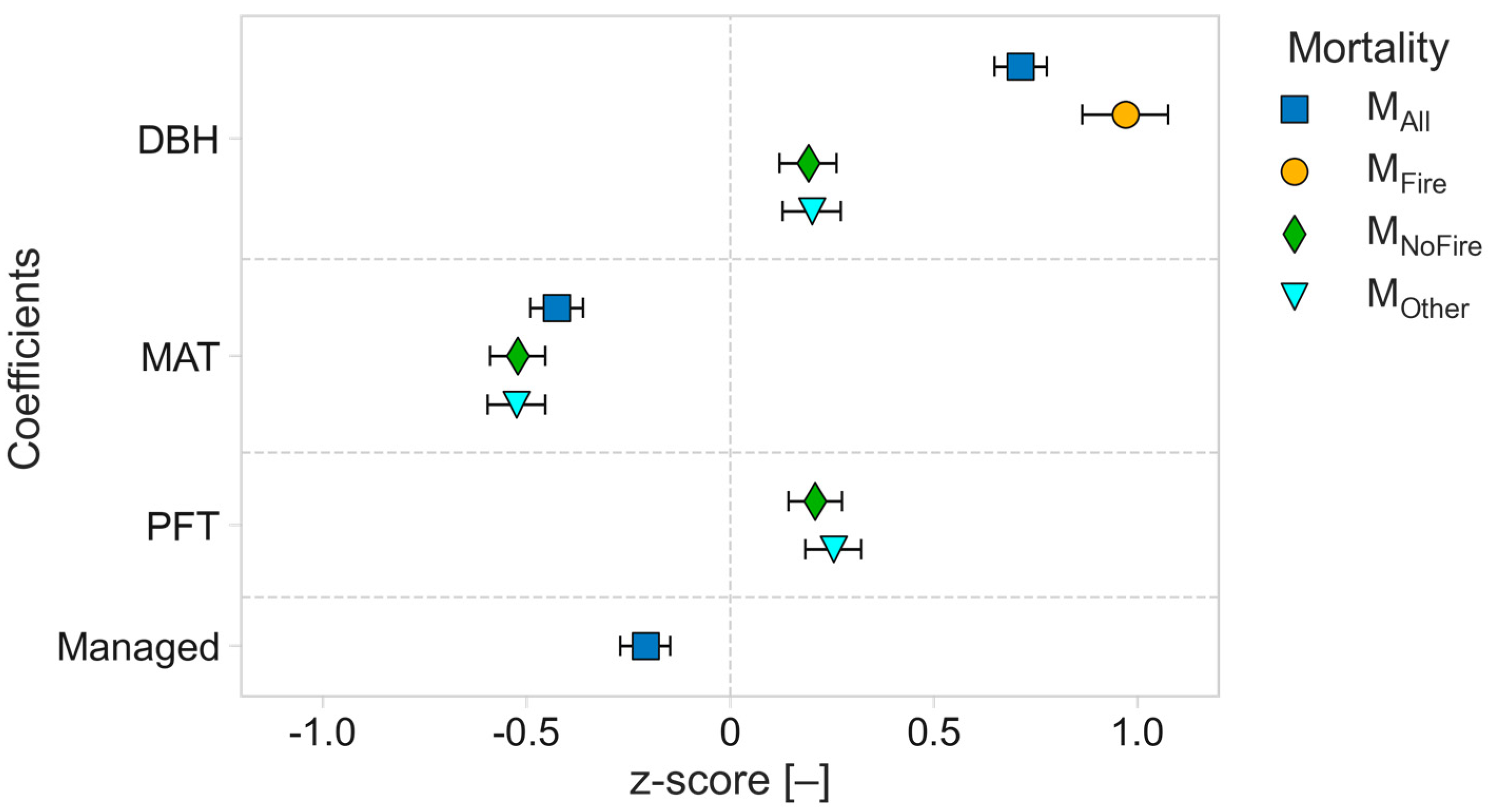
3.2. Drivers of Mean Time to Fall for Dead Standing Trees
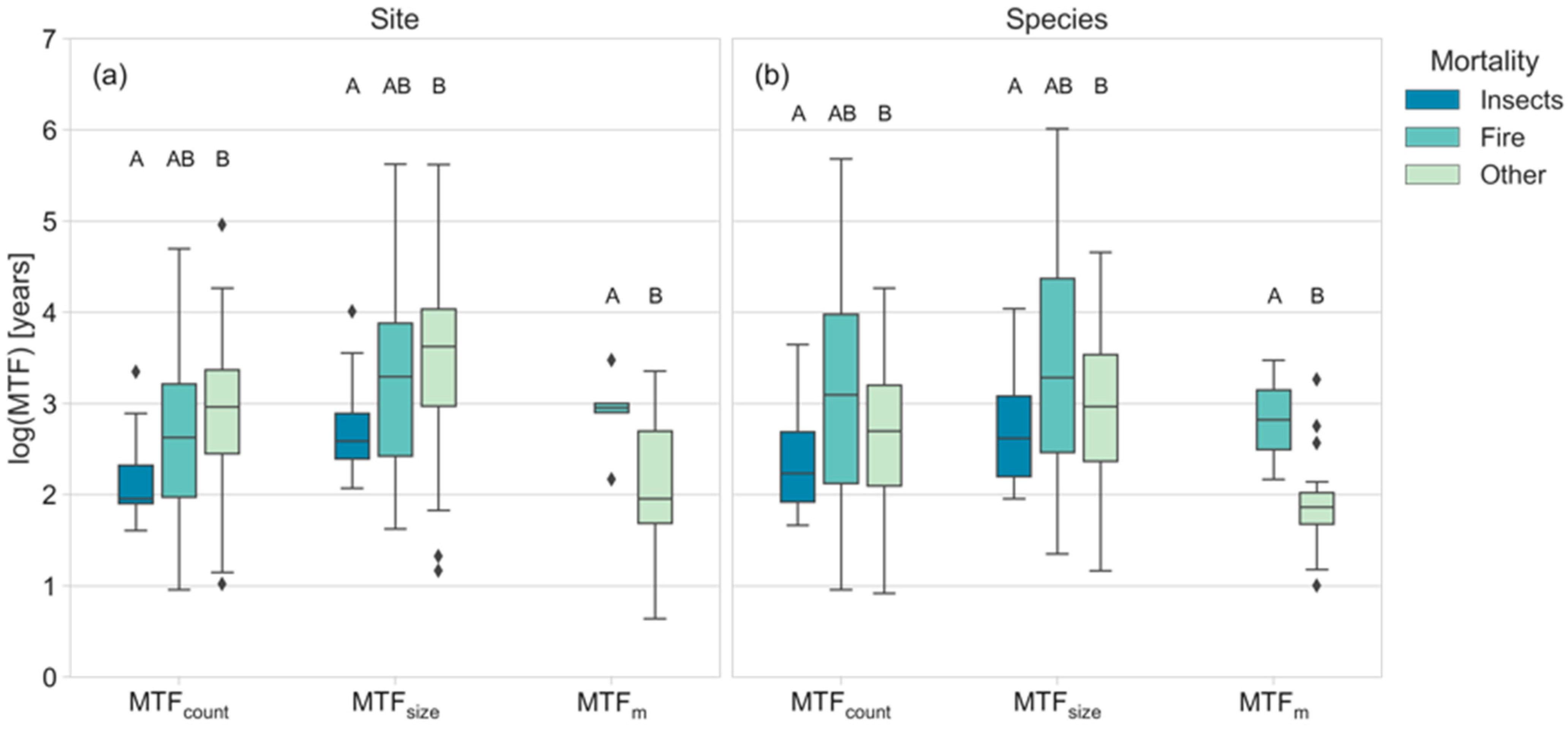
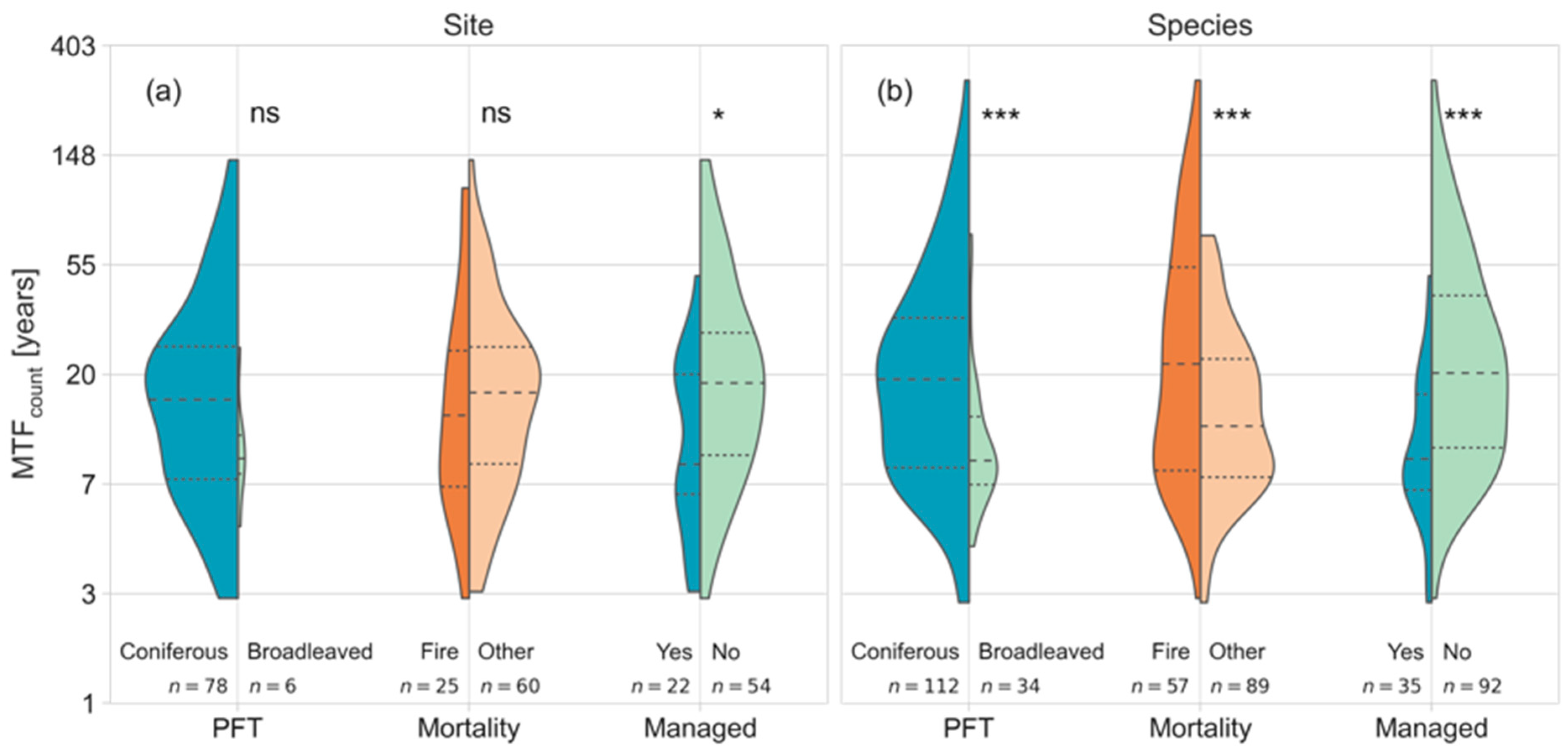
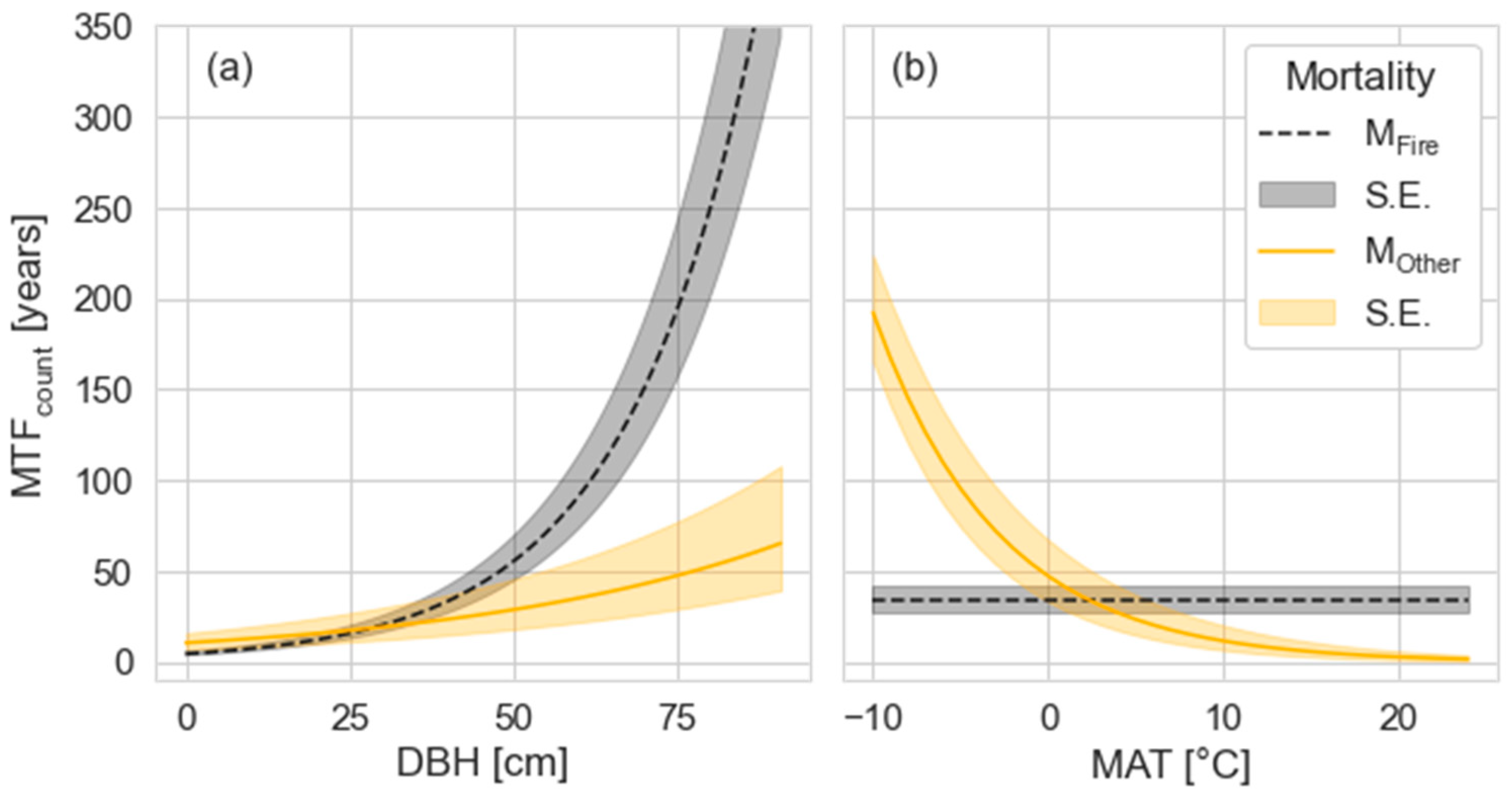
3.3. Driving Factors of the MTF Differ by DST Mortality Cause
3.4. Comparison of Regression Results with MTFm Data from the Tropics
4. Discussion
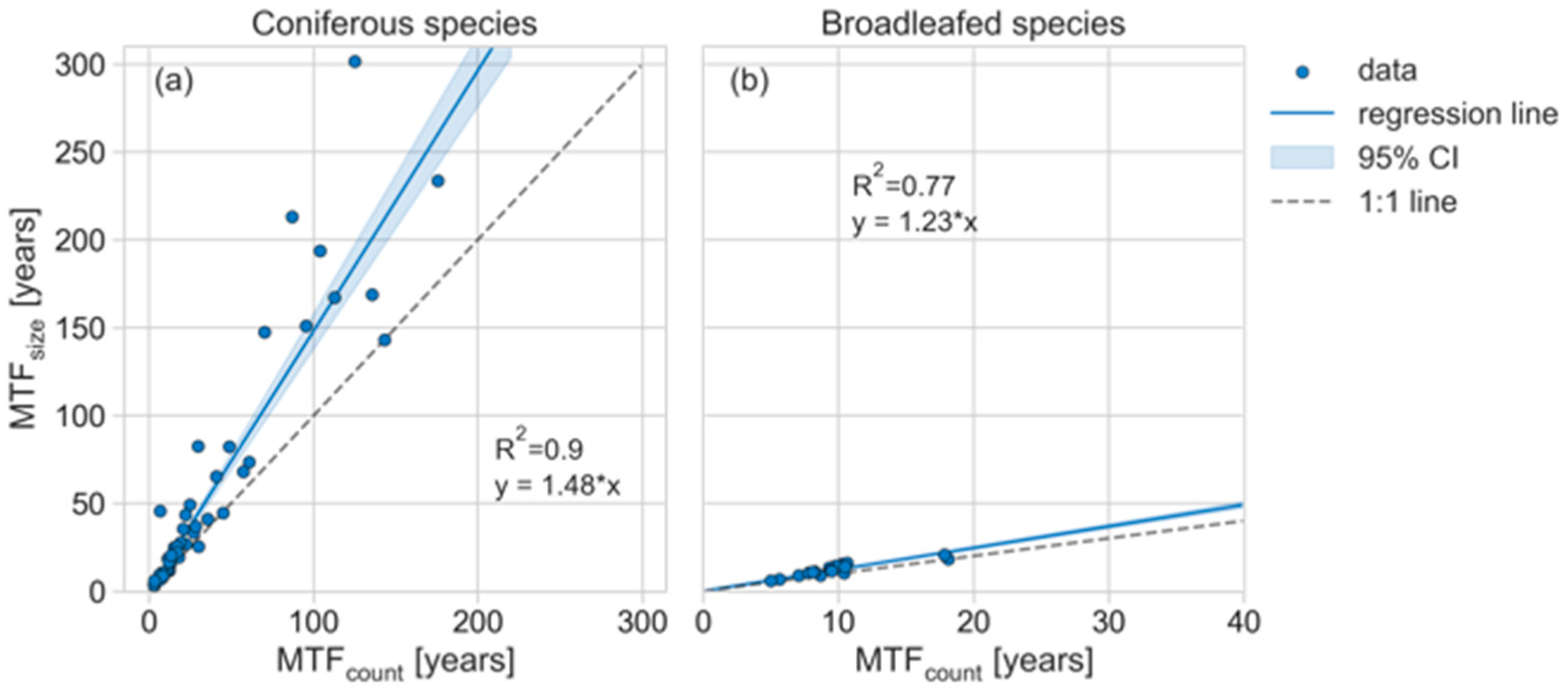
4.1. Site- and Species-Level Differences
4.2. Management Effect on the MTF
4.3. Climate Influence on the MTF
4.4. Substrate Quality Influence on the MTF
4.5. Analysis Limitations and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
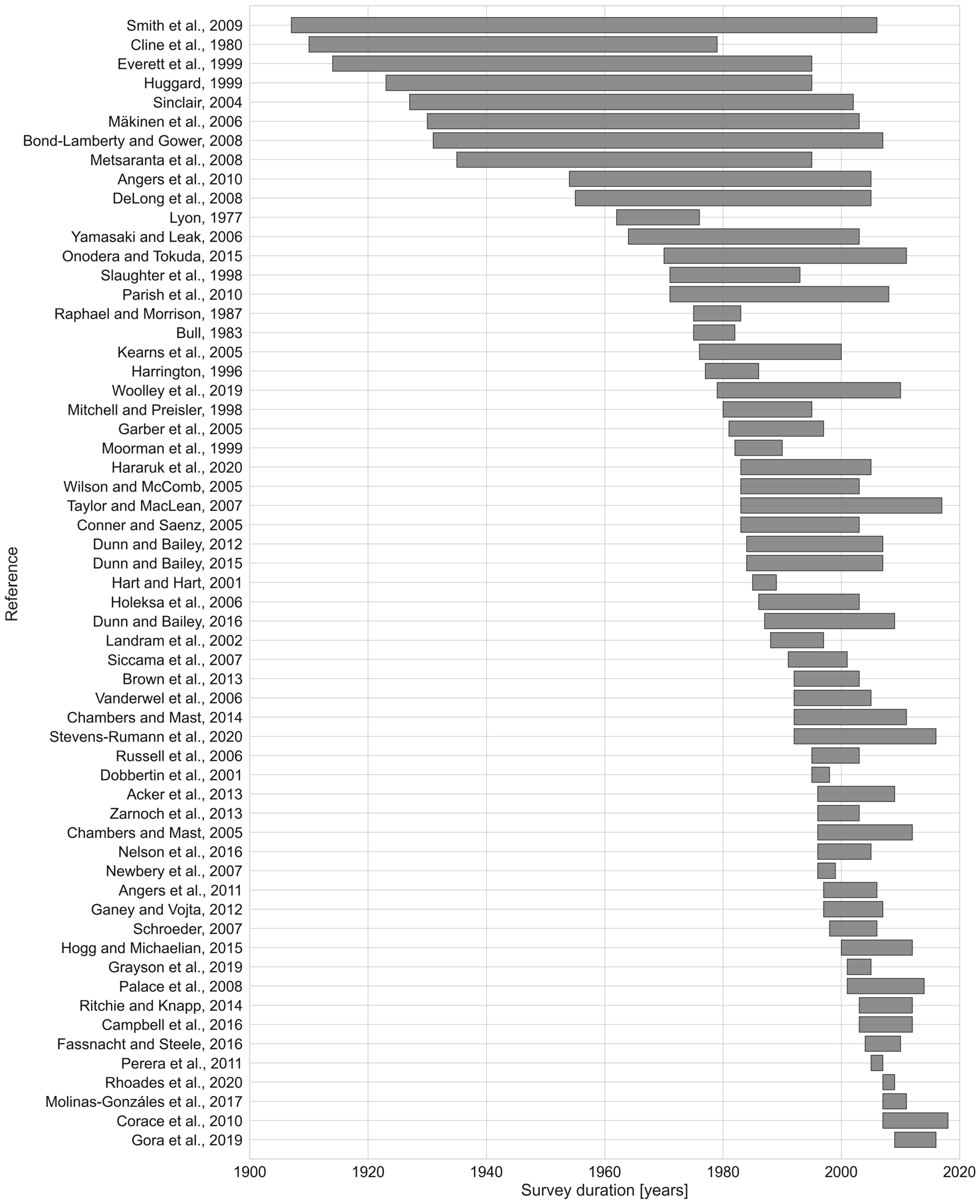


| MTF Source | Description | DBH Distribution Source | ||
|---|---|---|---|---|
| Reference | Survey Duration | Reference | Survey Year(s) Used | |
| Everett et al. (1999) | 1914–1995 * | Same dataset. Number of trees/DBH class and species digitized from Figure 3 in Lehmkul et al. (2003). | Lehmkuhl et al. (2003) | 1914–1995 * |
| Garber et al. (2005) | 1981–1997 | Tree based data from the NAT treatment (no intervention) from the 1999 inventory to estimate mean DBH by species for which Garber reported DBH specific DST survival curves and model coefficients (category 5; Table 2). DBH classes from grouping species’ DSTs into equally sized bins. Stand density to model DBH specific MTF by species also based on Kenefic et al. (2015). | Kenefic et al. (2015) | 1999 |
| Bull (1983) | 1975–1982 | DBH distribution based on Table 2 in Bull (1975) which includes all species. | Bull (1975) | 1973–1974 |
| Mortality | Managed | MTFcount [Years] | DBH [cm] | MAT [°C] | PFT [-] | Nobs | |
|---|---|---|---|---|---|---|---|
| Site | All | No | 22.0 (4.0–148.0) | 25.5 (9.4–73.6) | 3.95 (−3.1–9.8) | 0.93 | 46 |
| Yes | 9.0 (3.0–49.0) | 27.52 (14.7–49.0) | 6.66 (−0.3–19.2) | 0.89 | 18 | ||
| Fire | No | 20.0 (4.0–110.0) | 32.1 (14.5–73.6) | 5.81 (0.3–8.5) | 1.0 | 16 | |
| Yes | 7.0 (7.0–7.0) | 14.73 (14.7–14.7) | 0.9 (0.9–0.9) | 1.0 | 1 | ||
| Insects | No | 10.0 (7.0–18.0) | 27.52 (11.7–35.4) | 5.82 (1.0–9.1) | 0.80 | 5 | |
| Yes | 9.0 (7.0–27.0) | 29.81 (21.9–49.0) | 6.8 (−0.3–19.2) | 1.0 | 5 | ||
| No Fire | No | 22.0 (7.0–148.0) | 21.98 (9.4–50.9) | 2.96 (−3.1–9.8) | 0.90 | 30 | |
| Yes | 9.0 (3.0–49.0) | 28.27 (18.7–49.0) | 7.0 (−0.3–19.2) | 0.88 | 17 | ||
| Other | No | 26.0 (8.0–148.0) | 20.87 (9.4–50.9) | 2.38 (−3.1–9.8) | 0.92 | 25 | |
| Yes | 9.0 (3.0–49.0) | 27.63 (18.7–46.9) | 7.08 (3.1–18.0) | 0.83 | 12 | ||
| Species | All | No | 22.0 (4.0–299.0) | 30.44 (8.9–91.1) | 4.93 (−3.1–9.8) | 0.87 | 75 |
| Yes | 9.0 (2.0–49.0) | 25.69 (14.7–52.4) | 6.51 (−0.3–19.2) | 0.53 | 32 | ||
| Fire | No | 28.0 (6.0–299.0) | 40.18 (11.9–91.1) | 6.34 (1.3–8.5) | 0.97 | 36 | |
| Yes | 7.0 (7.0–7.0) | 14.73 (14.7–14.7) | 0.9 (0.9–0.9) | 1.0 | 1 | ||
| Insects | No | 10.0 (9.0–11.0) | 15.84 (11.7–20.0) | 7.8 (6.5–9.1) | 0.5 | 2 | |
| Yes | 11.0 (7.0–37.0) | 28.68 (21.1–49.0) | 5.71 (−0.3–19.2) | 1.0 | 7 | ||
| No Fire | No | 18.0 (4.0–74.0) | 21.44 (8.9–50.9) | 3.64 (−3.1–9.8) | 0.77 | 39 | |
| Yes | 9.0 (2.0–49.0) | 26.04 (16.6–52.4) | 6.69 (−0.3–19.2) | 0.52 | 31 | ||
| Other | No | 18.0 (4.0–74.0) | 21.74 (8.9–50.9) | 3.41 (−3.1–9.8) | 0.78 | 37 | |
| Yes | 9.0 (2.0–49.0) | 25.27 (16.6–52.4) | 6.98 (3.1–18.0) | 0.38 | 24 |
| Model | Climate | MTFcount | MTFsize | |||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | |||
| Site | MAT + DBH + Managed | CRUclim | 0.34 | 23.7 | 0.40 | 49.6 |
| CRUobs | 0.36 | 23.4 | 0.42 | 49.3 | ||
| CHELSA30s | 0.30 | 24.6 | 0.35 | 51.2 | ||
| WorldClim30s | 0.34 | 24.5 | 0.40 | 51.2 | ||
| WorldClim10m | 0.32 | 24.5 | 0.39 | 50.9 | ||
| MATSoil + DBH + Managed | SoilTemps | 0.34 | 24.4 | 0.40 | 51.1 | |
| Species | MAT + DBH + Managed | CRUclim | 0.61 | 26.0 | 0.57 | 44.0 |
| CRUobs | 0.61 | 26.0 | 0.59 | 44.0 | ||
| CHELSA30s | 0.58 | 26.3 | 0.57 | 43.3 | ||
| WorldClim30s | 0.58 | 27.3 | 0.56 | 46.4 | ||
| WorldClim10m | 0.55 | 27.4 | 0.54 | 45.4 | ||
| MATsoil + DBH + Managed | SoilTemps | 0.62 | 27.4 | 0.59 | 45.9 | |

| Mortality Group | Model | R2 | ∆AIC | RMSE | Nobs | Climate | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Count | Size | Count | Size | Count | Size | |||||
| Site | All | MAT + DBH + Managed | 0.36 | 0.42 | 0 | 0 | 23.4 | 49.2 | 64 | CRUobs |
| MATsoil + DBH + Managed | 0.34 | 0.40 | 1.6 | 1.3 | 24.4 | 51.1 | - | |||
| Fire | DBH | 0.40 | 0.29 | 0 | 0 | 20.8 | 54.0 | 17 | - | |
| No Fire | MATSoil | 0.32 | 0.38 | 0 | 0 | 23.9 | 47.1 | 47 | - | |
| MAT | 0.32 | 0.37 | 0.1 | 0.8 | 23.0 | 45.0 | CRUobs | |||
| Other | MATSoil | 0.37 | 0.46 | 0 | 0 | 25.9 | 51.1 | 37 | - | |
| MAT | 0.36 | 0.43 | 1.5 | 1.9 | 24.4 | 47.8 | CRUobs | |||
| Species | All | MATSoil + DBH + Managed | 0.62 | 0.59 | 0 | 0.2 | 26.8 | 46.0 | 107 | |
| MAT + DBH + Managed | 0.61 | 0.59 | 3.6 | 0 | 26.0 | 44.0 | CRU | |||
| Fire | DBH | 0.76 | 0.72 | 0 | 0 | 34.0 | 58.8 | 37 | - | |
| No Fire | MAT + DBH + PFT | 0.54 | 0.54 | 0 | 0 | 12.4 | 18.8 | 70 | CRUobs | |
| MAT + PFT | 0.49 | - | 5.6 | - | 12.4 | - | CRUobs | |||
| Other | MAT + DBH + PFT | 0.58 | 0.58 | 0 | 0 | 12.8 | 19.3 | 61 | CRUobs | |
| Level | Nobs | Mortality | Model | R2 | RMSE | Climate | |||
|---|---|---|---|---|---|---|---|---|---|
| Count | Size | Count | Size | ||||||
| MFire MAT range | Site | 17 | Fire | DBH | 0.54 | 0.47 | 21.0 | 54.9 | - |
| 38 | No Fire | MATSoil | 0.24 | 0.29 | 25.6 | 50.4 | - | ||
| 29 | Other | MATSoil | 0.22 | 0.28 | 18.7 | 37.6 | - | ||
| Species | 37 | Fire | DBH | 0.70 | 0.65 | 38.4 | 67.8 | - | |
| 53 | No Fire | MAT + DBH | 0.36 | 0.40 | 12.0 | 18.1 | CRUobs | ||
| 49 | Other | MAT + DBH + PFT | 0.43 | - | 11.4 | - | CRUobs | ||
| MAT + DBH | 0.35 | 0.38 | 12.4 | 18.8 | CRUobs | ||||
| Mortality | Model | R2 | ∆AIC | RMSE | Nobs | Climate | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Count | Size | Count | Size | Count | Size | |||||
| Species | Other | MAT + PFT + DBH | 0.55 | 0.55 | 0 | 0 | 13.0 | 19.7 | 60 | CRUobs |
| MATSoil + DBH | - | 0.50 | - | 5.2 | - | 21.8 | - | |||
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.A.M.; Arneth, A.; Kautz, M.; Poulter, B.; Smith, B. Important Role of Forest Disturbances in the Global Biomass Turnover and Carbon Sinks. Nat. Geosci. 2019, 12, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Hubau, W.; Lewis, S.L.; Phillips, O.L.; Affum-Baffoe, K.; Beeckman, H.; Cuní-Sanchez, A.; Daniels, A.K.; Ewango, C.E.N.; Fauset, S.; Mukinzi, J.M.; et al. Asynchronous Carbon Sink Saturation in African and Amazonian Tropical Forests. Nature 2020, 579, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Tagesson, T.; Schurgers, G.; Horion, S.; Ciais, P.; Tian, F.; Brandt, M.; Ahlström, A.; Wigneron, J.P.; Ardö, J.; Olin, S.; et al. Recent Divergence in the Contributions of Tropical and Boreal Forests to the Terrestrial Carbon Sink. Nat. Ecol. Evol. 2020, 4, 202–209. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Mackensen, J.; Bauhus, J.; Webber, E. Decomposition Rates of Coarse Woody Debris - A Review with Particular Emphasis on Australian Tree Species. Aust J Bot 2003, 51, 27–37. [Google Scholar] [CrossRef]
- Hararuk, O.; Kurz, W.A.; Didion, M. Dynamics of Dead Wood Decay in Swiss Forests. For. Ecosyst. 2020, 7, 36. [Google Scholar] [CrossRef]
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodall, C.W. Release of Coarse Woody Detritus-Related Carbon: A Synthesis across Forest Biomes. Carbon Balance Manag. 2020, 15, 1. [Google Scholar] [CrossRef]
- Yatskov, M.; Harmon, M.E.; Krankina, O.N. A Chronosequence of Wood Decomposition in the Boreal Forests of Russia. Can. J. For. Res. 2003, 33, 1211–1226. [Google Scholar] [CrossRef]
- Vanderwel, M.C.; Malcolm, J.R.; Smith, S.M. An Integrated Model for Snag and Downed Woody Debris Decay Class Transitions. Ecol. Manag. 2006, 234, 48–59. [Google Scholar] [CrossRef]
- Mukhortova, L.V.; Kirdianov, A.V.; Myglan, V.S.; Guggenberger, G. Wood Transformation in Dead-Standing Trees in the Forest-Tundra of Central Siberia. Biol. Bull. 2009, 36, 70–78. [Google Scholar] [CrossRef]
- Hararuk, O.; Shaw, C.; Kurz, W.A. Constraining the Organic Matter Decay Parameters in the CBM-CFS3 Using Canadian National Forest Inventory Data and a Bayesian Inversion Technique. Ecol. Model. 2017, 364, 1–12. [Google Scholar] [CrossRef]
- Song, Z.; Dunn, C.; Lü, X.-T.T.; Qiao, L.; Pang, J.-P.P.; Tang, J.-W.W. Coarse Woody Decay Rates Vary by Physical Position in Tropical Seasonal Rainforests of SW China. Ecol. Manag. 2017, 385, 206–213. [Google Scholar] [CrossRef]
- Gora, E.M.; Lucas, J.M.; Yanoviak, S.P. Microbial Composition and Wood Decomposition Rates Vary with Microclimate From the Ground to the Canopy in a Tropical Forest. Ecosystems 2019, 22, 1206–1219. [Google Scholar] [CrossRef]
- Oberle, B.; Covey, K.R.; Dunham, K.M.; Hernandez, E.J.; Walton, M.L.; Young, D.F.; Zanne, A.E. Dissecting the Effects of Diameter on Wood Decay Emphasizes the Importance of Cross-Stem Conductivity in Fraxinus Americana. Ecosystems 2018, 21, 85–97. [Google Scholar] [CrossRef]
- Woodall, C.W.; Domke, G.M.; MacFarlane, D.W.; Oswalt, C.M. Comparing Field-and Model-Based Standing Dead Tree Carbon Stock Estimates across Forests of the US. Forestry 2012, 85, 125–133. [Google Scholar] [CrossRef]
- Aakala, T.; Kuuluvainen, T.; Gauthier, S.; De Grandpré, L. Standing Dead Trees and Their Decay-Class Dynamics in the Northeastern Boreal Old-Growth Forests of Quebec. Ecol. Manag. 2008, 255, 410–420. [Google Scholar] [CrossRef]
- Linder, P. Structural Changes in Two Virgin Boreal Forest Stands in Central Sweden over 72 Years. Scand. J. For. Res. 1998, 13, 451–461. [Google Scholar] [CrossRef]
- Josefsson, T.; Gunnarson, B.; Liedgren, L.; Bergman, I.; Östlund, L. Historical Human Influence on Forest Composition and Structure in Boreal Fennoscandia. Can. J. For. Res. 2010, 40, 872–884. [Google Scholar] [CrossRef]
- Cook, G.D.; Liedloff, A.C.; Meyer, C.P.M.; Richards, A.E.; Bray, S.G. Standing Dead Trees Contribute Significantly to Carbon Budgets in Australian Savannas. Int. J. Wildland Fire 2020, 29, 215–228. [Google Scholar] [CrossRef]
- Delaney, M.; Brown, S.; Lugo, A.E.; Torres-Lezama, A.; Quintero, N.B. The Quantity and Turnover of Dead Wood in Permanent Forest Plots in Six Life Zones of Venezuela 1. Biotropica 1998, 30, 2–11. [Google Scholar] [CrossRef]
- Gora, E.M.; Kneale, R.C.; Larjavaara, M.; Muller-Landau, H.C. Dead Wood Necromass in a Moist Tropical Forest: Stocks, Fluxes, and Spatiotemporal Variability. Ecosystems 2019, 22, 1189–1205. [Google Scholar] [CrossRef]
- Harmon, M.E.; Krankina, O.N.; Sexton, J. Decomposition Vectors: A New Approach to Estimating Woody Detritus Decomposition Dynamics. Can. J. For. Res. 2000, 30, 76–84. [Google Scholar] [CrossRef]
- Bradford, M.A.; Warren, R.J.; Baldrian, P.; Crowther, T.W.; Maynard, D.S.; Oldfield, E.E.; Wieder, W.R.; Wood, S.A.; King, J.R. Climate Fails to Predict Wood Decomposition at Regional Scales. Nat. Clim. Chang. 2014, 4, 625–630. [Google Scholar] [CrossRef]
- Krankina, O.N.; Harmon, M.E. Dynamics of the Dead Wood Carbon Pool in Northwestern Russian Boreal Forests. Water Air Soil Pollut. 1995, 82, 227–238. [Google Scholar] [CrossRef]
- Storaunet, K.O.; Rolstad, J. Time since Death and Fall of Norway Spruce Logs in Old-Growth and Selectively Cut Boreal Forest. Can. J. For. Res. 2002, 32, 1801–1812. [Google Scholar] [CrossRef]
- Boulanger, Y.; Sirois, L. Postfire Dynamics of Black Spruce Coarse Woody Debris in Northern Boreal Forest of Quebec. Can. J. For. Res. 2006, 36, 1770–1780. [Google Scholar] [CrossRef]
- Angers, V.A.; Drapeau, P.; Bergeron, Y. Snag Degradation Pathways of Four North American Boreal Tree Species. Ecol. Manag. 2010, 259, 246–256. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Phillips, O.L.; Feldpausch, T.R.; Gloor, E.; Baker, T.R.; Lloyd, J.; Lopez-Gonzalez, G.; Monteagudo-Mendoza, A.; Malhi, Y.; Lewis, S.L.; et al. Long-Term Decline of the Amazon Carbon Sink. Nature 2015, 519, 344–348. [Google Scholar] [CrossRef] [PubMed]
- van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.M. Mechanical Stability of Trees under Static Loads. Am. J. Bot. 2006, 93, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. Adv. Ecol. Res. 1986, 15, 135–305. [Google Scholar] [CrossRef]
- Zanne, A.E.; Oberle, B.; Dunham, K.M.; Milo, A.M.; Walton, M.L.; Young, D.F. A Deteriorating State of Affairs: How Endogenous and Exogenous Factors Determine Plant Decay Rates. J. Ecol. 2015, 103, 1421–1431. [Google Scholar] [CrossRef]
- Hu, Z.; Michaletz, S.T.; Johnson, D.J.; McDowell, N.G.; Huang, Z.; Zhou, X.; Xu, C. Traits Drive Global Wood Decomposition Rates More than Climate. Glob. Chang. Biol. 2018, 24, 5259–5269. [Google Scholar] [CrossRef]
- Tuomi, M.; Laiho, R.; Repo, A.; Liski, J. Wood Decomposition Model for Boreal Forests. Ecol. Model. 2011, 222, 709–718. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.C.C.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global Meta-Analysis of Wood Decomposition Rates: A Role for Trait Variation among Tree Species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef]
- Kahl, T.; Baber, K.; Otto, P.; Wirth, C.; Bauhus, J. Drivers of CO2 Emission Rates from Dead Wood Logs of 13 Tree Species in the Initial Decomposition Phase. Forests 2015, 6, 2484–2504. [Google Scholar] [CrossRef]
- Kipping, L.; Gossner, M.M.; Koschorreck, M.; Muszynski, S.; Maurer, F.; Weisser, W.W.; Jehmlich, N.; Noll, M. Emission of CO2 and CH4 From 13 Deadwood Tree Species Is Linked to Tree Species Identity and Management Intensity in Forest and Grassland Habitats. Glob. Biogeochem. Cycles 2022, 36, e2021GB007143. [Google Scholar] [CrossRef]
- Scheffer, T.C.; Cowling, E.B. Natural Resistance of Wood to Microbial Deterioration. Annu. Rev. Phytopathol. 1966, 4, 147–168. [Google Scholar] [CrossRef]
- Scheffer, T.C.; Morrell, J.J. Natural Durability of Wood: A Worldwide Checklist of Species; Research Contribution 22; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1998. [Google Scholar]
- Oberle, B.; Ogle, K.; Zanne, A.E.; Woodall, C.W. When a Tree Falls: Controls on Wood Decay Predict Standing Dead Tree Fall and New Risks in Changing Forests. PLoS ONE 2018, 13, e0196712. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, T.; Olsson, J.; Östlund, L. Linking Forest History and Conservation Efforts: Long-Term Impact of Low-Intensity Timber Harvest on Forest Structure and Wood-Inhabiting Fungi in Northern Sweden. Biol. Conserv. 2010, 143, 1803–1811. [Google Scholar] [CrossRef]
- Pastorelli, R.; Paletto, A.; Agnelli, A.E.; Lagomarsino, A.; De Meo, I. Microbial Diversity and Ecosystem Functioning in Deadwood of Black Pine of a Temperate Forest. Forests 2021, 12, 1418. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Preisler, H.K. Fall Rate of Lodgepole Pine Killed by the Mountain Pine Beetle in Central Oregon. West. J. Appl. For. 1998, 13, 23–26. [Google Scholar] [CrossRef]
- Wei, X.; Kimmins, J.P. Asymbiotic Nitrogen Fixation in Harvested and Wildfire-Killed Lodgepole Pine Forests in the Central Interior of British Columbia. Ecol. Manag. 1998, 109, 343–353. [Google Scholar] [CrossRef]
- Hanula, J.L.; Ulyshen, M.D.; Wade, D.D. Impacts of Prescribed Fire Frequency on Coarse Woody Debris Volume, Decomposition and Termite Activity in the Longleaf Pine Flatwoods of Florida. Forests 2012, 3, 317–331. [Google Scholar] [CrossRef]
- Schroeder, L.M. Retention or Salvage Logging of Standing Trees Killed by the Spruce Bark Beetle Ips Typographus: Consequences for Dead Wood Dynamics and Biodiversity. Scand. J. For. Res. 2007, 22, 524–530. [Google Scholar] [CrossRef]
- Hilger, A.B.; Shaw, C.H.; Metsaranta, J.M.; Kurz, W.A. Estimation of Snag Carbon Transfer Rates by Ecozone and Lead Species for Forests in Canada. Ecol. Appl. 2012, 22, 2078–2090. [Google Scholar] [CrossRef]
- Smith, C.Y.; Moroni, M.T.; Warkentin, I.G. Snag Dynamics in Post-Harvest Landscapes of Western Newfoundland Balsam Fir-Dominated Boreal Forests. Ecol. Manag. 2009, 258, 832–839. [Google Scholar] [CrossRef]
- Cline, S.P.; Berg, A.B.; Wight, H.M. Snag Characteristics and Dynamics in Douglas-Fir Forests, Western Oregon. J. Wildl. Manag. 1980, 44, 773. [Google Scholar] [CrossRef]
- Everett, R.; Lehmkuhl, J.; Schellhaas, R.; Ohlson, P.; Keenum, D.; Riesterer, H.; Spurbeck, D. Snag Dynamics in a Chronosequence of 26 Wildfires on the East Slope of the Cascade Range in Washington State, USA. Int. J. Wildland Fire 1999, 9, 223–234. [Google Scholar] [CrossRef]
- Huggard, D.J. Static Life-Table Analysis of Fall Rates of Subalpine Fir Snags. Ecol. Appl. 1999, 9, 1009–1016. [Google Scholar] [CrossRef]
- Sinclair, R. Persistence of Dead Trees and Fallen Timber in the Arid Zone: 76 Years of Data from the T.G.B. Osborn Vegetation Reserve, Koonamore, South Australia. Rangel. J. 2004, 26, 111–122. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J.; Siitonen, J.; Sievänen, R. Predicting the Decomposition of Scots Pine, Norway Spruce, and Birch Stems in Finland. Ecol. Appl. 2006, 16, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Gower, S.T. Decomposition and Fragmentation of Coarse Woody Debris: Re-Visiting a Boreal Black Spruce Chronosequence. Ecosystems 2008, 11, 831–840. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Lieffers, V.J.; Wein, R.W. Dendrochronological Reconstruction of Jack Pine Snag and Downed Log Dynamics in Saskatchewan and Manitoba, Canada. Ecol. Manag. 2008, 255, 1262–1270. [Google Scholar] [CrossRef]
- DeLong, S.C.; Sutherland, G.D.; Daniels, L.D.; Heemskerk, B.H.; Storaunet, K.O. Temporal Dynamics of Snags and Development of Snag Habitats in Wet Spruce-Fir Stands in East-Central British Columbia. Ecol. Manag. 2008, 255, 3613–3620. [Google Scholar] [CrossRef]
- Lyon, L.J. Attrition of Lodgepole Pine Snags on the Sleeping Child Burn, Montana; Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1977; Volume 219.
- Yamasaki, M.; Leak, W.B. Snag Longevity in Managed Northern Hardwoods. North. J. Appl. For. 2006, 23, 215–217. [Google Scholar] [CrossRef]
- Onodera, K.; Tokuda, S. Do Larger Snags Stand Longer?—Snag Longevity in Mixed Conifer–Hardwood Forests in Hokkaido, Japan. Ann. Sci. 2015, 72, 621–629. [Google Scholar] [CrossRef]
- Slaughter, K.W.; Grigal, D.F.; Ohmann, L.F. Carbon Storage in Southern Boreal Forests Following Fire. Scand. J. For. Res. 1998, 13, 119–127. [Google Scholar] [CrossRef]
- Parish, R.; Antos, J.A.; Ott, P.K.; Lucca, C.M. Di Snag Longevity of Douglas-Fir, Western Hemlock, and Western Redcedar from Permanent Sample Plots in Coastal British Columbia. Ecol. Manag. 2010, 259, 633–640. [Google Scholar] [CrossRef]
- Raphael, M.; Morrison, M. Decay and Dynamics of Snags in the Sierra Nevada, California. For. Sci. 1987, 33, 774–783. [Google Scholar]
- Bull, E.B. Longevity of Snags and Their Use by Woodpeckers. In Snag Habitat Management: Proceedings of the Symposium, USDA Forestry Service General Technical Report RM-99; Coordinators, T., Davis, J., Goodwin, G., Ockenfels, R., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1983; pp. 64–67. [Google Scholar]
- Kearns, H.S.J.; Jacobi, W.R.; Johnson, D.W. Persistence of Pinyon Pine Snags and Logs in Southwestern Colorado. West. J. Appl. For. 2005, 20, 247–252. [Google Scholar] [CrossRef]
- Harrington, M.G. Fall Rates of Prescribed Fire-Killed Ponderosa Pine; United States Department of Agriculture-Forest Service: Washington, DC, USA, 1996.
- Woolley, T.; Shaw, D.C.; Hollingsworth, L.W.T.; Agne, M.C.; Fitzgerald, S.; Eglitis, A.; Kurth, L. Beyond Red Crowns: Complex Changes in Surface and Crown Fuels and Their Interactions 32 Years Following Mountain Pine Beetle Epidemics in South-Central Oregon, USA. Fire Ecol. 2019, 15, 4. [Google Scholar] [CrossRef]
- Garber, S.M.; Brown, J.P.; Wilson, D.S.; Maguire, D.A.; Heath, L.S. Snag Longevity under Alternative Silvicultural Regimes in Mixed-Species Forests of Central Maine. Can. J. For. Res. 2005, 35, 787–796. [Google Scholar] [CrossRef]
- Moorman, C.E.; Russell, K.R.; Sabin, G.R.; Guynn, D.C., Jr. Snag Dynamics and Cavity Occurrence in the South Carolina Piedmont. Ecol. Manag. 1999, 118, 37–48. [Google Scholar] [CrossRef]
- Wilson, B.F.; McComb, B.C. Dynamics of Dead Wood over 20 Years in a New England Oak Forest. Can. J. For. Res. 2005, 35, 682–692. [Google Scholar] [CrossRef]
- Taylor, S.L.; MacLean, D.A. Dead Wood Dynamics in Declining Balsam Fir and Spruce Stands in New Brunswick, Canada. Can. J. For. Res. 2007, 37, 750–762. [Google Scholar] [CrossRef]
- Conner, R.N.; Saenz, D. The Longevity of Large Pine Snags in Eastern Texas. Wildl. Soc. Bull. 2005, 33, 700–705. [Google Scholar] [CrossRef]
- Dunn, C.J.; Bailey, J.D. Temporal Dynamics and Decay of Coarse Wood in Early Seral Habitats of Dry-Mixed Conifer Forests in Oregon’s Eastern Cascades. Ecol. Manag. 2012, 276, 71–81. [Google Scholar] [CrossRef]
- Dunn, C.J.; Bailey, J.D. Temporal Fuel Dynamics Following High-Severity Fire in Dry Mixed Conifer Forests of the Eastern Cascades, Oregon, USA. Int. J. Wildland Fire 2015, 24, 470–483. [Google Scholar] [CrossRef]
- Hart, J.H.; Hart, D.L. Heartrot Fungi’s Role in Creating Picid Nesting Sites in Living Aspen. In Sustaining Aspen in Western Landscapes: Symposium; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2001; pp. 207–213. [Google Scholar]
- Holeksa, J.; Zielonka, T.; Zywiec, M. Modeling the Decay of Coarse Woody Debris in a Subalpine Norway Spruce Forest of the West Carpathians, Poland. Can. J. For. Res. 2008, 38, 415–428. [Google Scholar] [CrossRef]
- Dunn, C.J.; Bailey, J.D. Tree Mortality and Structural Change Following Mixed-Severity Fire in Pseudotsuga Forests of Oregon’s Western Cascades, USA. Ecol. Manag. 2016, 365, 107–118. [Google Scholar] [CrossRef]
- Landram, F.M.; Laudenslayer, W.F.; Atzet, T. Demography of Snags in Eastside Pine Forests of California. In General Technical Report PSW-GTR-181, Proceedings of the Symposium on the Ecology and Management of Dead Wood in Western Forests, Reno, NV, USA, 2–4 November 1999; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2002. [Google Scholar]
- Siccama, T.G.; Fahey, T.J.; Johnson, C.E.; Sherry, T.W.; Denny, E.G.; Girdler, E.B.; Likens, G.E.; Schwarz, P.A. Population and Biomass Dynamics of Trees in a Northern Hardwood Forest at Hubbard Brook. Can. J. For. Res. 2007, 37, 737–749. [Google Scholar] [CrossRef]
- Brown, M.J.; Kertis, J.; Huff, M.H. Natural Tree Regeneration and Coarse Woody Debris Dynamics after a Forest Fire in the Western Cascade Range; United States Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2013; Volume 592, pp. 1–50. [CrossRef]
- Chambers, C.L.; Mast, J.N. Snag Dynamics and Cavity Excavation after Bark Beetle Outbreaks in Southwestern Ponderosa Pine Forests. For. Sci. 2014, 60, 713–723. [Google Scholar] [CrossRef]
- Stevens-Rumann, C.S.; Hudak, A.T.; Morgan, P.; Arnold, A.; Strand, E.K. Fuel Dynamics Following Wildfire in US Northern Rockies Forests. Front. For. Glob. Chang. 2020, 3, 51. [Google Scholar] [CrossRef]
- Russell, R.E.; Saab, V.A.; Dudley, J.G.; Rotella, J.J. Snag Longevity in Relation to Wildfire and Postfire Salvage Logging. Ecol. Manag. 2006, 232, 179–187. [Google Scholar] [CrossRef]
- Dobbertin, M.; Baltensweiler, A.; Rigling, D. Tree Mortality in an Unmanaged Mountain Pine (Pinus Mugo Var. Uncinata) Stand in the Swiss National Park Impacted by Root Rot Fungi. Ecol. Manag. 2001, 145, 79–89. [Google Scholar] [CrossRef]
- Acker, S.A.; Kertis, J.; Bruner, H.; O’Connell, K.; Sexton, J. Dynamics of Coarse Woody Debris Following Wildfire in a Mountain Hemlock (Tsuga Mertensiana) Forest. Ecol. Manag. 2013, 302, 231–239. [Google Scholar] [CrossRef]
- Zarnoch, S.J.; Vukovich, M.A.; Kilgo, J.C.; Blake, J.I. Snag Characteristics and Dynamics Following Natural and Artificially Induced Mortality in a Managed Loblolly Pine Forest. Can. J. For. Res. 2013, 43, 817–825. [Google Scholar] [CrossRef]
- Chambers, C.L.; Mast, J.N. Ponderosa Pine Snag Dynamics and Cavity Excavation Following Wildfire in Northern Arizona. Ecol. Manag. 2005, 216, 227–240. [Google Scholar] [CrossRef]
- Nelson, K.N.; Turner, M.G.; Romme, W.H.; Tinker, D.B. Landscape Variation in Tree Regeneration and Snag Fall Drive Fuel Loads in 24-Year Old Post-Fire Lodgepole Pine Forests. Ecol. Appl. 2016, 26, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Newbery, J.E.; Lewis, K.J.; Walters, M.B. Inonotus Tomentosus and the Dynamics of Unmanaged and Partial-Cut Wet Sub-Boreal Spruce-Fir Forests. Can. J. For. Res. 2007, 37, 2663–2676. [Google Scholar] [CrossRef]
- Angers, V.A.; Gauthier, S.; Drapeau, P.; Jayen, K.; Bergeron, Y. Tree Mortality and Snag Dynamics in North American Boreal Tree Species after a Wildfire: A Long-Term Study. Int. J. Wildland Fire 2011, 20, 751. [Google Scholar] [CrossRef]
- Ganey, J.L.; Vojta, S.C. Trends in Snag Populations in Drought-Stressed Mixed-Conifer and Ponderosa Pine Forests (1997–2007). Int. J. For. Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Hogg, E.H.; Michaelian, M. Factors Affecting Fall down Rates of Dead Aspen (Populus Tremuloides) Biomass Following Severe Drought in West-Central Canada. Glob. Chang. Biol. 2015, 21, 1968–1979. [Google Scholar] [CrossRef]
- Grayson, L.M.; Cluck, D.R.; Hood, S.M. Persistence of Fire-Killed Conifer Snags in California, USA. Fire Ecol. 2019, 15, 1–14. [Google Scholar] [CrossRef]
- Palace, M.; Keller, M.; Silva, H. Necromass Production: Studies in Undisturbed and Logged Amazon Forests. Ecol. Appl. 2008, 18, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.W.; Knapp, E.E. Establishment of a Long-Term Fire Salvage Study in an Interior Ponderosa Pine Forest. J. For. 2014, 112, 395–400. [Google Scholar] [CrossRef]
- Campbell, J.L.; Fontaine, J.B.; Donato, D.C. Carbon Emissions from Decomposition of Fire-Killed Trees Following a Large Wildfire in Oregon, United States. J. Geophys. Res. Biogeosci. 2016, 121, 718–730. [Google Scholar] [CrossRef]
- Fassnacht, K.S.; Steele, T.W. Snag Dynamics in Northern Hardwood Forests under Different Management Scenarios. Ecol. Manag. 2016, 363, 267–276. [Google Scholar] [CrossRef]
- Perera, A.H.; Dalziel, B.D.; Buse, L.J.; Routledge, R.G.; Brienesse, M. What Happens to Tree Residuals in Boreal Forest Fires and What Causes the Changes? Forest Research Report No. 174; Ontario Ministry of Natural Resources: Sault Ste. Marie, ON, Canada, 2011; ISBN 0381-3924 978-1-4435-7268-2. [Google Scholar]
- Rhoades, C.C.; Hubbard, R.M.; Hood, P.R.; Starr, B.J.; Tinker, D.B.; Elder, K. Snagfall the First Decade after Severe Bark Beetle Infestation of High-Elevation Forests in Colorado, USA. Ecol. Appl. 2020, 30, e02059. [Google Scholar] [CrossRef] [PubMed]
- Molinas-González, C.R.; Leverkus, A.B.; Marañón-Jiménez, S.; Castro, J. Fall Rate of Burnt Pines across an Elevational Gradient in a Mediterranean Mountain. Eur. J. Res. 2017, 136, 401–409. [Google Scholar] [CrossRef]
- Corace, R.G.; Seefelt, N.E.; Goebel, P.C.; Shaw, H.L. Snag Longevity and Decay Class Development in a Recent Jack Pine Clearcut in Michigan. North. J. Appl. For. 2010, 27, 125–131. [Google Scholar] [CrossRef]
- Aakala, T. Coarse Woody Debris in Late-Successional Picea Abies Forests in Northern Europe: Variability in Quantities and Models of Decay Class Dynamics. Ecol. Manag. 2010, 260, 770–779. [Google Scholar] [CrossRef]
- Alexeyev, V.A.; Birdsey, R.A. (Eds.) Carbon Storage in Forests and Peatlands of Russia; General Technical Report NE-244; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Radnor, PA, USA, 1998; 137p.
- Hagemann, U.; Moroni, M.T.; Shaw, C.H.; Kurz, W.A.; Makeschin, F. Comparing Measured and Modelled Forest Carbon Stocks in High-Boreal Forests of Harvest and Natural-Disturbance Origin in Labrador, Canada. Ecol. Model. 2010, 221, 825–839. [Google Scholar] [CrossRef]
- Hall, S.A.; Burke, C.; Hobbs, N.T. Litter and Dead Wood Dynamics in Ponderosa Pine Forests along a 160-Year Chronosequence. Ecol. Appl. 2006, 16, 2344–2355. [Google Scholar] [CrossRef]
- Lee, P. Dynamics of Snags in Aspen-Dominated Midboreal Forests. Ecol. Manag. 1998, 105, 263–272. [Google Scholar] [CrossRef]
- Mellen, K.; Ager, A. A Coarse Wood Dynamics Model for the Western Cascades. In General Technical Report PSW-GTR-181, Proceedings of the Symposium on the Ecology and Management of Dead Wood in Western Forests, Reno, NV, USA, 2–4 November 1999; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2002; Volume 97801, pp. 503–516. [Google Scholar]
- Moroni, M.T. Disturbance History Affects Dead Wood Abundance in Newfoundland Boreal Forests. Can. J. For. Res. 2006, 36, 3194–3208. [Google Scholar] [CrossRef]
- Storaunet, K.O.; Rolstad, J. How Long Do Norway Spruce Snags Stand? Evaluating Four Estimation Methods. Can. J. For. Res. 2004, 34, 376–383. [Google Scholar] [CrossRef]
- Vanderwel, M.C.; Caspersen, J.P.; Woods, M.E. Snag Dynamics in Partially Harvested and Unmanaged Northern Hardwood Forests. Can. J. For. Res. 2006, 36, 2769–2779. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.W.; Knapp, E.E.; Skinner, C.N. Snag Longevity and Surface Fuel Accumulation Following Post-Fire Logging in a Ponderosa Pine Dominated Forest. Ecol. Manag. 2013, 287, 113–122. [Google Scholar] [CrossRef]
- Lehmkuhl, J.F.; Everett, R.L.; Schellhaas, R.; Ohlson, P.; Keenum, D.; Riesterer, H.; Spurbeck, D. Cavities in Snags along a Wildfire Chronosequence in Eastern Washington. J. Wildl. Manag. 2003, 219–228. [Google Scholar] [CrossRef]
- Kenefic, L.; Brissette, J.; Russell, M.; Puhlick, J. Overstory Tree and Regeneration Data from the “Silvicultural Effects on Composition, Structure, and Growth”. In Penobscot Experimental Forest, 2nd ed.; Forest Service Research Data Archive; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2015. [Google Scholar]
- Bull, E.L. Habitat Utilization of the Pileated Woodpecker, Blue Mountains, Oregon. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1975. [Google Scholar]
- Viovy, N. CRUNCEP Version 7—Atmospheric Forcing Data for the Community Land Model. Available online: https://rda.ucar.edu/datasets/ds314.3/ (accessed on 27 April 2022).
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas [Data Set]. EnviDat. Available online: https://envidat.ch/#/metadata/chelsa-climatologies (accessed on 21 April 2022).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; van den Hoogen, J.; Aalto, J.; Ashcroft, M.B.; De Frenne, P.; Kemppinen, J.; Kopecký, M.; Luoto, M.; Maclean, I.M.D.; Crowther, T.W.; et al. Global Maps of Soil Temperature. Glob. Chang. Biol. 2022, 28, 3110–3144. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, J.; Lembrechts, J.; Nijs, I.; Lenoir, J. Global Soil Bioclimatic Variables at 30 Arc Second Resolution Version 1 [Data Set]. Available online: https://zenodo.org/record/4558732#.ZGIJLqVByUk (accessed on 21 April 2022).
- Guglielmo, M.; Tang, F.H.M.; Pasut, C.; Maggi, F. SOIL-WATERGRIDS, Mapping Dynamic Changes in Soil Moisture and Depth of Water Table from 1970 to 2014. Sci. Data 2021, 8, 263. [Google Scholar] [CrossRef]
- Maggi, F.; Guglielmo, M.; Tang, F.H.M.M.; Pasut, C. SOIL-WATERGRIDS v1, Mapping Dynamic Changes in Soil Moisture and Depth of Water Table from 1970 to 2014, Dataset and Modelling (Version v1) [Data Set]. Available online: https://zenodo.org/record/4997453#.ZGIJ3qVByUk (accessed on 25 January 2023).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY Plant Trait Database – Enhanced Coverage and Open Access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Rohatgi, A. Webplotdigitizer: Version 4.6 [Computer Software]. Available online: https://automeris.io/WebPlotDigitizer (accessed on 22 September 2021).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Chojnacky, D.C.; Heath, L.S.; Jenkins, J.C. Updated Generalized Biomass Equations for North American Tree Species. Forestry 2014, 87, 129–151. [Google Scholar] [CrossRef]
- Kershaw, J.A.; Ducey, M.J.; Beers, T.W.; Husch, B. Forest Mensuration; Wiley-Blackwell: Oxford, UK, 2017; ISBN 9781118902035. [Google Scholar]
- Chambers, J.Q.; Higuchi, N.; Schimel, J.P.; Ferreira, L.V.; Melack, J.M. Decomposition and Carbon Cycling of Dead Trees in Tropical Forests of the Central Amazon. Oecologia 2000, 122, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Burnham, K.P.; Anderson, D. Model Selection and Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Richards, S.A. Testing Ecological Theory Using the Information-Theoretic Approach: Examples and Cautionary Results. Ecology 2005, 86, 2805–2814. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities and Ecosystems; Macmillan: New York, NY, USA, 1970. [Google Scholar]
- Lustenhouwer, N.; Maynard, D.S.; Bradford, M.A.; Lindner, D.L.; Oberle, B.; Zanne, A.E.; Crowther, T.W. A Trait-Based Understanding of Wood Decomposition by Fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 11551–11558. [Google Scholar] [CrossRef]
- Jurgensen, M.; Reed, D.; Page-Dumroese, D.; Laks, P.; Collins, A.; Mroz, G.; Degórski, M. Wood Strength Loss as a Measure of Decomposition in Northern Forest Mineral Soil. Eur. J. Soil Biol. 2006, 42, 23–31. [Google Scholar] [CrossRef]
- Bradford, M.A.; Maynard, D.S.; Crowther, T.W.; Frankson, P.T.; Mohan, J.E.; Steinrueck, C.; Veen, G.F.; King, J.R.; Warren, R.J. Belowground Community Turnover Accelerates the Decomposition of Standing Dead Wood. Ecology 2021, 102, e03484. [Google Scholar] [CrossRef]
- Oettel, J.; Zolles, A.; Gschwantner, T.; Lapin, K.; Kindermann, G.; Schweinzer, K.M.; Gossner, M.M.; Essl, F. Dynamics of Standing Deadwood in Austrian Forests under Varying Forest Management and Climatic Conditions. J. Appl. Ecol. 2023, 60, 696–713. [Google Scholar] [CrossRef]
- Wang, C.; Bond-Lamberty, B.; Gower, S.T. Environmental Controls on Carbon Dioxide Flux from Black Spruce Coarse Woody Debris. Oecologia 2002, 132, 374–381. [Google Scholar] [CrossRef]
- Oettel, J.; Lapin, K.; Kindermann, G.; Steiner, H.; Schweinzer, K.M.; Frank, G.; Essl, F. Patterns and Drivers of Deadwood Volume and Composition in Different Forest Types of the Austrian Natural Forest Reserves. Ecol. Manag. 2020, 463, 118016. [Google Scholar] [CrossRef]
- Loescher, H.; Ayres, E.; Duffy, P.; Luo, H.; Brunke, M. Spatial Variation in Soil Properties among North American Ecosystems and Guidelines for Sampling Designs. PLoS ONE 2014, 9, e83216. [Google Scholar] [CrossRef] [PubMed]
- Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi. J. Fungi 2022, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Cantera, L.; Alonso, R.; Lupo, S.; Bettucci, L.; Amilivia, A.; Martínez, J.; Dieste, A. Decay Resistance of Thermally Modified Eucalyptus Grandis Wood against Wild Strains of Trametes Versicolor and Pycnoporus Sanguineus. Wood Mater. Sci. Eng. 2022, 17, 478–487. [Google Scholar] [CrossRef]
- Ananyev, V.A.; Timofeeva, V.V.; Kryshen’, A.M.; Pekkoev, A.N.; Kostina, E.E.; Ruokolainen, A.V.; Moshnikov, S.A.; Medvedeva, M.V.; Polevoi, A.V.; Humala, A.E. Fire Severity Controls Successional Pathways in a Fire-Affected Spruce Forest in Eastern Fennoscandia. Forests 2022, 13, 1775. [Google Scholar] [CrossRef]
- Hernández, D.L.; Hobbie, S.E. Effects of Fire Frequency on Oak Litter Decomposition and Nitrogen Dynamics. Oecologia 2008, 158, 535–543. [Google Scholar] [CrossRef]
- Edman, M.; Eriksson, A.M. Competitive Outcomes between Wood-Decaying Fungi Are Altered in Burnt Wood. FEMS Microbiol. Ecol. 2016, 92, fiw068. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Gérardin, P.; Zoulalian, A. Investigations of the Reasons for Fungal Durability of Heat-Treated Beech Wood. Polym. Degrad. Stab. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Wengel, M.; Kothe, E.; Schmidt, C.M.; Heide, K.; Gleixner, G. Degradation of Organic Matter from Black Shales and Charcoal by the Wood-Rotting Fungus Schizophyllum Commune and Release of DOC and Heavy Metals in the Aqueous Phase. Sci. Total Environ. 2006, 367, 383–393. [Google Scholar] [CrossRef]
- Monleon, V.J.; Cromack, K.J.; Landsberg, J.D. Short- and Long-Term Effects of Prescribed Underburning on Nitrogen Availability in Ponderosa Pine Stands in Central Oregon. Can. J. For. Res. 1997, 27, 369–378. [Google Scholar] [CrossRef]
- Ascough, P.L.; Sturrock, C.J.; Bird, M.I. Investigation of Growth Responses in Saprophytic Fungi to Charred Biomass. Isot. Environ. Health Stud. 2010, 46, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kymäläinen, M.; Havimo, M.; Keriö, S.; Kemell, M.; Solio, J. Biological Degradation of Torrefied Wood and Charcoal. Biomass Bioenergy 2014, 71, 170–177. [Google Scholar] [CrossRef]
- Presley, G.; Cappellazzi, J.; Eastin, I. Durability of Thermally Modified Western Hemlock Lumber Against Wood Decay Fungi. Front. For. Glob. Chang. 2022, 5, 30. [Google Scholar] [CrossRef]
- Boulanger, Y.; Sirois, L.; Hébert, C. Fire Severity as a Determinant Factor of the Decomposition Rate of Fire-Killed Black Spruce in the Northern Boreal Forest. Can. J. For. Res. 2011, 41, 370–379. [Google Scholar] [CrossRef]


| Climate | Wood Traits | ||
|---|---|---|---|
| Temperature | Moisture | Substrate Quality | Tree Size |
| MAT | MAP | PFT * | DBH |
| MATsoil | Soil water ‡ | Wood durability † | |
| Soil water max ‡ | |||
| Reporting Type | Key Assumption | np | |
|---|---|---|---|
| 1 | mean residence time | steady state | 4 |
| 2 | decay constant k | single negative exponential decay | 12 |
| 3 | mean annual fall rate (F, % y−1) | 8 | |
| 4 | single observations of percent of dead standing tree count, volume or mass remaining; single estimate of the probability to remain after n years (f) | 15 | |
| 5 | survival or persistence curves; data | other | 33 |
| Continent | np | Site | Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | PFT | Mortality | Managed | DBH | All | PFT | Mortality | Managed | DBH | ||
| Conifer | Fire | Conifer | Fire | ||||||||
| (n) | (n) | (%) | (%) | (%) | (cm) | (n) | (%) | (%) | (%) | (cm) | |
| North America | 57 | 113 | 84.1 | 25.7 | 15.9 | 23.2 | 153 | 68.6 | 40.5 | 22.2 | 26.9 |
| Europe | 10 | 15 | 93.3 | 13.3 | 33.3 | 26.2 | 17 | 70.6 | 11.8 | 23.5 | 23.6 |
| Asia | 1 | 1 | 100 | 0 | 0 | 27.6 | 7 | 28.6 | 0 | 0 | 26.4 |
| Oceania | 1 | 1 | 0 | 0 | 0 | - | 2 | 0 | 0 | 0 | - |
| South America | 1 | 1 | 0 | 0 | 0 | - | - | - | - | - | - |
| All | 70 | 131.0 | 84.0 | 23.7 | 17.6 | 23.5 | 179.0 | 66.5 | 35.8 | 24.1 | 26.8 |
| Nobs | Mortality | Model | R2 | RMSE | Climate | |||
|---|---|---|---|---|---|---|---|---|
| Count | Size | Count | Size | |||||
| Site | 64 | All | MAT + DBH + Managed | 0.36 | 0.42 | 23.4 | 49.2 | CRUobs |
| 17 | Fire | DBH | 0.54 | 0.47 | 21.0 | 54.9 | - | |
| 47 | No Fire | MAT | 0.32 | 0.37 | 23.0 | 45.0 | CRUobs | |
| 37 | Other | MAT | 0.36 | 0.43 | 24.4 | 47.8 | CRUobs | |
| Species | 107 | All | MAT + DBH + Managed | 0.61 | 0.60 | 25.9 | 43.9 | CRU |
| 37 | Fire | DBH | 0.70 | 0.65 | 38.4 | 67.8 | - | |
| 70 | No Fire | MAT + DBH + PFT | 0.54 | 0.54 | 12.4 | 18.8 | CRUobs | |
| 61 | Other | MAT + DBH + PFT | 0.58 | 0.58 | 12.5 | 19.0 | CRUobs | |
| Mortality | Intercept | DBH | MAT | PFT | Managed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Size | Count | Size | Count | Size | Count | Size | Count | Size | ||
| Site | All | 2.64 (0.22) | 3.25 (0.23) | 0.03 (0.01) | 0.03 (0.01) | −0.09 (0.02) | −0.11 (0.02) | - | - | −0.69 (0.21) | −0.78 (0.22) |
| Fire | 1.44 (0.37) | 2.13 (0.41) | 0.05 (0.01) | 0.05 (0.01) | - | - | - | - | - | - | |
| No Fire | 3.25 (0.15) | 3.90 (0.16) | - | - | −0.11 (0.02) | −0.13 (0.02) | - | - | - | - | |
| Other | 3.40 (0.16) | 4.07 (0.17) | - | - | −0.12 (0.03) | −0.15 (0.03) | - | - | - | - | |
| Species | All | 2.40 (0.13) | 2.71 (0.15) | 0.04 (0.00) | 0.04 (0.00) | −0.12 (0.02) | −0.13 (0.02) | - | - | −0.45 (0.13) | −0.49 (0.15) |
| Fire | 1.51 (0.22) | 1.83 (0.25) | 0.05 (0.00) | 0.05 (0.01) | - | - | - | - | - | - | |
| No Fire | 2.52 (0.16) | 2.83 (0.18) | 0.02 (0.01) | 0.02 (0.01) | −0.13 (0.02) | −0.14 (0.02) | 0.44 (0.14) | 0.43 (0.15) | - | - | |
| Other | 2.54 (0.17) | 2.87 (0.19) | 0.02 (0.01) | 0.02 (0.01) | −0.14 (0.02) | −0.15 (0.02) | 0.52 (0.14) | 0.52 (0.15) | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gärtner, A.; Jönsson, A.M.; Metcalfe, D.B.; Pugh, T.A.M.; Tagesson, T.; Ahlström, A. Temperature and Tree Size Explain the Mean Time to Fall of Dead Standing Trees across Large Scales. Forests 2023, 14, 1017. https://doi.org/10.3390/f14051017
Gärtner A, Jönsson AM, Metcalfe DB, Pugh TAM, Tagesson T, Ahlström A. Temperature and Tree Size Explain the Mean Time to Fall of Dead Standing Trees across Large Scales. Forests. 2023; 14(5):1017. https://doi.org/10.3390/f14051017
Chicago/Turabian StyleGärtner, Antje, Anna Maria Jönsson, Daniel B. Metcalfe, Thomas A. M. Pugh, Torbern Tagesson, and Anders Ahlström. 2023. "Temperature and Tree Size Explain the Mean Time to Fall of Dead Standing Trees across Large Scales" Forests 14, no. 5: 1017. https://doi.org/10.3390/f14051017
APA StyleGärtner, A., Jönsson, A. M., Metcalfe, D. B., Pugh, T. A. M., Tagesson, T., & Ahlström, A. (2023). Temperature and Tree Size Explain the Mean Time to Fall of Dead Standing Trees across Large Scales. Forests, 14(5), 1017. https://doi.org/10.3390/f14051017







