Survey Study Reveals High Prevalence of Heterobasidion Root Rot Infection in Scots Pine (Pinus sylvestris) Stands Established on Seemingly Low-Risk Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Collection and Identification of Infected Trees
2.3. Microscopy Analysis
2.4. Defoliation Assessment
2.5. Statistical Analysis
3. Results
3.1. The Prevalence of Heterobasidion Infection on Low-Risk Sites
3.2. Relation between Infection Frequency and Stand Characters (Site Index, Stand Age)
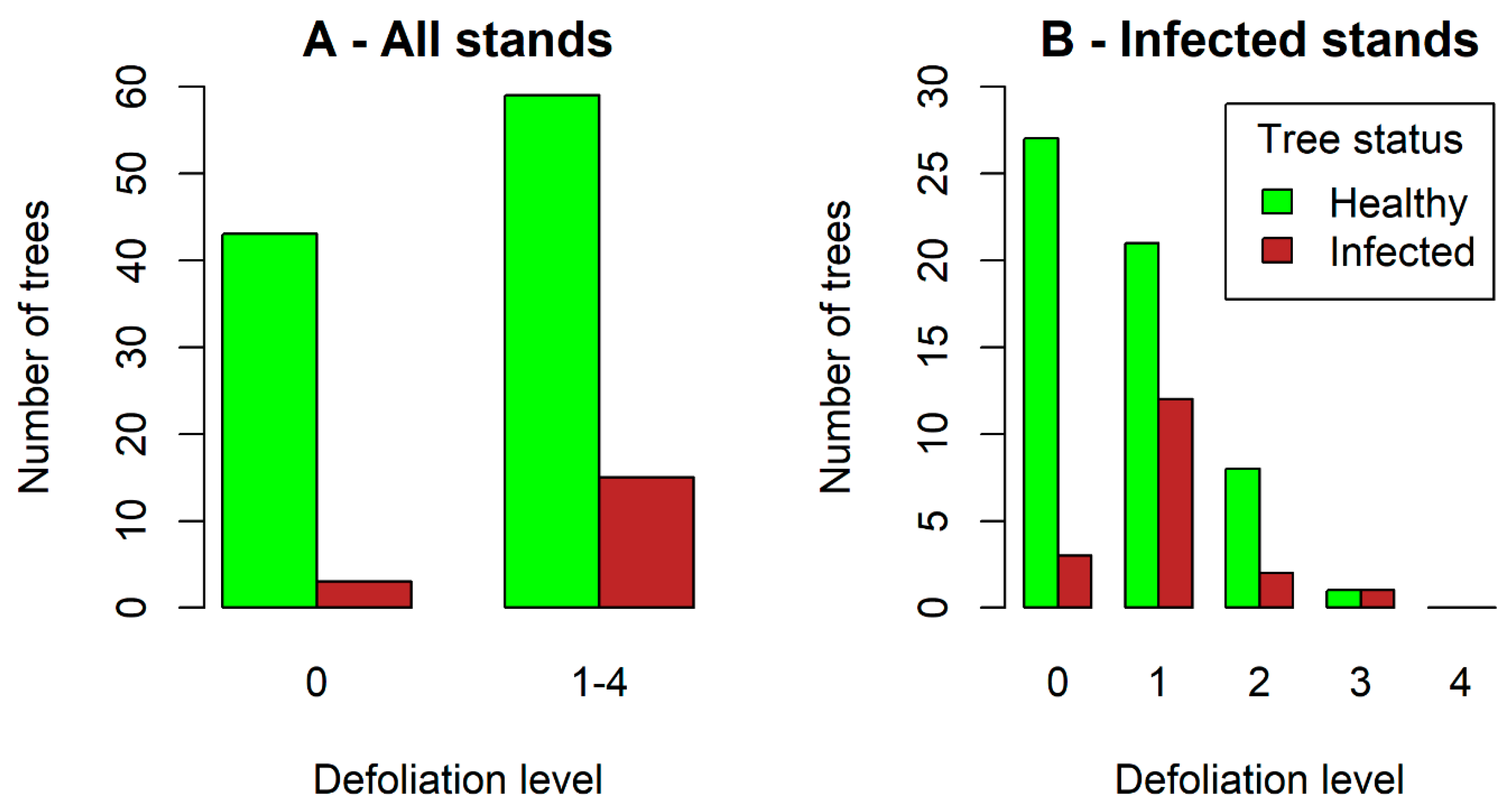
3.3. Relationship between Infection Frequency and Tree Characteristics (Tree Volume, Root Diameter, Crown Defoliation)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidakovic, M. Conifers morphology and variation. 1. überarb. Aufl. Zagreb 1991, 179–185. Available online: https://books.google.se/books/about/Conifers_Morphology_and_Variation.html?id=D7y1nQEACAAJ&redir_esc=y (accessed on 20 March 2023).
- Nilsson, P.; Roberge, C.; Fridman, J.; Wulff, S. Skogsdata 2020. Official Statistics of Sweden; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2020. [Google Scholar]
- Durrant, T.H.; De Rigo, D.; Caudullo, G. Pinus sylvestris in Europe: Distribution, habitat, usage and threats. Eur. Atlas For. Tree Species 2016, 14, 845–846. [Google Scholar]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. S.L. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef]
- Korhonen, K. Intersterility Groups of Heterobasidion annosum; 1978; Volume 94, No. 6; ISBN 9514003659. Available online: https://www.cabdirect.org/cabdirect/abstract/19820678136 (accessed on 20 March 2023).
- Werner, A.; Lakomy, P. Host specialization of is-group isolates of Heterobasidion annosum to Scots pine, Norway spruce and common fir in field inoculation experiments. Dendrobiology 2002, 47, 59–68. [Google Scholar]
- Müller, M.M.; Henttonen, H.M.; Penttilä, R.; Kulju, M.; Helo, T.; Kaitera, J. Distribution of Heterobasidion butt rot in northern finland. For. Ecol. Manag. 2018, 425, 85–91. [Google Scholar] [CrossRef]
- Piri, T. The spreading of the s type of Heterobasidion annosum from Norway spruce stumps to the subsequent tree stand. Eur. J. For. Pathol. 1996, 26, 193–204. [Google Scholar] [CrossRef]
- Rishbeth, J. Observations on the biology of Fomes annosus, with particular reference to east anglian pine plantations: II. Spore production, stump infection, and saprophytic activity in stumps. Ann. Bot. 1951, 15, 1–22. [Google Scholar] [CrossRef]
- Brandtberg, P.; Johansson, M.; Seeger, P. Effects of season and urea treatment on infection of stumps of Picea abies by Heterobasidion annosum in stands on former arable land. Scand. J. For. Res. 1996, 11, 261–268. [Google Scholar] [CrossRef]
- Isomäki, A.; Kallio, T. Consequences of Injury Caused by Timber Harvesting Machines on the Growth and Decay of Spruce (Picea abies (L.) Karst.). Acta For. Fenn. 1974, 7570. [Google Scholar] [CrossRef]
- Rishbeth, J. Observations on the biology of Fomes annosus, with particular reference to east anglian pine plantations: III. Natural and experimental infection of pines, and some factors affecting severity of the disease. Ann. Bot. 1951, 15, 221–246. [Google Scholar] [CrossRef]
- Paludan, F. Infektion og spredning af Fomes annosus i ung rødgran (Infection and spread of Fomes annosus in young Norway spruce). Det Forstl. Dan. 1966, 30, 21–47, (Danish with English Summary). [Google Scholar]
- Stener, L.-G.; Ahlberg, G. Study of Root and Butt Rot Frequency in Hybrid Larch Stands in Southern Sweden; Skogforsk: Uppsala, Sweden, 2002. [Google Scholar]
- Stenlid, J.; Wästerlund, I. Estimating the frequency of stem rot in Picea abies using an increment borer. Scand. J. For. Res. 1986, 1, 303–308. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Drobyshev, I.; Cleary, M.; Rönnberg, J. Incidence and impact of root infection by Heterobasidion spp., and the justification for preventative silvicultural measures on Scots pine trees: A case study in southern Sweden. For. Ecol. Manag. 2014, 315, 153–159. [Google Scholar] [CrossRef]
- Kurkela, T. Crown condition as an indicator of the incidence of root rot caused by Heterobasidion annosum in Scots pine stands. Silva Fenn. 2002, 36, 451–457. [Google Scholar] [CrossRef]
- Gibbs, J.N.; Greig, B.J.W.; Pratt, J.E. Fomes root rot in Thetford forest, East Anglia: Past, present and future. Forestry 2002, 75, 191–202. [Google Scholar] [CrossRef]
- Burdekin, D.A. A study of losses in Scots pine caused by Fomes annosus. Forestry 1972, 45, 189–196. [Google Scholar] [CrossRef]
- Rönnberg, J.; Petrylaite, E.; Nilsson, G.; Pratt, J. Two studies to assess the risk to Pinus sylvestris from Heterobasidion spp. in southern Sweden. Scand. J. For. Res. 2006, 21, 405–413. [Google Scholar] [CrossRef]
- Alexander, S.A.; Skelly, J.M.; Morris, C.L. Edaphic factors associated with the incidence and severity of disease caused by Fomes annosus in Loblolly pine plantations in Virginia. Phytopathology 1975, 65, 585–591. [Google Scholar] [CrossRef]
- Baker, F.A.; Verbyla, D.L.; Hodges, C.S., Jr.; Ross, E.W. Classification and regression tree analysis for assessing hazard of pine mortality caused by Heterobasidion annosum. Plant Dis. 1993, 77, 136–139. [Google Scholar] [CrossRef]
- Froelich, R.C.; Dell, T.R.; Walkinshaw, C.H. Soil factors associated with Fomes annosus in the Gulf States. For. Sci. 1966, 12, 356–361. [Google Scholar]
- Morris, C.L.; Frazier, D.H. Development of a hazard rating for Fomes annosus in Virginia. Plant Dis. Report. 1966, 50, 510. [Google Scholar]
- Redfern, D.B.; Pratt, J.E.; Hendry, S.J.; Low, J.D. Development of a policy and strategy for controlling infection by Heterobasidion annosum in british forests: A review of supporting research. Forestry 2010, 83, 207–218. [Google Scholar] [CrossRef]
- Wang, L. Impact of Heterobasidion spp. Root Rot in Conifer Trees and Assessment of Stump Treatment; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2012; Volume 97, ISBN 9157677441. [Google Scholar]
- Thor, M. Sverige Trea i Europa på Stubbehandling Mot Rotröta; Skogforsk: Uppsala, Sweden, 2001. [Google Scholar]
- Rishbeth, J. Stump protection against Fomes annosus: III. Inoculation with Peniophora gigantea. Ann. Appl. Biol. 1963, 52, 63–77. [Google Scholar] [CrossRef]
- Pellicciaro, M.; Lione, G.; Ongaro, S.; Gonthier, P. Comparative efficacy of state-of-the-art and new biological stump treatments in forests infested by the native and the alien invasive Heterobasidion species present in Europe. Pathogens 2021, 10, 1272. [Google Scholar] [CrossRef]
- Näslund, M. Skogsforskningsinstitutets Större Tabeller för Kubering av Stående Träd. 1950. V1. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201300214936 (accessed on 20 March 2023).
- Hanisch, B.; Kilz, E. Monitoring of Forest Damage: Spruce and Pine; Verlag Eugen Ulmer: Stuttgart, Germany, 1990; ISBN 3800133083. Available online: https://www.cabdirect.org/cabdirect/abstract/19910655657 (accessed on 20 March 2023).
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Siev, R. Predicting the activity of Heterobasidion parviporum on Norway spruce in warming climate from its respiration rate at different temperatures. For. Path. 2014, 44, 325–336. [Google Scholar] [CrossRef]
- Hägglund, B. Samband Mellan Ståndortsindex h100 Och Bonitet för Tall Och Gran i Sverige; Sveriges Lantbruksuniversitet: Uppsala, Sweden, 1981. [Google Scholar]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of spatial and temporal variability in natural site conditions. Forestry 2013, 86, 305–315. [Google Scholar] [CrossRef]
- Woodward, S. Heterobasidion annosum: Biology, Ecology, Impact, and Control; CABI: Wallingford, UK, 1998. [Google Scholar]
- Mattila, U.; Nuutinen, T. Assessing the Incidence of Butt Rot in Norway Spruce in Southern Finland. 2007. Available online: https://urn.fi/URN:NBN:fi-fe2016101425267 (accessed on 20 March 2023).
- Rishbeth, J. Some further observations on Fomes annosus Fr. Forestry 1957, 30, 69–89. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Julio Camarero, J.; Stenlid, J. Understanding the role of sapwood loss and reaction zone formation on radial growth of Norway spruce (Picea abies) trees decayed by Heterobasidion annosum s.l. For. Ecol. Manag. 2012, 274, 201–209. [Google Scholar] [CrossRef]
- Froelich, R.C.; Cowling, E.B.; Collicott, L.V.; Dell, T.R. Fomes annosus reduces height and diameter growth of planted slash pine. For. Sci. 1977, 23, 299–306. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Chapter 11. Absorption of water and ascent of sap. In Physiology of Woody Plants; Academic Press Inc.: San Diego, CA, USA, 1997; pp. 237–268. [Google Scholar]
- Bradford, B.; Skelly, J.M.; Alexander, S.A. Incidence and severity of annosus root rot in loblolly pine plantations in Virginia. Eur. J. For. Pathol. 1978, 8, 135–145. [Google Scholar] [CrossRef]
- Morrison, D.J.; Redfern, D.B. Long-term development of Heterobasidion annosum in basidiospore-infected Sitka spruce stumps. Plant Pathol. 1994, 43, 897–906. [Google Scholar] [CrossRef]
- Piri, T.; Vainio, E.J.; Nuorteva, H.; Hantula, J. High seedling mortality of Scots pine caused by Heterobasidion annosum s.s. Forests 2021, 12, 1289. [Google Scholar] [CrossRef]
- Bergmüller, K.O.; Vanderwel, M.C. Predicting tree mortality using spectral indices derived from multispectral UAV imagery. Remote Sens. 2022, 14, 2195. [Google Scholar] [CrossRef]
- Korhonen, K.; Lipponen, K.; Bendz, M.; Johansson, M.; Ryen, I.; Venn, K.; Seiskari, P.; Niemi, M. Control of Heterobasidion annosum by stump treatment with ’rotstop’, a new commercial formulation of Phlebiopsis gigantea. In Proceedings of the 8th International Conference on Root and Butt Rots, Uppsala, Sweden, 9–16 August 1993; Sveriges Lantbruksuniv: Uppsala, Sweden, 1994. [Google Scholar]
- Korhonen, K. Simulated stump treatment experiments for monitoring the efficacy of Phlebiopsis gigantea against Heterobasidion. In Proceedings of the 10th International Conference of Root and Butt Rots. Proceedings of the IUFRO Working Party 7.02.01, Quebec City, QC, Canada, 16–22 September 2001; pp. 206–210. [Google Scholar]
- Thor, M. Stump Treatment against Toot and Butt Rot Caused by Root Fomes (Heterobasidion annosum)—A Study of the Literature; Redogoerelse-Skogforsk: Uppsala, Sweden, 1996. [Google Scholar]
- Sennerda, B. Massaveden hämmar prisuppgång. Skogen 2021, 2, 32–34. [Google Scholar]
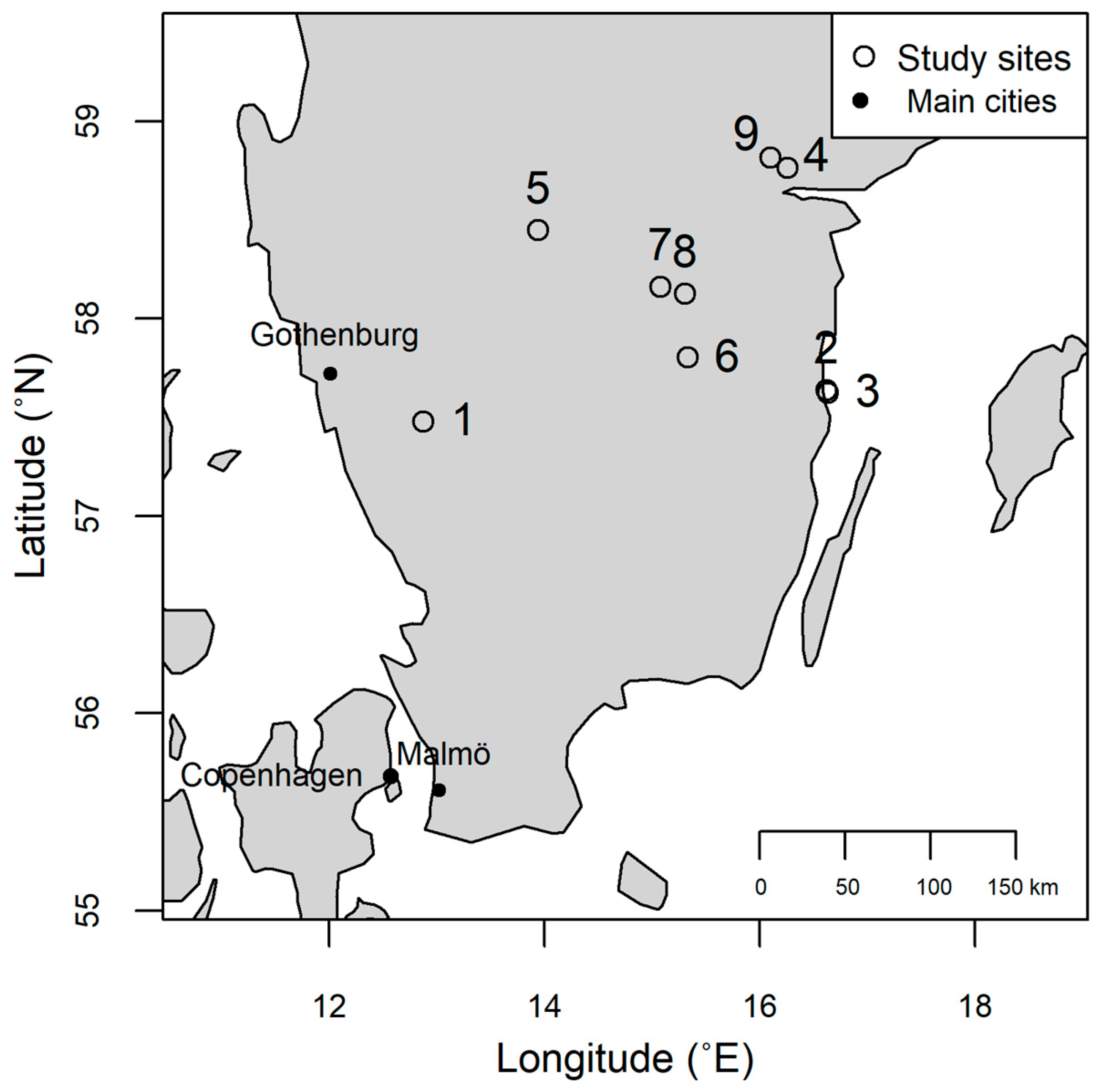

| Stand Id | Site Name | Location | Stand Age (yr) | Site Index | Soil Type | Previous Tree Species | Number of Thinnings | Number of Uprooted Trees | Number of Infected Trees | Infection Frequency (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Svenljunga | 57°28′42.0″ N 12°52′27.0″ E | 38 | T25 | Moraine | Scots pine + Norway spruce | 3 | 15 | 0 | 0 |
| 2 | Västervik | 57°38′15.3″ N 16°37′00.1″ E | 52 | T18 | Moraine | - * | - ** | 15 | 0 | 0 |
| 3 | Västervik | 57°37′24.2″ N 16°38′03.8″ E | 47 | T21 | Moraine | - * | - ** | 15 | 0 | 0 |
| 4 | Norrköping | 58°45′48.3″ N 16°15′22.4″ E | 42 | T29 | Silty clay | - * | 1 | 15 | 5 | 33.33 |
| 5 | Skövde | 58°26′53.2″ N 13°56′35.3″ E | 37 | T24 | Glacial sediments | Scots pine | 1 | 15 | 2 | 13.33 |
| 6 | Österbymo | 57°48′05.4″ N 15°19′48.4″ E | 62 | T25 | Moraine | Scots pine | 2 | 15 | 2 | 13.33 |
| 7 | Boxholm | 58°09′40.4″ N 15°04′27.3″ E | 45 | T27 | Moraine | Norway spruce | 1 | 15 | 3 | 20 |
| 8 | London Grytfall | 58°07′29.8″ N 15°18′09.8″ E | 58 | T26 | Moraine | Scots pine | 2 | 15 | 3 | 20 |
| 9 | Simonstorp | 58°48′51.4″ N 16°06′05.7″ E | 36 | T28 | Moraine | Scots pine | 1 | 15 | 5 | 33.33 |
| total | 135 | 20 | 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, K.; Dambrauskaite, M.; Witzell, J.; Rönnberg, J. Survey Study Reveals High Prevalence of Heterobasidion Root Rot Infection in Scots Pine (Pinus sylvestris) Stands Established on Seemingly Low-Risk Sites. Forests 2023, 14, 1018. https://doi.org/10.3390/f14051018
Youssef K, Dambrauskaite M, Witzell J, Rönnberg J. Survey Study Reveals High Prevalence of Heterobasidion Root Rot Infection in Scots Pine (Pinus sylvestris) Stands Established on Seemingly Low-Risk Sites. Forests. 2023; 14(5):1018. https://doi.org/10.3390/f14051018
Chicago/Turabian StyleYoussef, Khaled, Milda Dambrauskaite, Johanna Witzell, and Jonas Rönnberg. 2023. "Survey Study Reveals High Prevalence of Heterobasidion Root Rot Infection in Scots Pine (Pinus sylvestris) Stands Established on Seemingly Low-Risk Sites" Forests 14, no. 5: 1018. https://doi.org/10.3390/f14051018
APA StyleYoussef, K., Dambrauskaite, M., Witzell, J., & Rönnberg, J. (2023). Survey Study Reveals High Prevalence of Heterobasidion Root Rot Infection in Scots Pine (Pinus sylvestris) Stands Established on Seemingly Low-Risk Sites. Forests, 14(5), 1018. https://doi.org/10.3390/f14051018







