Adult Trees Cryptomeria japonica (Thunb. ex L.f.) D. Don Micropropagation: Factors Involved in the Success of the Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sterilization
2.3. Micropropagation Process
2.3.1. Experiment 1
2.3.2. Root Induction and Acclimatization of Rooted Plants
2.3.3. Experiment 2
2.3.4. Root Induction and Acclimatization of Rooted Plants
2.4. Data collection and Statistical Analysis
2.4.1. Experiment 1
2.4.2. Experiment 2
3. Results
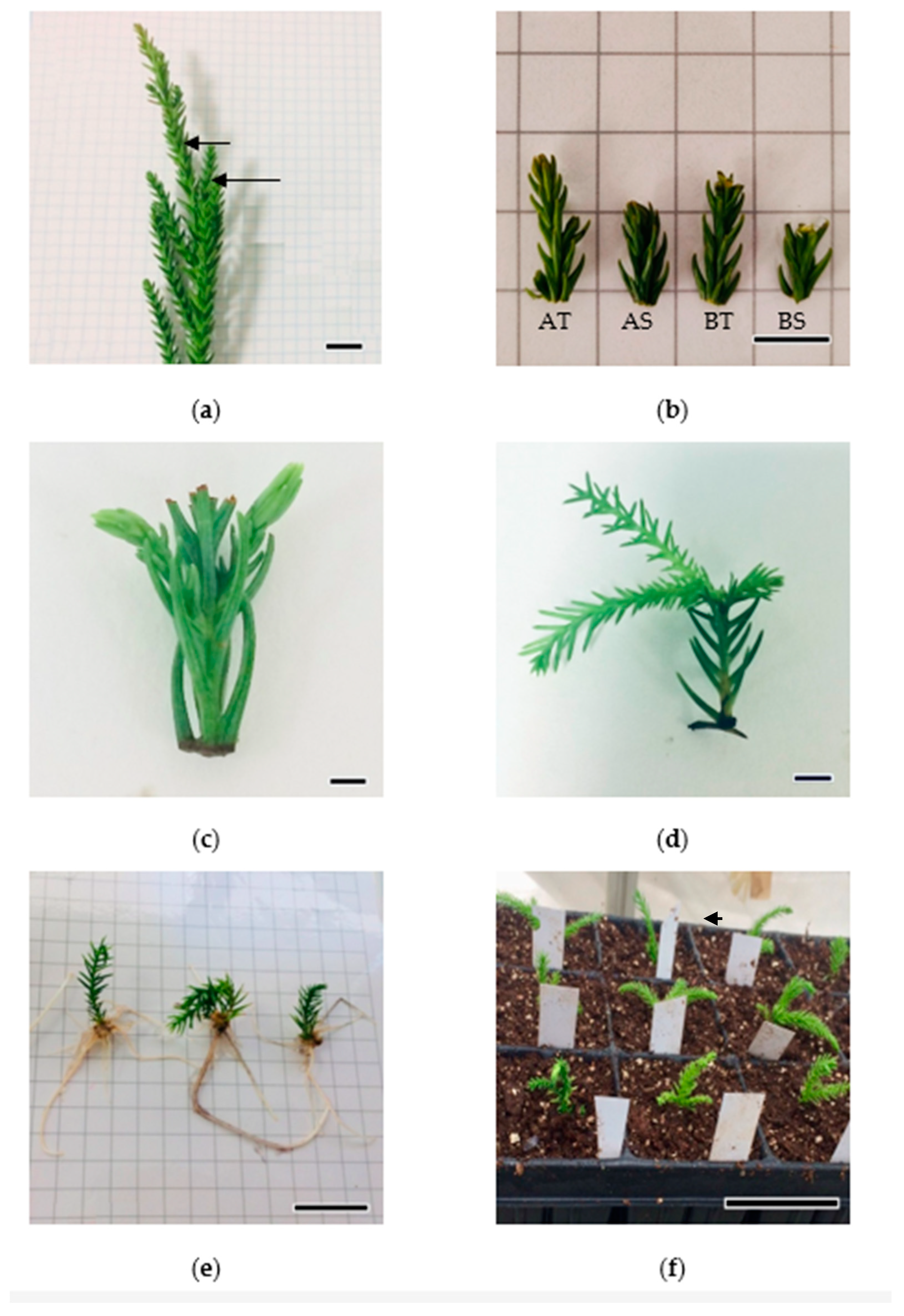
3.1. Micropropagation Process
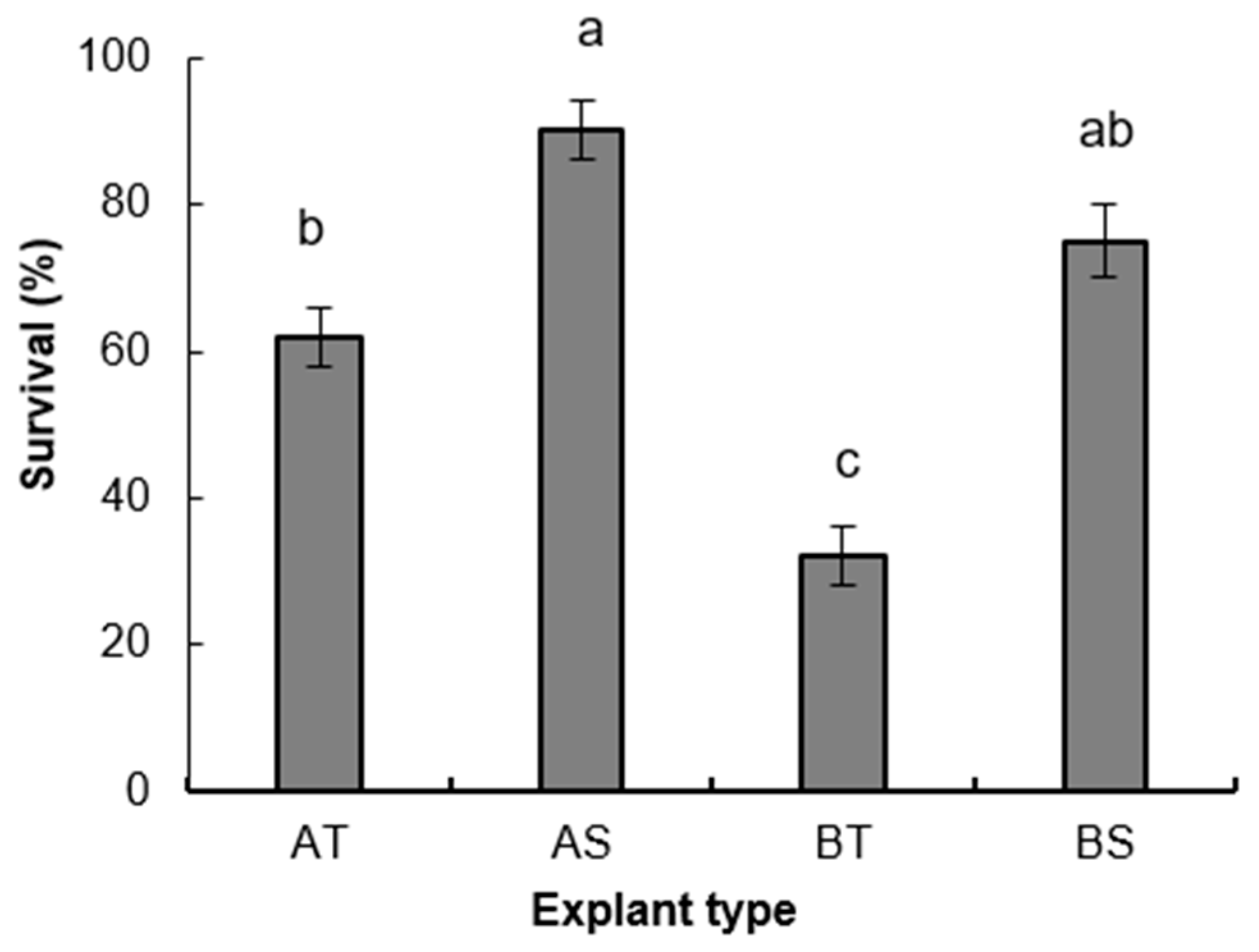
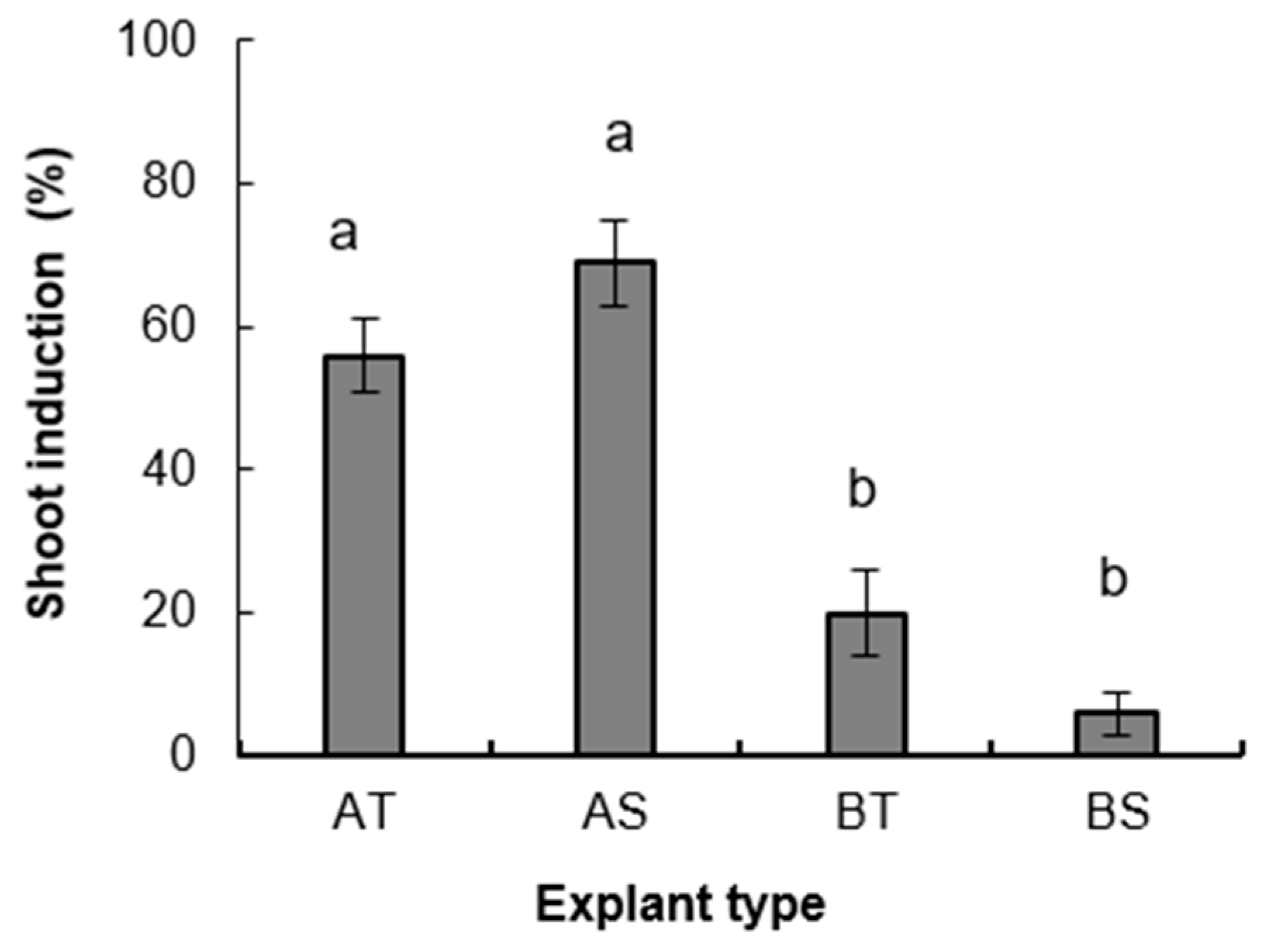
3.1.1. Experiment 1
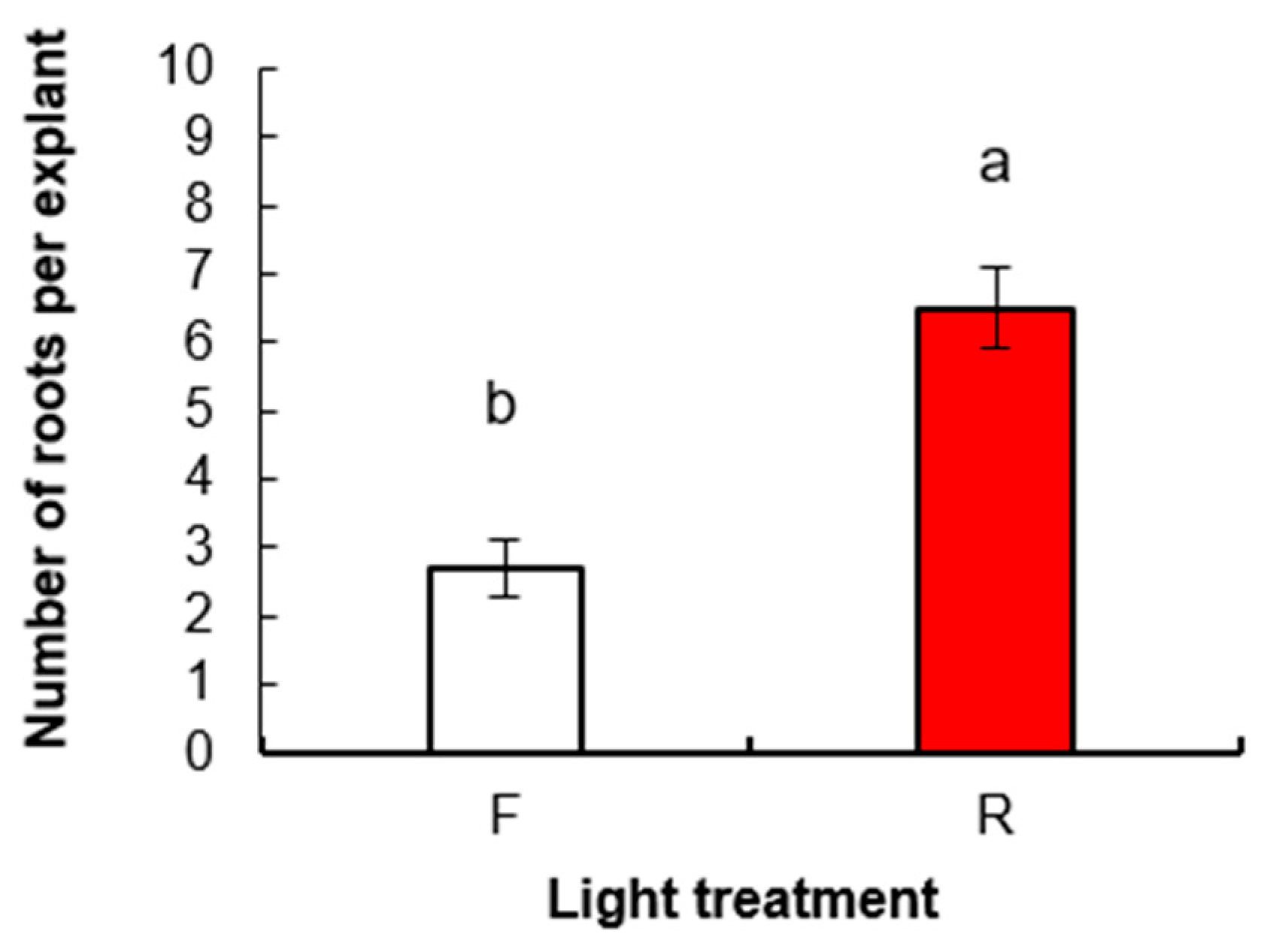
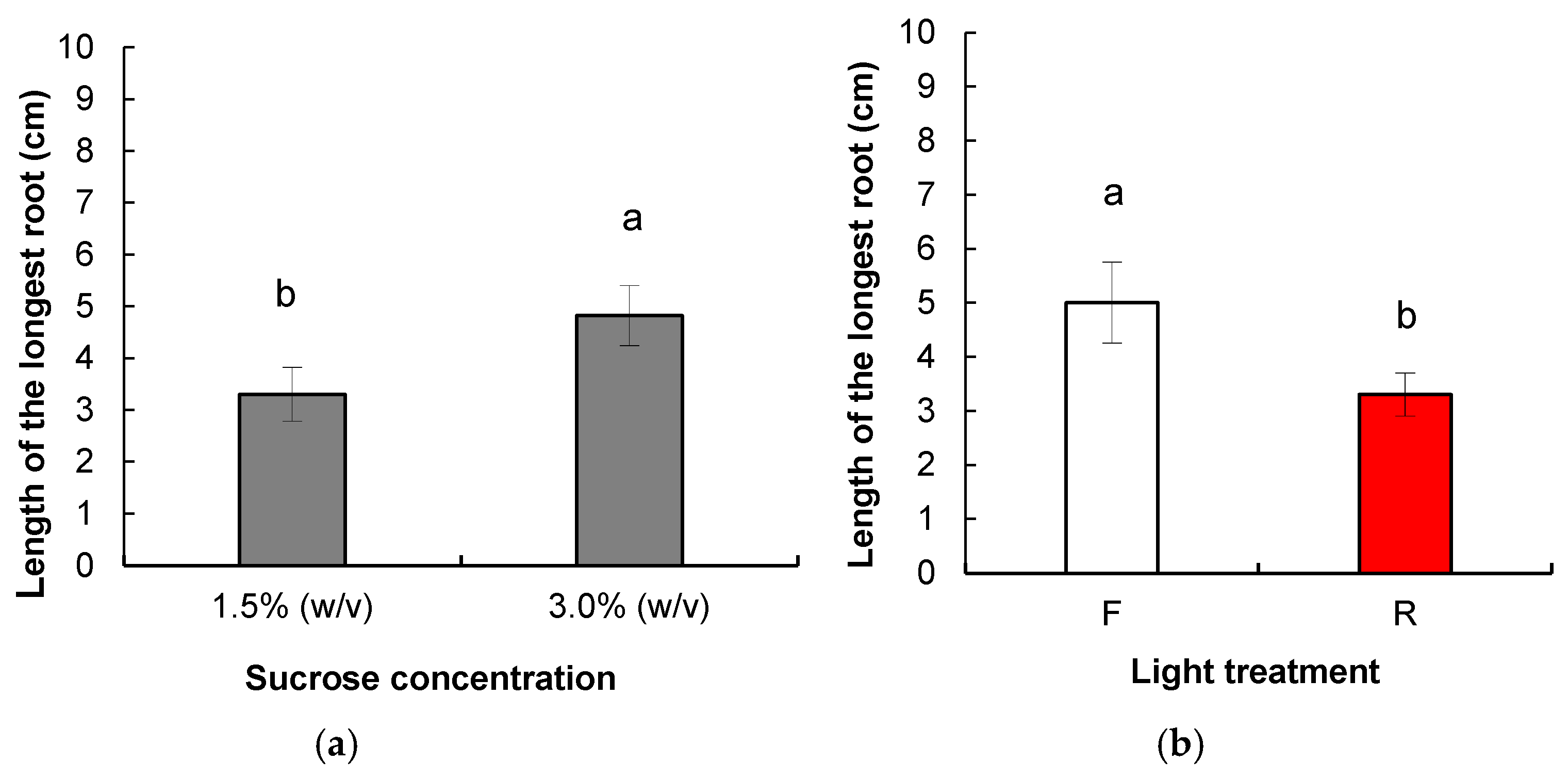
3.1.2. Root Induction and Acclimatization of Rooted Plants
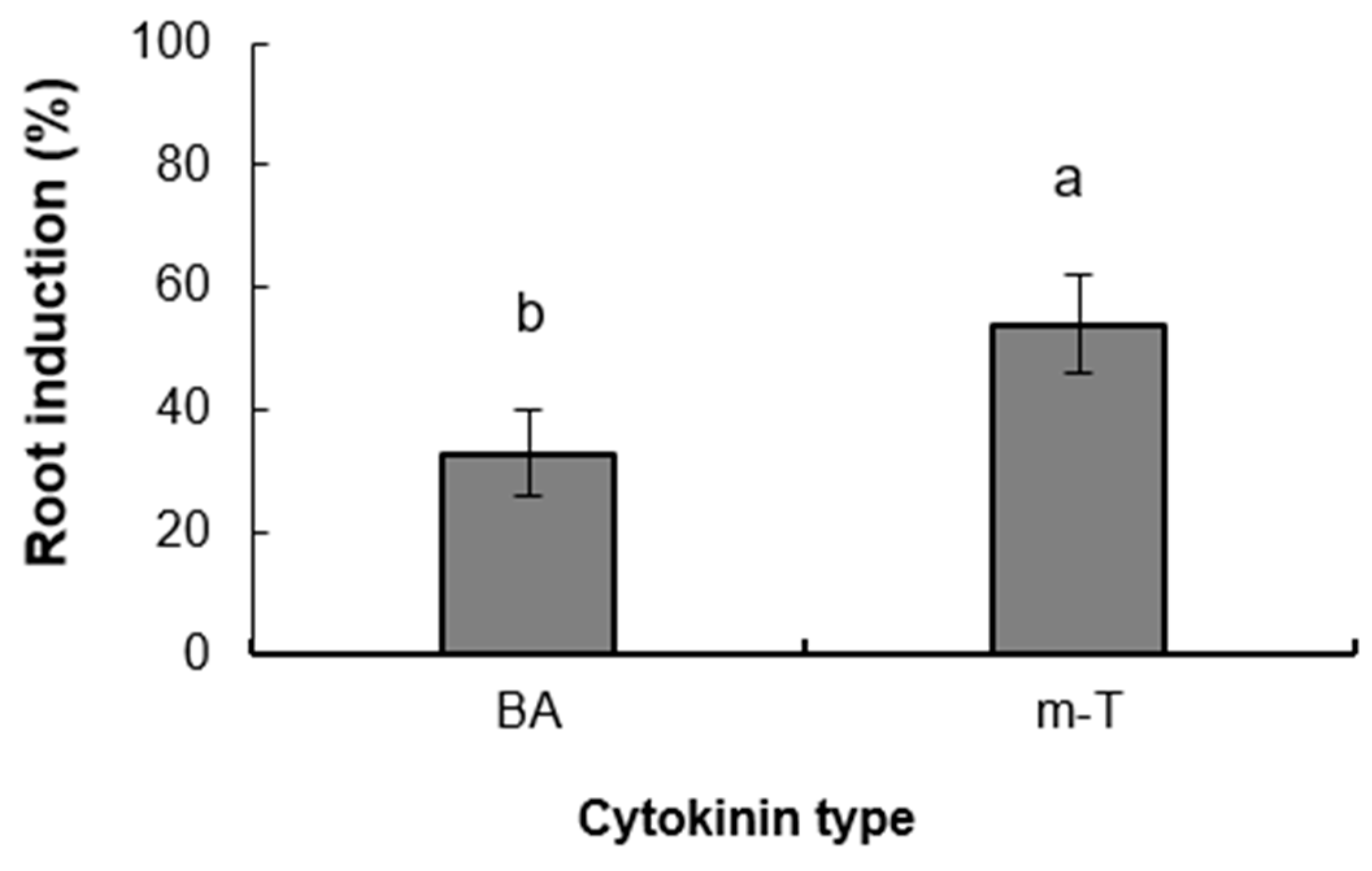
3.2. Experiment 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kusumi, J.; Tsumura, Y.; Yoshimaru, H.; Tachida, H. Phylogenetic relationships in Taxodiaceae and Cupressaceae sensu stricto based on matK gene, chlL gene, trnLtrnF IGS region, and trnL intron sequences. Am. J. Bot. 2000, 87, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Uchiyama, K.; Kimura, M.; Tsumura, Y. Genetic analysis distinguished new natural population and old plantations of Cryptomeria japonica. Trees For. People 2023, 11, 100365. [Google Scholar] [CrossRef]
- Forestry Agency. Statistical Handbook of Forest and Forestry; Forestry Agency, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2020; pp. 8–9. (In Japanese) [Google Scholar]
- Taniguchi, T.; Konagaya, K.; Nanasato, Y. Somatic embryogenesis in artificially pollinated seed families of 2nd generation plus trees and cryopreservation of embryogenic tissue in Cryptomeria japonica D. Don (sugi). Plant Biotechnol. 2020, 37, 239–245. [Google Scholar] [CrossRef]
- Tsubomura, M.; Taniguchi, T. Micropropagation of the male sterile ‘Soushun’ Japanese cedar. Comb. Proc. Int. Plant. Prop. Soc. 2008, 58, 421–427. [Google Scholar]
- Phillips, G.C. In vitro morphogenesis in plants-recent advances. In Vitro Cell. Dev. Biol. Plant. 2004, 40, 342–345. [Google Scholar] [CrossRef]
- Park, J.S.; Barret, J.D.; Bonga, J.M. Application of somatic embryogenesis in high-value clonal forestry: Deployment, genetic control, and stability of cryopreserves clones. Cell. Dev. Biol. Plant. 1998, 34, 231–239. [Google Scholar] [CrossRef]
- Sahu, J.; Sahu, R.K. A review on low cost methods for in vitro micropropagation of plant through tissue culture technique. UK J. Pharm. Biosci. 2013, 1, 38–41. [Google Scholar] [CrossRef]
- Narváez, I.; Martín, C.; Jiménez-Díaz, R.M.; Mercado, J.A.; Pliego-Alfaro, F. Plant regeneration via somatic embryogenesis in mature wild olive genotypes resistant to the defoliating pathotype of Verticillium dahliae. Front. Plant Sci. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Rojas-Vargas, A.; Castander-Olarieta, A.; Moncaleán, P.; Montalbán, I.A. Optimization of the micropropagation of elite adult trees of Sequoia sempervirens: Forest species of interest in the Basque Country, Spain. Rev. Bionatura. 2021, 6, 1511–1519. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Serrano-Martinez, F.; Cano-Castillo, M.; Casas, J.L. In vitro propagation, genetic assessment, and, medium-term conservation of coastal endangered species Tetraclinis articulata (Vahl) Masters (Cupressaceae) from adult trees. Plants 2022, 11, 187. [Google Scholar] [CrossRef]
- Rai, M.K.; Asthana, P.; Jaiswal, V.S.; Jaiswa, U. Biotechnological advances in guava (Psidium guajava L.): Recent developments and prospects for further research. Trees 2010, 24, 1–12. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 1962, 15, 474–497. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Études des milieux adaptés aux cultures in vitro de Prunus. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Shojaeiyan, A.; Shokri, S.; Ghojah, S.M. Effects of nutrient media, different cytokinin types and their concentrations on in vitro multiplication of G × N15 (hybrid of almond × peach) vegetative rootstock. J. Genet. Eng. Biotechnol. 2014, 12, 81–87. [Google Scholar] [CrossRef]
- De Diego, N.; Montalbán, I.A.; Moncaleán, P. In vitro regeneration of adult Pinus sylvestris L. trees. S. Afr. J. Bot. 2010, 76, 158–162. [Google Scholar] [CrossRef]
- De Almeida, M.; de Almeida, C.V.; Graner, E.M.; Brondani, G.E.; de Abreu-Tarazi, M.F. Pre-procambial cells are niches for pluripotent and totipotent stem-like cells for organogenesis and somatic embryogenesis in the peach palm: A histological study. Plant Cell Rep. 2012, 31, 1495–1515. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.I. Plant sugar-response pathways. part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef]
- Calamar, A.; De Klerk, G.J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Murthy, H.N.; Ammar, M.H.; Alghamdi, S.S.; Al-Suhaibani, N.A.; Alsadon, A.A.; Paek, K.Y. In vitro rooting of leguminous plants: Difficulties, alternatives, and strategies for improvement. Hortic. Environ. Biotechnol. 2016, 57, 311–322. [Google Scholar] [CrossRef]
- Alallaq, S.; Ranjan, A.; Brunoni, F.; Novák, O.; Lakehal, A.; Bellini, C. Red light controls adventitious root regeneration by modulating hormone homeostasis in Picea abies seedlings. Front. Plant Sci. 2020, 11, 586140. [Google Scholar] [CrossRef]
- Larraburu, E.E.; Correa, G.S.; Llorente, B.E. In vitro development of yellow lapacho (Bignoniaceae) using high-power lightemitting diode. Rev. Arvore. 2018, 42, e420508. [Google Scholar] [CrossRef]
- Rocha, P.S.G.; Oliveira, R.P.; Scivittaro, W.B.; Santos, U.L. Diodos emissores de luz e concentrações de BAP na multiplicaçãoin vitro de morangueiro. Ciênc. Rural 2010, 40, 1922–1928. [Google Scholar] [CrossRef]
- Kulus, D.; Woźny, A. Influence of light conditions on the morphogenetic and biochemical response of selected ornamental plant species under in vitro conditions: A mini-review. BioTechnologia 2020, 101, 75–83. [Google Scholar] [CrossRef]
- Marín-Martínez, L.A.; Iglesias-Andreu, L.G. Effect of LED lights on the in vitro growth of Pinus pseudostrobus Lindl., plants. J. For. Sci. 2022, 68, 311–317. [Google Scholar] [CrossRef]
- Río-Álvarez, I.; Rodríguez-Herva, J.J.; Martínez, P.M.; González-Melendi, P.; García-Casado, G.; Rodríguez-Palenzuela, P.; López-Solanilla, E. Light regulates motility, attachment, and virulence in the plant pathogen Pseudomonas syringae pv tomato DC3000. Environ. Microbiol. 2014, 16, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Payghamzadeh, K.; Kazemitabar, S.K. In vitro propagation of walnut—A review. Afr. J. Biotechnol. 2011, 10, 290–311. [Google Scholar]
- Hine-Gómez, A.; Valverde-Cerdas, L. Establecimiento in vitro de Cryptomeria japónica (Taxocidaceae). Rev. Biol. Trop. 2003, 51, 683–690, (In Espanish with English Abstract). [Google Scholar]
- Da Silva, J.A.T.; Winarto, B.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Tissue disinfection for preparation of Dendrobium in vitro culture. Folia Horticult. 2016, 28, 57–75. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Ollero, J.; Arrillaga, I.; Segura, J. Factors influencing axillary shoot proliferation and adventitious budding in cedar. Tree Physiol. 2005, 25, 477–486. [Google Scholar] [CrossRef]
- Chang, S.H.; Ho, C.K.; Chen, Z.Z.; Tsay, J.Y. Micropropagation of Taxus mairei from mature trees. Plant Cell Rep. 2001, 20, 496–502. [Google Scholar] [CrossRef]
- Sathyagowri, S.; Seran, T.H. In vitro plant regeneration of ginger (Zingiber officinale Rosc.) with emphasis on initial culture establishment. J. Med. Aromat. Plants 2011, 1, 195–202. [Google Scholar]
- George, E.F.; Debergh, P.C. Micropropagation: Uses and Methods. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 29–64. [Google Scholar] [CrossRef]
- Desai, C.; Inghalihalli, R.; Krishnamurthy, R. Micropropagation of Anthurium andraeanum an important tool in floriculture. J. Pharmacogn. Phytochem. 2015, 4, 112–117. [Google Scholar]
- Rodríguez, S.M.; Ordás, R.J.; Alvarez, J.M. Conifer Biotechnology: An Overview. Forests 2022, 13, 1061. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant. 2019, 55, 242–257. [Google Scholar] [CrossRef]
- De Diego, N.; Montalbán, I.A.; Fernandez de Larrinoa, E.; Moncaleán, P. In vitro regeneration of Pinus pinaster adult trees. Can. J. For. Res. 2008, 38, 2607–2615. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Cortizo, M.; de Diego, N.; Moncaleán, P.; Ordás, R.J. Micropropagation of adult stone pine (Pinus pinea L.). Trees Struct. Funct. 2009, 23, 835–842. [Google Scholar] [CrossRef]
- Montalbán, I.A.; Novák, O.; Rolčik, J.; Strnad, M.; Moncaleán, P. Endogenous cytokinin and auxin profiles during in vitro organogenesis from vegetative buds of Pinus radiata Adult Trees. Physiol. Plant. 2013, 148, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Montalbán, I.A.; Pedrosa, A.; Tavares, J.; Pestryakov, A.; Bogdanchikova, N.; Canhoto, J.; Moncaleán, P. Regeneration of Pinus halepensis (Mill.) through organogenesis from apical shoot buds. Forests 2021, 12, 363. [Google Scholar] [CrossRef]
- Rojas-Vargas, A.; Castander-Olarieta, A.; Montalbán, I.A.; Moncaleán, P. Influence of physico-chemical factors on the efficiency and metabolite profile of adult Pinus radiata D. Don bud organogenesis. Forests 2022, 13, 1455. [Google Scholar] [CrossRef]
- Rafi, Z.N.; Salehi, H. Factors affecting in-vitro propagation of some genotypes of Himalayan cedar [Cedrus deodara (Roxb. ex Lamb) G. Don.]. Adv Hortic Sci. 2018, 32, 479–486. [Google Scholar]
- Maruyama, T.E.; Ueno, S.; Mori, H.; Kaneeda, T.; Moriguchi, Y. Factors influencing somatic embryo maturation in sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants 2021, 10, 874. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Sargent, W.A.; Rensema, T.; Walla, J.A. Influence of plant growth regulators, basal media and carbohydrate levels on the in vitro development of Pinus ponderosa (Dougl. Ex Law.) cotyledon explants. Plant Cell Tissue Organ Cult. 1990, 20, 47–52. [Google Scholar] [CrossRef]
- Rojas-Vargas, A.; Castander-Olarieta, A.; do Nascimento, A.M.M.; Vélez, M.L.; Pereira, C.; Martins, J.; Zuzarte, M.; Canhoto, J.; Montalbán, I.A.; Moncaleán, P. Testing Explant Sources, Culture Media, and Light Conditions for the Improvement of Organogenesis in Pinus ponderosa (P. Lawson and C. Lawson). Plants 2023, 12, 850. [Google Scholar] [CrossRef]
- Arteta, T.A.; Hameg, R.; Landin, M.; Gallego, P.P.; Barreal, M.E. Artificial Neural Networks Elucidated the Essential Role of Mineral Nutrients versus Vitamins and Plant Growth Regulators in Achieving Healthy Micropropagated Plants. Plants 2022, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Moncaleán, P. Testing novel cytokinins for improved in vitro adventitious shoots formation and subsequent ex vitro performance in Pinus radiata. Forestry 2011, 84, 363–373. [Google Scholar] [CrossRef]
- Zarei, M.; Salehi, H.; Jowkar, A. Controlling the barriers of cloning mature Picea abies (L.) H. Karst. via tissue culture and co-cultivation with Agrobacterium rhizogenes. Trees 2020, 34, 637–647. [Google Scholar] [CrossRef]
- Vinoth, A.; Ravindhran, R. In vitro morphogenesis of woody plants using thidiazuron. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 211–230. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; da Silva, J.A.T. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Koguta, K.V.; Flores, P.C.; Alcantara, G.B.; Higa, A.R. Serial micropropagation and cuttings as rejuvenation methods for Cryptomeria japonica (L.F) D.Don. Rev. Espac. 2017, 38, 7. [Google Scholar]
- Gantait, S.; Mitra, M. Role of Meta-topolin on in vitro shoot regeneration: An insight. In Me-Tatopolin: A Growth Regulator for plant Biotechnology and Agriculture; Ahmad, N., Strnad, M., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Anis, M.; Alatar, A.A. Meta-topolin improved micropropagation in Syzygium cumini and acclimatization to ex vitro conditions. Biol. Plant. 2019, 63, 174–182. [Google Scholar] [CrossRef]
- Jayaprakash, K.; Manokari, M.; Cokulraj, M.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Shekhawat, M.S. Improved organogenesis and micro-structural traits in micropropagated plantlets of Caralluma umbellata Haw. in response to Meta-Topolin. Plant Cell Tissue Organ Cult. 2023, in press. [Google Scholar] [CrossRef]
- Ioannidis, K.; Tomprou, I.; Panayiotopoulou, D.; Boutsios, S.; Daskalakou, E.N. Potential and Constraints on In Vitro Micropropagation of Juniperus drupacea Labill. Forests 2023, 14, 142. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Sperdouli, I.; Adamakis, I.D.; Grigoriadou, K. The use of different LEDs wavelength and light intensities for in vitro proliferation of cherry rootstock: Influence on photosynthesis and photomorphogenesis. Plant Cell Tissue Organ Cult. 2023, 152, 317–330. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.; Gong, X.; Wang, H.; Gao, Y.; Wang, X.; Zeng, Z.; Hu, Y. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integr. Agric. 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Ragonezi, C.; Klimaszewska, K.; Castro, M.R.; Lima, M.; de Oliveira, P.; Zavattieri, M.A. Adventitious rooting of conifers: Influence of physical and chemical factors. Trees Struct. Funct. 2010, 24, 975–992. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hashimoto, K.; Ohba, E.; Kurata, Y. Light-emitting Diode–Induced Root Photomorphogenesis and Root Retention in Populus. HortScience 2022, 57, 872–876. Available online: https://journals.ashs.org/hortsci/view/journals/hortsci/57/8/article-p872.xml (accessed on 28 February 2023). [CrossRef]
- Ishii, K.; Hosoi, Y.; Taniguchi, T.; Tsubomura, M.; Kondo, T.; Yamada, H.; Saito, M.; Suda, T.; Fukisawa, T.; Tanaka, K. In vitro culture of various genotypes of male sterile Japanese cedar (Cryptomeria japonica D. Don). Plant Biotechnol. 2011, 28, 103–106. [Google Scholar] [CrossRef]
- Lai, C.S.; Kho, Y.H.; Chew, B.L.; Raja, P.B.; Subramaniam, S. Organogenesis of Cucumis metuliferus plantlets under the effects of LEDs and silver nanoparticles. S. Afr. J. Bot. 2022, 148, 78–87. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Vargas, A.; Montalbán, I.A.; Moncaleán, P. Adult Trees Cryptomeria japonica (Thunb. ex L.f.) D. Don Micropropagation: Factors Involved in the Success of the Process. Forests 2023, 14, 743. https://doi.org/10.3390/f14040743
Rojas-Vargas A, Montalbán IA, Moncaleán P. Adult Trees Cryptomeria japonica (Thunb. ex L.f.) D. Don Micropropagation: Factors Involved in the Success of the Process. Forests. 2023; 14(4):743. https://doi.org/10.3390/f14040743
Chicago/Turabian StyleRojas-Vargas, Alejandra, Itziar A. Montalbán, and Paloma Moncaleán. 2023. "Adult Trees Cryptomeria japonica (Thunb. ex L.f.) D. Don Micropropagation: Factors Involved in the Success of the Process" Forests 14, no. 4: 743. https://doi.org/10.3390/f14040743
APA StyleRojas-Vargas, A., Montalbán, I. A., & Moncaleán, P. (2023). Adult Trees Cryptomeria japonica (Thunb. ex L.f.) D. Don Micropropagation: Factors Involved in the Success of the Process. Forests, 14(4), 743. https://doi.org/10.3390/f14040743





