Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition
Abstract
1. Introduction
2. Materials and Methods
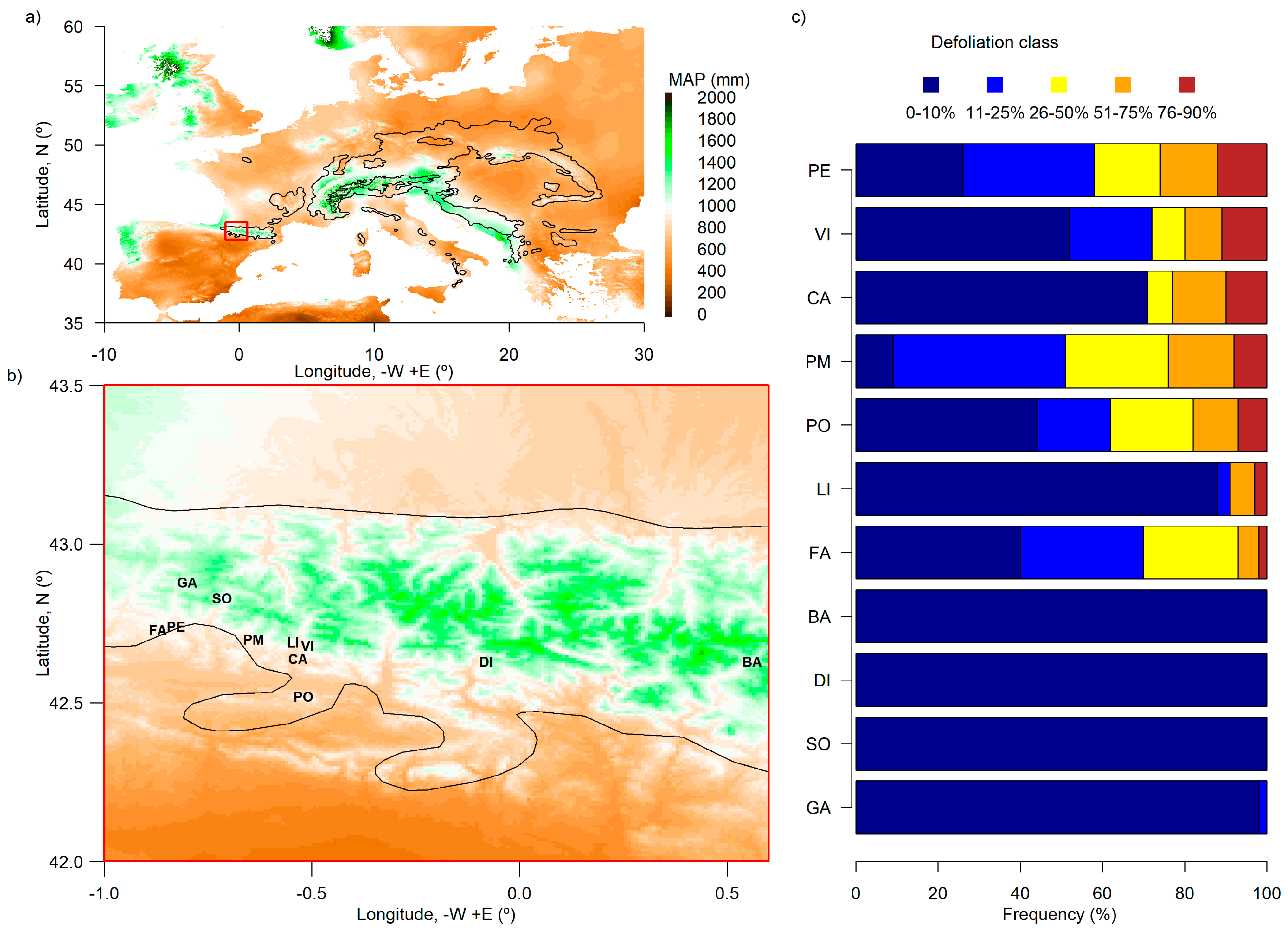
2.1. Study Area
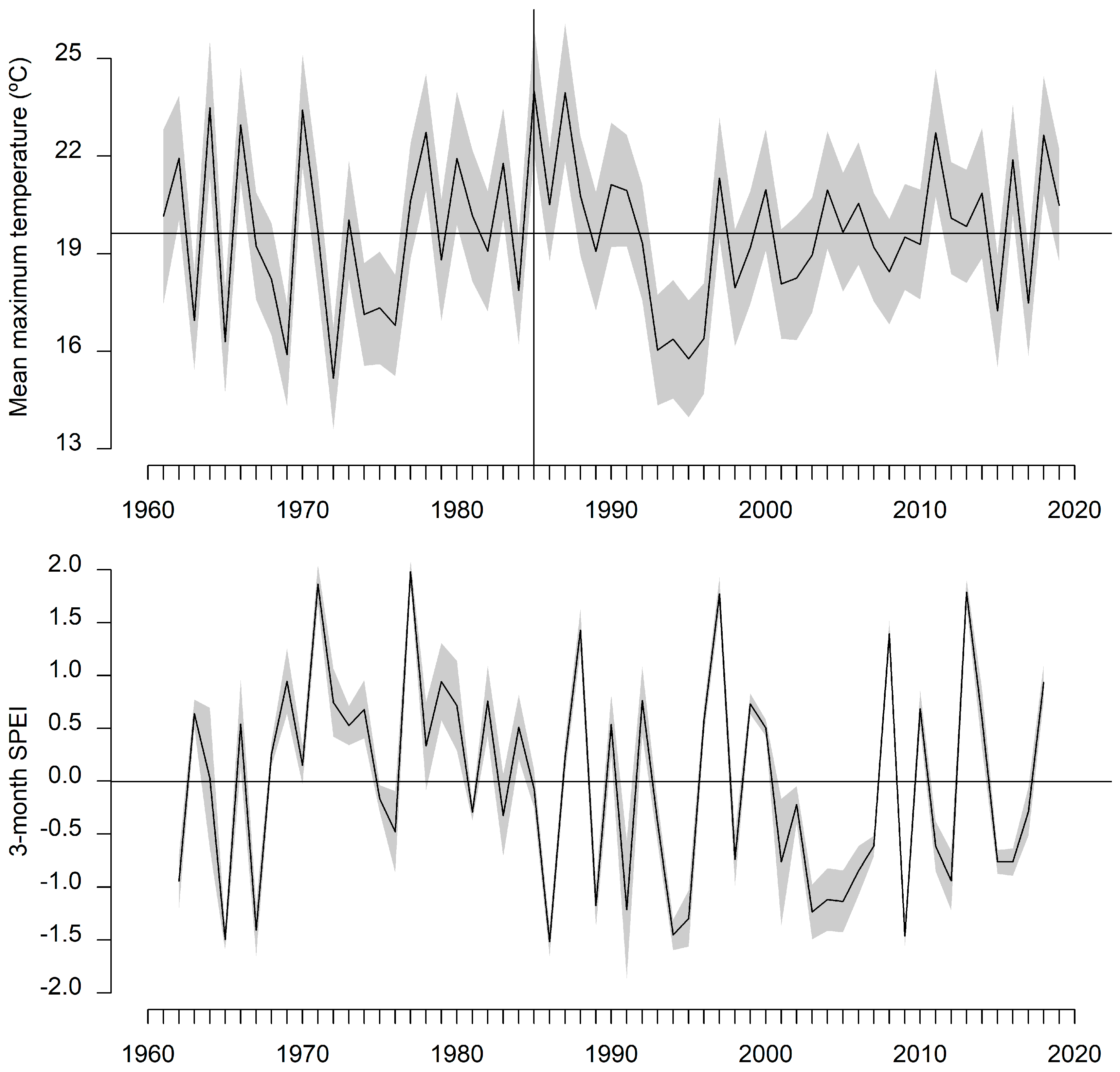
2.2. Field Sampling and Climate Data
2.3. Measuring Lying Deadwood, Stumps and Snags
2.4. Processing Tree Ring Data
2.5. Statistical Analyses
3. Results
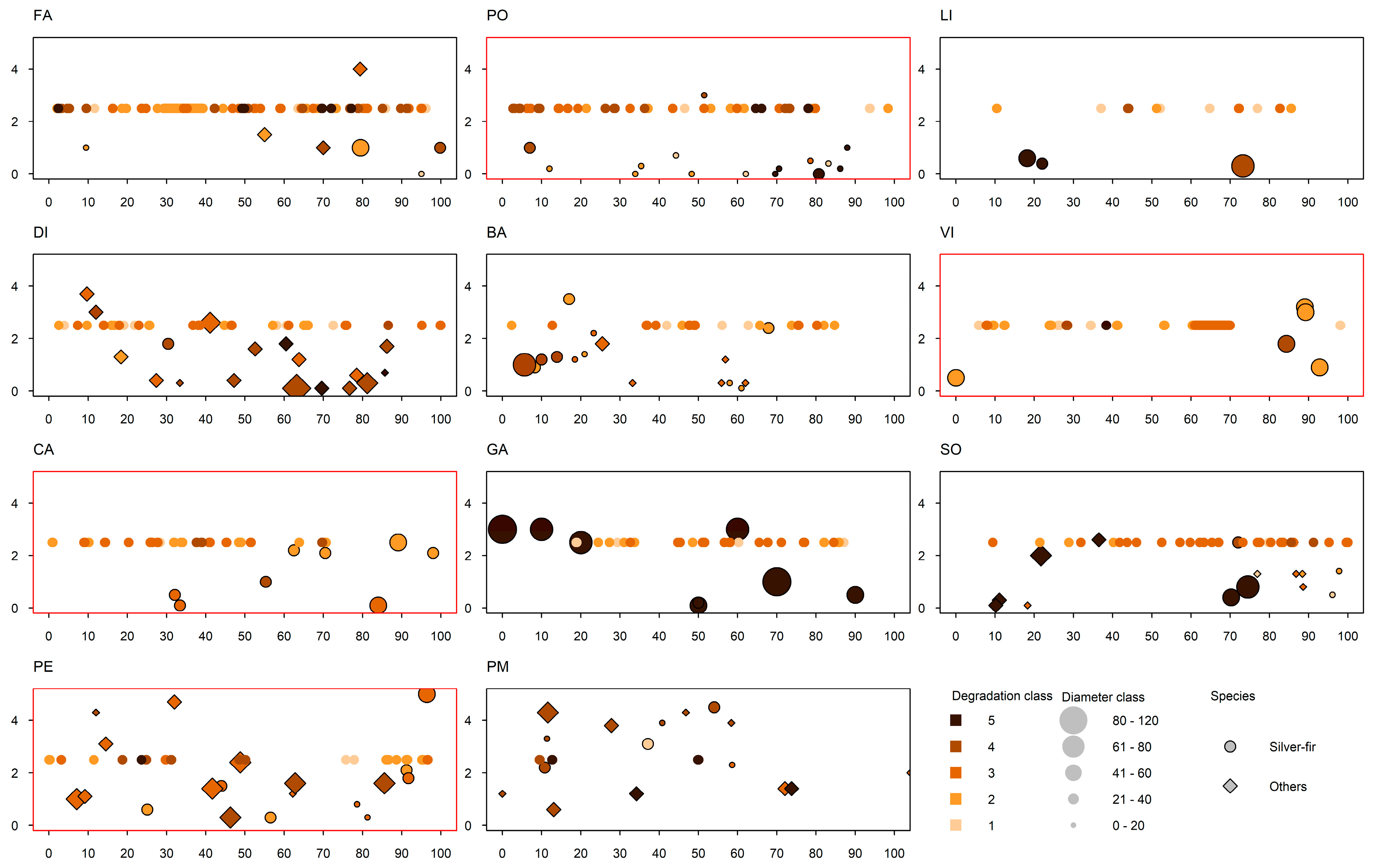
3.1. Silver Fir Stand Characteristics
3.2. Legacies of Past Management: Major Growth Releases
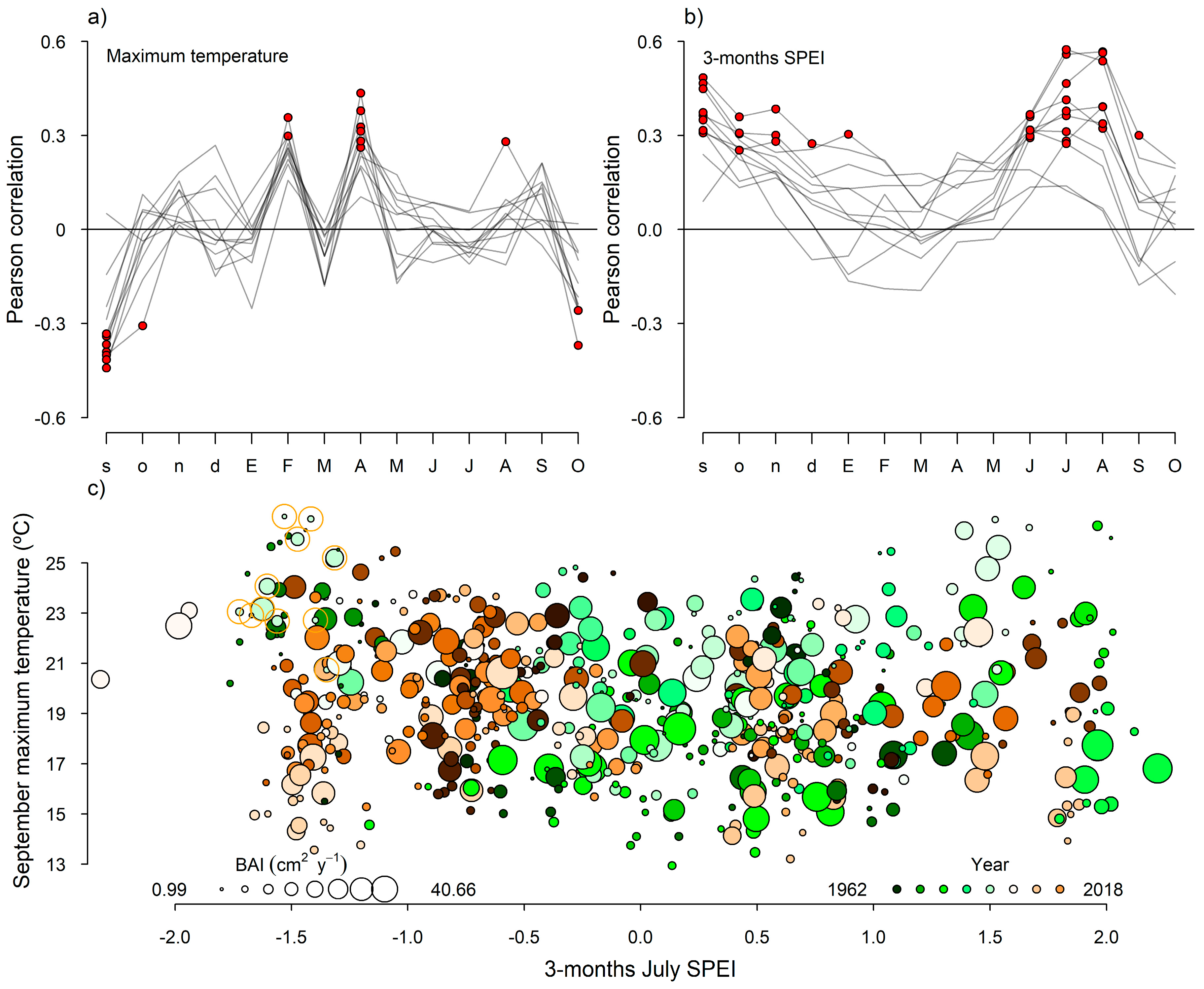
3.3. Tree Growth and Response to Climate
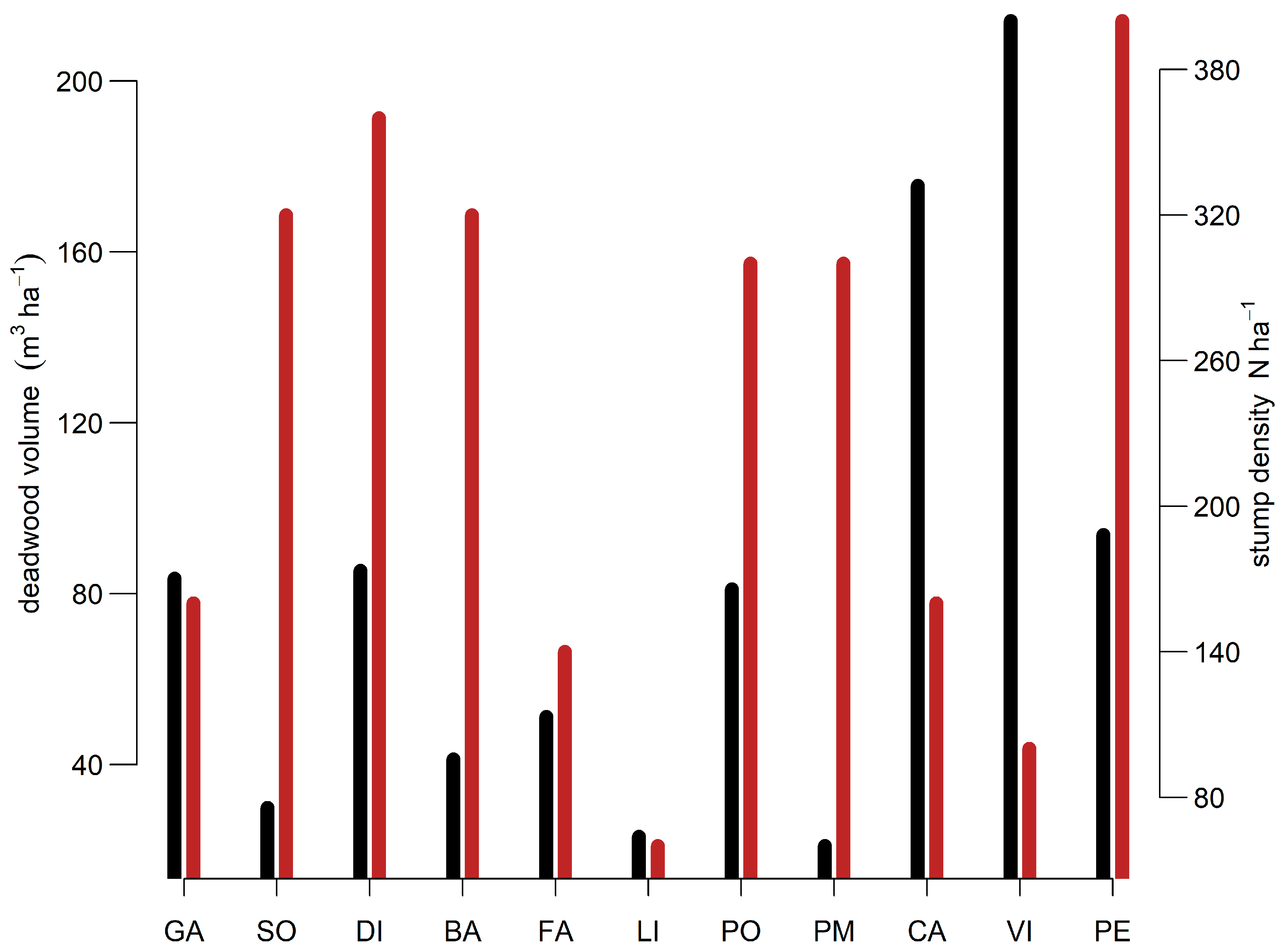
3.4. Deadwood Type, Volume and Coverage
3.5. Relationship between Variables across Stands
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- FOREST EUROPE, 2020: State of Europe’s Forests 2020; Liaison Unit Bratislava: Bratislava, Slovakia, 2020.
- Senf, C.; Pflugmacher, D.; Zhiqiang, Y.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef] [PubMed]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef] [PubMed]
- Senf, C.; Sebald, J.; Seidl, R. Increasing canopy mortality affects the future demographic structure of Europe’s forests. One Earth 2021, 4, 749–755. [Google Scholar] [CrossRef]
- Potočić, N.; Timmermann, V.; Ognjenović, M.; Kirchner, T.; Prescher, A.-K.; Ferretti, M. Tree Health Is Deteriorating in the European Forests (ICP Forests Brief No. 5); Programme Co-ordinating Centre of ICP Forests, Thünen Institute of Forest Ecosystems: Brunswick, Germany, 2021. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Change Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Taccoen, A.; Piedallu, C.; Seynave, I.; Perez, V.; Gégout-Petit, A.; Nageleisen, L.M.; Gégout, J.C. Background mortality drivers of European tree species: Climate change matters. Proc. Royal Soc. B 2019, 286, 20190386. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.; Widmann, M.; Bevacqua, E.; Van Loon, A.F.; Maraun, D.; Vrac, M. Increased probability of compound long-duration dry and hot events in Europe during summer (1950–2013). Env. Res. Lett. 2019, 14, 094006. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J. Compound climate events increase tree drought mortality across European forests. Sci. Tot. Env. 2022, 816, 151604. [Google Scholar] [CrossRef] [PubMed]
- Brus, D.J.; Hengeveld, G.M.; Walvoort, D.J.J.; Goedhart, P.W.; Heidema, A.H.; Nabuurs, G.J.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2011, 131, 145–157. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Change Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Caudullo, G.; Barredo, J.I. A georeferenced dataset of drought and heat-induced tree mortality in Europe. One Ecosyst. 2019, 4, e37753. [Google Scholar] [CrossRef]
- Camarero, J.J.; Bigler, C.; Linares, J.C.; Gil-Pelegrín, E. Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For. Ecol. Manage. 2011, 262, 759–769. [Google Scholar] [CrossRef]
- Cabrera, M. Evolución de abetales del Pirineo aragonés. Cuad. De La Soc. Española De Cienc. For. 2001, 11, 43–52. [Google Scholar]
- Camarero, J.J.; Padró, A.; Martín-Bernal, E.; Gil-Pelegrín, E. Aproximación dendroecológica al decaimiento del abeto (Abies alba Mill.) en el Pirineo aragonés. Montes 2002, 70, 26–33. [Google Scholar]
- Camarero, J.J.; Martín-Bernal, E.; Gil-Pelegrín, E. The impact of a needleminer (Epinotia subsequana) outbreak on radial growth of silver fir (Abies alba) in the Aragón Pyrenees: A dendrochronological assessment. Dendrochronologia 2003, 21, 3–12. [Google Scholar] [CrossRef]
- Macias, M.; Andreu, L.; Bosch, O.; Camarero, J.J.; Gutiérrez, E. Increasing aridity is enhancing silver fir (Abies alba Mill.) water stress in its south-western distribution limit. Clim. Change 2006, 79, 289–313. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Camarero, J.J.; Abadía, A.; Martín, E.; González-Cascón, R.; Morales, F.; Gil-Pelegrín, E. Physiological performance of silver-fir (Abies alba Mill.) populations under contrasting climates near the south-western distribution limit of the species. Flora 2007, 202, 226–236. [Google Scholar] [CrossRef]
- Oliva, J.; Colinas, C. Decline of silver fir (Abies alba Mill.) stands in the Spanish Pyrenees: Role of management, historic dynamics and pathogens. For. Ecol. Manage. 2007, 252, 84–97. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Change Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Hernández, L.; Camarero, J.J.; Gil-Peregrín, E.; Sánchez, M.Á.S.; Cañellas, I.; Montes, F. Biotic factors and increasing aridity shape the altitudinal shifts of marginal Pyrenean silver fir populations in Europe. For. Ecol. Manage. 2019, 432, 558–567. [Google Scholar] [CrossRef]
- Rolland, C.; Michalet, R.; Desplanque, C.; Petetin, A.; Aime, S. Ecological requirements of Abies alba in the French Alps derived from dendro-ecological analysis. J. Veg. Sci. 1999, 10, 297–306. [Google Scholar] [CrossRef]
- Mauri, A.; de Rigo, D.; Caudullo, G. Abies alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; p. e01493b+. [Google Scholar]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Bottero, A.; Forrester, D.I.; Cailleret, M.; Kohnle, U.; Gessler, A.; Michel, D.; Bose, A.K.; Bauhus, J.; Bugmann, H.; Cuntz, M.; et al. Growth resistance and resilience of mixed silver fir and Norway spruce forests in central Europe: Contrasting responses to mild and severe droughts. Glob. Change Biol. 2021, 27, 4403–4419. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Rebetez, M.; Conedera, M.; Augustin, S.; Brang, P.; Tinner, W. What is the potential of silver fir to thrive under warmer and drier climate? Eur. J. For. Res. 2019, 138, 547–560. [Google Scholar] [CrossRef]
- Walder, D.; Krebs, P.; Bugmann, H.; Manetti, M.C.; Pollastrini, M.; Anzillotti, S.; Conedera, M. Silver fir (Abies alba Mill.) is able to thrive and prosper under meso-Mediterranean conditions. For. Ecol. Manage. 2021, 498, 119537. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A. Will silver fir be under higher risk due to drought? A comment on Walder et al. (2021). For. Ecol. Manage. 2022, 503, 119826. [Google Scholar] [CrossRef]
- Aussenac, G. Ecology and ecophysiology of circum-Mediterranean firs in the context of climate change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- Tardif, J.; Camarero, J.J.; Ribas, M.; Gutiérrez, E. Spatiotemporal variability in tree ring growth in the Central Pyrenees: Climatic and site influences. Ecol. Monogr. 2003, 73, 241–257. [Google Scholar] [CrossRef]
- Tikvić, I.; Seletković, Z.; Ugarković, D.; Posavec, S.; Španjol, Ž. Dieback of Silver Fir (Abies alba Mill.) on Northern Velebit (Croatia). Period. Biol. 2008, 110, 137–143. [Google Scholar]
- Ficko, A.; Poljanec, A.; Bončina, A. Do changes in spatial distribution, structure and abundance of silver fir (Abies alba Mill.) indicate its decline? For. Ecol. Manag. 2001, 261, 844–854. [Google Scholar] [CrossRef]
- Čavlović, J.; Bončina, A.; Božić, M.; Goršić, E.; Simončič, T.; Teslak, K. Depression and growth recovery of silver fir in uneven-aged Dinaric forests in Croatia from 1901 to 2001. Forestry 2015, 88, 586–598. [Google Scholar] [CrossRef]
- Piedallu, C.; Dallery, D.; Bresson, C.; Legay, M.; Gégout, J.C.; Pierrat, R. Spatial vulnerability assessment of silver fir and Norway spruce dieback driven by climate warming. Landsc. Ecol. 2022, 38, 341–361. [Google Scholar] [CrossRef]
- Gazol, A.; Sangüesa-Barreda, G.; Camarero, J.J. Forecasting forest vulnerability to drought in Pyrenean silver fir forests showing dieback. Front. For. Glob. Change 2020, 3, 36. [Google Scholar] [CrossRef]
- Hereş, A.M.; Petritan, I.C.; Bigler, C.; Curtu, A.L.; Petrea, Ş.; Petritan, A.M.; Yuste, J.C. Legacies of past forest management determine current responses to severe drought events of conifer species in the Romanian Carpathians. Sci. Tot. Environ. 2021, 751, 141851. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, T.; Trentanovi, G.; Dainese, M.; Gobbo, G.; Lingua, E.; Sommacal, M. Stand structure and plant species diversity in managed and abandoned silver fir mature woodlands. For. Ecol. Manag. 2012, 270, 232–238. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; Oliva, J.; Montes, F.; Gazol, A. Past logging, drought and pathogens interact and contribute to forest dieback. Agric. For. Meteorol. 2015, 208, 85–94. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Molina-Valero, J.A.; Pérez-Cruzado, C.; Bigler, C.; Bugmann, H. Development and long-term dynamics of old-growth beech-fir forests in the Pyrenees: Evidence from dendroecology and dynamic vegetation modelling. For. Ecol. Manag. 2022, 524, 120541. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Kuemmerle, T. Where are Europe’s last primary forests? Div. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Santopuoli, G.; Vizzarri, M.; Spina, P.; Maesano, M.; Mugnozza, G.S.; Lasserre, B. How individual tree characteristics and forest management influence occurrence and richness of tree-related microhabitats in Mediterranean mountain forests. For. Ecol. Manag. 2022, 503, 119780. [Google Scholar] [CrossRef]
- Petritan, I.C.; Commarmot, B.; Hobi, M.L.; Petritan, A.M.; Bigler, C.; Abrudan, I.V.; Rigling, A. Structural patterns of beech and silver fir suggest stability and resilience of the virgin forest Sinca in the Southern Carpathians, Romania. For. Ecol. Manag. 2015, 356, 184–195. [Google Scholar] [CrossRef]
- Horvat, V.; García De Vicuña, J.; Biurrun, I.; García-Mijangos, I. Managed and unmanaged silver fir-beech forests show similar structural features in the western Pyrenees. iForest-Biogeosci. For. 2018, 11, 698. [Google Scholar] [CrossRef]
- Molina-Valero, J.A.; Camarero, J.J.; Alvarez-Gonzalez, J.G.; Cerioni, M.; Hevia, A.; Sanchez-Salguero, R.; Pérez-Cruzado, C. Mature forests hold maximum live biomass stocks. For. Ecol. Manag. 2021, 480, 118635. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J. Functional diversity enhances silver fir growth resilience to an extreme drought. J. Ecol. 2016, 104, 1063–1075. [Google Scholar] [CrossRef]
- Pardos, M.; Del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K.; et al. The greater resilience of mixed forests to drought mainly depends on their composition: Analysis along a climate gradient across Europe. For. Ecol. Manag. 2021, 481, 118687. [Google Scholar] [CrossRef]
- Grossiord, C. Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytol. 2020, 228, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gillerot, L.; Forrester, D.I.; Bottero, A.; Rigling, A.; Lévesque, M. Tree neighbourhood diversity has negligible effects on drought resilience of European beech, silver fir and Norway spruce. Ecosystems 2021, 24, 20–36. [Google Scholar] [CrossRef]
- Jourdan, M.; Kunstler, G.; Morin, X. How neighbourhood interactions control the temporal stability and resilience to drought of trees in mountain forests. J. Ecol. 2020, 108, 666–677. [Google Scholar] [CrossRef]
- Asbeck, T.; Großmann, J.; Paillet, Y.; Winiger, N.; Bauhus, J. The use of tree-related microhabitats as forest biodiversity indicators and to guide integrated forest management. Curr. For. Rep. 2021, 7, 59–68. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Sangüesa-Barreda, G.; Vicente-Serrano, S.M. Post-drought resilience after forest die-off: Shifts in regeneration, composition, growth and productivity. Front. Plant Sci. 2018, 9, 1546. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Rosinger, C.; Gorfer, M.; Berger, H.; Deltedesco, E.; Bässler, C.; Müller, J.; Seifert, L.; Rewald, B.; Godbold, D.L. Surviving trees and deadwood moderate changes in soil fungal communities and associated functioning after natural forest disturbance and salvage logging. Soil Biol. Biochem. 2022, 166, 108558. [Google Scholar] [CrossRef]
- Vigo, J.; Ninot, J.M. Los Pirineos; Peinado Lorca, M., Rivas-Martínez, S., Eds.; La vegetación de España; Publicaciones de la Universidad de Alcalá de Henares: Alcalá de Henares, Spain, 1987; pp. 351–384. [Google Scholar]
- Martín, E.; Cobos, J.M. Graves ataques de perforadores en los abetales de Ansó (Huesca). Boletín Sanid. Veg. Y Plagas 1986, 12, 297–298. [Google Scholar]
- Vicente-Serrano, S.M.; Tomas-Burguera, M.; Beguería, S.; Reig, F.; Latorre, B.; Peña-Gallardo, M.; Luna, M.Y.; Morata, A.; González-Hidalgo, J.C. A high resolution dataset of drought indices for Spain. Data 2017, 2, 22. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Cottam, G.; Curtis, J.T. The use of distance measure in phytosociological sampling. Ecology 1956, 37, 451–460. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Müller, E.H.R.; Stierlin, H.R. Sanasilva Tree Crown Photos with Percentages of Foliage Loss; WSL-Swiss Federal Institute for Forest, Snow and Landscape Research: Birmensdorf, Switzerland, 1990. [Google Scholar]
- Redfern, D.B.; Boswell, R.C. Assessment of crown condition in forest trees: Comparison of methods, sources of variation and observer bias. For. Ecol. Manag. 2004, 188, 149–160. [Google Scholar] [CrossRef]
- Solberg, S. Summer drought: A driver for crown condition and mortality of Norway spruce in Norway. For. Pathol. 2004, 34, 93–104. [Google Scholar] [CrossRef]
- Van Wagner, C.E. The line intersect method for forest fuel sampling. For. Sci. 1968, 14, 20–26. [Google Scholar]
- De Long, S.C.; Sutherland, G.D.; Daniels, L.D.; Heemskerk, B.H.; Storaunet, K.O. Temporal dynamics of snags and development of snag habitats in wet spruce-fir stands in east-central British Columbia. For. Ecol. Manag. 2008, 255, 3613–3620. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Klesse, S. Critical note on the application of the “two-third” spline. Dendrochronologia 2021, 65, 125786. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Abrams, M.D. Radial-growth averaging criteria for reconstructing disturbance histories from presettlement-origin oaks. Ecol. Monogr. 1997, 67, 225–249. [Google Scholar] [CrossRef]
- Guiot, J. The boostrapped response function. Tree-Ring Bull. 1991, 51, 39–41. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 10 January 2023).
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. 2022, R Package Version 1.7.4. Available online: https://CRAN.R-project.org/package=dplR (accessed on 10 January 2023).
- Altman, J.; Fibich, P.; Dolezal, J.; Aakala, T. TRADER: A package for Tree Ring Analysis of Disturbance Events in R. Dendrochonologia 2014, 32, 107–112. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. 2021, R Package Version 0.92. Available online: https://github.com/taiyun/corrplot (accessed on 10 January 2023).
- Hijmans, R. Raster: Geographic Data Analysis and Modeling. 2022, R Package Version 3.5-29. Available online: https://CRAN.R-project.org/package=raster (accessed on 10 January 2023).
- Bujoczek, L.; Bujoczek, M.; Zięba, S. How much, why and where? Deadwood in forest ecosystems: The case of Poland. Ecol. Indic. 2021, 121, 107027. [Google Scholar] [CrossRef]
- Kermavnar, J.; Marinšek, A.; Eler, K.; Kutnar, L. Evaluating short-term impacts of forest management and microsite conditions on understory vegetation in temperate fir-beech forests: Floristic, ecological, and trait-based perspective. Forests 2019, 10, 909. [Google Scholar] [CrossRef]
- Valerio, M.; Ibáñez, R.; Gazol, A. The role of canopy cover dynamics over a decade of changes in the understory of an Atlantic beech-oak forest. Forests 2021, 12, 938. [Google Scholar] [CrossRef]
- Horvat, V.; Heras, P.; García-Mijangos, I.; Biurrun, I. Intensive forest management affects bryophyte diversity in the western Pyrenean silver fir-beech forests. Biol. Conserv. 2017, 215, 81–91. [Google Scholar] [CrossRef]
- Chevaux, L.; Mårell, A.; Baltzinger, C.; Boulanger, V.; Cadet, S.; Chevalier, R.; Debaive, N.; Dumas, Y.; Gosselin, M.; Gosselin, F.; et al. of stand structure and ungulates on understory vegetation in managed and unmanaged forests. Ecol. Appl. 2022, 32, e2531. [Google Scholar] [CrossRef]
- Horvat, V.; Biurrun, I.; García-Mijangos, I. Herb layer in silver fir–beech forests in the western Pyrenees: Does management affect species diversity? For. Ecol. Manag. 2017, 385, 87–96. [Google Scholar] [CrossRef]


| Site (Code) | Latitude (N) | Longitude (−W, +E) | Elevation (m a.s.l.) | Diameter at 1.3 m (cm) | Basal Area of Silver Fir/Other Species 1 (m2 ha−1) | Density of Silver Fir/Other Species 1 (inds. ha−1) | Percentage of Severely Defoliated (Dead) Trees | Understory Plant Cover 1 (%) |
|---|---|---|---|---|---|---|---|---|
| Fago (FA) | 42.730 | −0.870 | 918 | 33.79 ± 15.68 | 68.7/1.6 (Ps) | 1273/20 (Fs, Ps) | 7.7 (0.0) | 2.30 (Bs), 0.90 (Hn) |
| Paco Ezpela (PE) | 42.741 | −0.827 | 1232 | 27.84 ± 10.23 | 21.7/17.3 (Fs)/16.8 (Ps) | 659/119 (Fs, Ps, Qs) | 26.3 (10.6) | 9.20 (Bs), 1.80 (Ia) |
| Gamueta (GA) | 42.880 | −0.800 | 1400 | 62.07 ± 17.17 | 37.6/18.1 (Fs) | 775/1867 (Fs) | 0.0 (0.0) | --- |
| Selva de Oza (SO) | 42.830 | −0.716 | 1195 | 35.67 ± 9.31 | 34.0/9.1 (Fs) | 1939/470 (Fs) | 0.0 (0.0) | --- |
| Paco Mayor (PM) | 42.700 | −0.640 | 1353 | 23.48 ± 9.77 | 22.0/14.4 (Ps)/4.2 (Fs) | 454/20 (Ps, Fs, Qs) | 24.1 (17.2) | 8.00 (Bs), 0.50 (Ia) |
| Lierde (LI) | 42.691 | −0.545 | 1222 | 43.18 ± 21.23 | 109.2/16.5 (Ps) | 910/68 (Ps) | 9.1 (0.0) | 6.60 (Bs), 0.50 (Ia) |
| Castiello de Jaca (CA) | 42.639 | −0.534 | 1175 | 39.35 ± 9.71 | 71.6/1.3 (QSf) | 564/47 (Ps, Qs) | 23.7 (21.1) | 8.40 (Bs), 0.60 (Ia) |
| Peña Oroel (PO) | 42.520 | −0.520 | 1604 | 29.05 ± 8.59 | 47.9/9.2 (Ps) | 1948/9 (Ps) | 18.2 (9.1) | 33.10 (Bs), 1.48 (Hn) |
| Paco de Villanúa (VI) | 42.680 | −0.510 | 1270 | 33.48 ± 9.43 | 68.0/2.1 (Ps) | 578/50 (Ps) | 18.2 (15.9) | 4.20 (Ia), 2.10 (Bs) |
| Diazas (DI) | 42.630 | −0.080 | 1528 | 31.41 ± 12.53 | 53.4/7.3 (Ps) | 917/35 (Ps, Sa) | 0.0 (0.0) | 4.20 (Bs), 0.30 (Dl) |
| Ballibierna (BA) | 42.630 | 0.560 | 1600 | 36.42 ± 14.41 | 88.1 | 927 | 0.0 (0.0) | 1.45 (Bs) |
| Site | No. Trees | No. Radii | Age at 1.3 m (y) | TRW (mm) | SD (mm) | BAI (cm2) | SD (cm2) | Trend BAI (cm2 y−1) | AR1 |
|---|---|---|---|---|---|---|---|---|---|
| FA | 25 | 50 | 137 | 2.50 | 1.01 | 17.38 | 0.84 | −0.35 | 0.75 |
| PE | 39 | 70 | 188 | 1.35 | 0.74 | 8.81 | 0.55 | 0.05 | 0.81 |
| GA | 29 | 59 | 462 | 1.52 | 0.76 | 21.16 | 0.65 | 0.13 | 0.86 |
| SO | 20 | 40 | 201 | 2.38 | 1.10 | 21.26 | 0.43 | 0.04 | 0.84 |
| PM | 38 | 74 | 170 | 1.53 | 0.93 | 12.94 | 0.55 | −0.57 | 0.81 |
| LI | 34 | 67 | 95 | 3.11 | 1.36 | 31.59 | 0.55 | −0.05 | 0.71 |
| CA | 17 | 34 | 225 | 1.14 | 0.68 | 7.51 | 0.37 | −0.14 | 0.83 |
| PO | 12 | 24 | 150 | 1.45 | 0.58 | 8.87 | 0.20 | −0.03 | 0.79 |
| VI | 33 | 55 | 140 | 1.82 | 0.85 | 12.22 | 0.71 | −0.31 | 0.84 |
| DI | 16 | 27 | 112 | 2.88 | 1.22 | 19.50 | 0.97 | 0.33 | 0.83 |
| BA | 32 | 54 | 173 | 2.36 | 1.05 | 24.33 | 0.53 | −0.15 | 0.81 |
| Site | Presence of Snags | Average Decomposition Grade (1–5) | Deadwood Volume (m3 ha−1) | Deadwood Cover (%) | Stump Basal Area of All Species–Silver Fir/Other Tree Species 1 (m2 ha−1) | Stump Density of All Species–Silver Fir/Other Tree Species 1 (No. ha−1) |
|---|---|---|---|---|---|---|
| FA | Yes | 2 | 51.2 | 3.8 | 9.9–5.2/3.7 (Ps) | 140–80/40 (Ps) |
| PE | Yes | 2 | 93.8 | 3.2 | 40.1–12.8/27.2 (Ps) | 400–180/200 (Ps) |
| GA | Yes | 3 | 83.5 | 2.5 | 70.9 | 160 |
| SZ | Yes | 3 | 29.9 | 1.7 | 27.2–14.7/1.29 (Fs) | 320–180/120 (Fs) |
| PM | Yes | 4 | 21.0 | 0.7 | 21.7–7.8/1.9 (Ps) | 300–120/20 (Ps) |
| LI | No | 1 | 23.1 | 1.2 | 10.4 | 60 |
| CA | Yes | 2.5 | 175.5 | 4.6 | 18.8 | 160 |
| PO | Yes | 2.5 | 81.1 | 3.0 | 5.6 | 300 |
| VI | Yes | 2 | 214.1 | 4.4 | 14.4 | 100 |
| DI | Yes | 3 | 85.4 | 3.1 | 37.55–2.0/26.4 (Ps) | 360–20/200 (Ps) |
| BA | No | 3 | 41.2 | 2.7 | 16.9–14.8/2.1 (Ps) | 320–220/100 (Ps) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazol, A.; González de Andrés, E.; Colangelo, M.; Valeriano, C.; Camarero, J.J. Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition. Forests 2023, 14, 713. https://doi.org/10.3390/f14040713
Gazol A, González de Andrés E, Colangelo M, Valeriano C, Camarero JJ. Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition. Forests. 2023; 14(4):713. https://doi.org/10.3390/f14040713
Chicago/Turabian StyleGazol, Antonio, Ester González de Andrés, Michele Colangelo, Cristina Valeriano, and Jesús Julio Camarero. 2023. "Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition" Forests 14, no. 4: 713. https://doi.org/10.3390/f14040713
APA StyleGazol, A., González de Andrés, E., Colangelo, M., Valeriano, C., & Camarero, J. J. (2023). Pyrenean Silver Fir Forests Retain Legacies of Past Disturbances and Climate Change in Their Growth, Structure and Composition. Forests, 14(4), 713. https://doi.org/10.3390/f14040713








