Impacts of Exotic Pests on Forest Ecosystems: An Update
Abstract
1. Background
- (1)
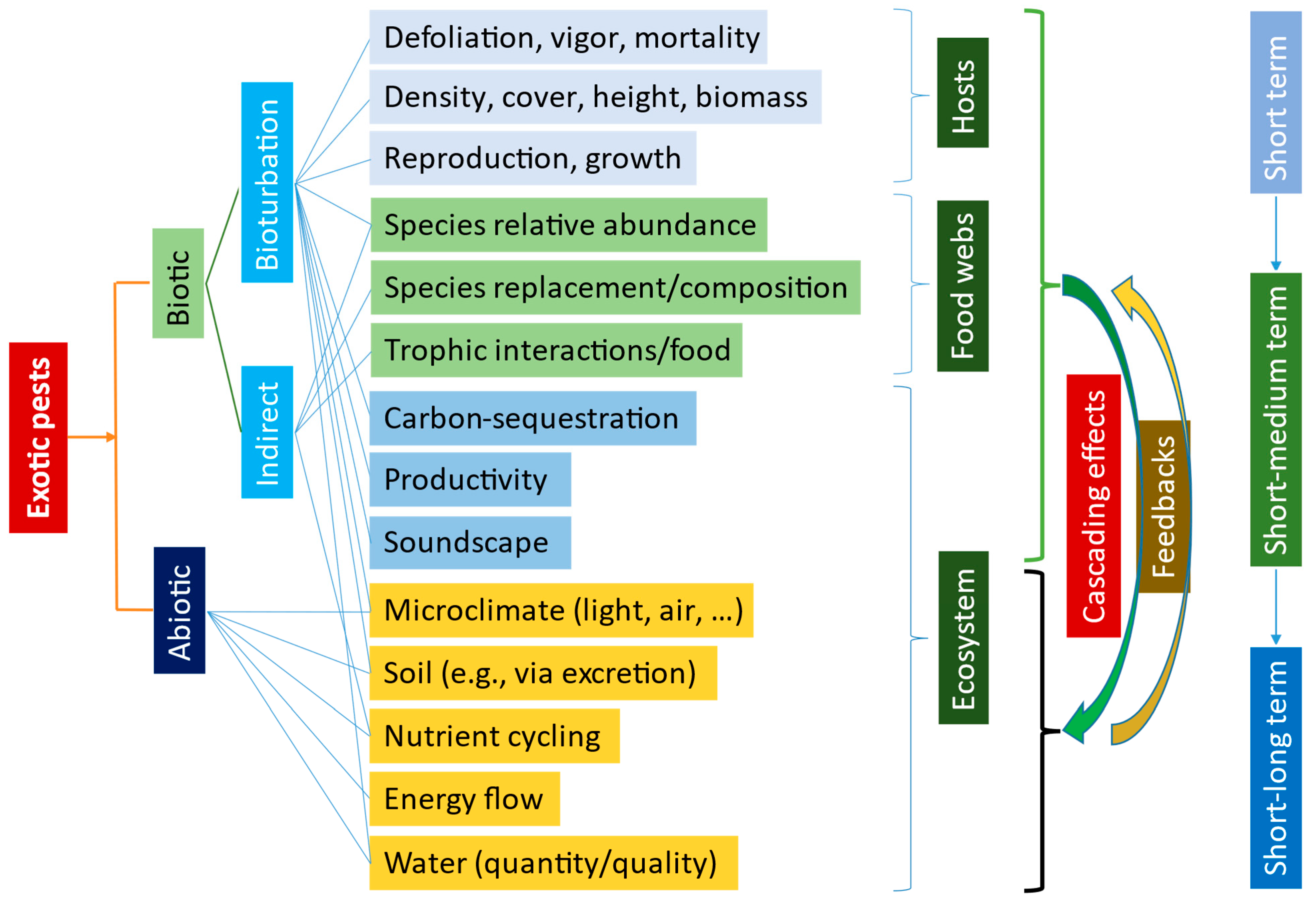
- Examining the direct and indirect biotic vs. abiotic effects of pests on forest ecosystems, followed by an assessment of short- and long-term impacts and feedback;
- (2)
- Assessing and discussing how climate change and major disturbances may facilitate such effects;
- (3)
- Proposing future research perspectives with a list of urgent questions and tasks for current and future studies;
- (4)
- Recommending corresponding management strategies following new research findings.
2. Biotic Effects
2.1. Direct Effects
2.2. Indirect Effects
3. Abiotic Effects
3.1. Direct Effects
3.2. Indirect Effects
4. Feedback Effects
5. Short- vs. Long-Term Effects
6. Climate Change May Enhance the Impacts of Pests on Forest Ecosystems
7. Perspectives on Future Ecosystem-Level Research
- (1)
- More rigorous studies are needed to examine exotic pests’ direct and indirect impacts on native insect species, especially those that provide key ecosystem functions necessary to maintain healthy ecosystems;
- (2)
- Large-scale studies are needed to examine regional, latitudinal, and elevational variations of ecosystem consequences due to exotic pest infestation;
- (3)
- Wherever possible, long-term studies that are newly initiated or are based on ongoing research (especially short-term projects) are needed to continue to detect chronic changes at the ecosystem level. Because invasion impacts can be highly context-dependent, the most helpful studies would include repeated observations and experiments at multiple sites that differ in the abundance of invertebrate invasive species [27];
- (4)
- More research to delineate exotic pests’ native ranges is critical for developing more effective biocontrol and management policies and practices [65];
- (5)
- (6)
- Future research should assess the feedback effects of altered soil and microbial communities due to exotic pest invasion on further pest and plant invasions;
- (7)
- We need to study whether affected ecosystems can recover to pre-invasion status, and if so, how fast, assuming the target pest can be successfully eradicated. Similarly, if the target pest cannot be eradicated, we need to better understand how cycles of pest infestation are related to regeneration of the host species;
- (8)
- Better and constantly improved models and tools are needed to predict the spread of invasive forest insects and diseases [70];
- (9)
- We need a better understanding of how exotic pest infestations directly and indirectly affect the services provided by forest ecosystems.
8. Urgent Tasks for Ecosystem-Level Management
- (1)
- In some regions and for some habitats, complete baseline information on exotic pests (e.g., the number of species, species identities, abundance, and distribution) is still lacking. Contributing to this is the difficulty of systematically monitoring the presence of exotic pests when their detection typically requires field surveys across broad scales, over which the pests may be spreading at a relatively high rate. Such information/knowledge gaps could be filled by enhanced efforts and investments in field surveys, inventories, and timely assessments at all levels (i.e., local, regional, national, and international), in addition to the wider incorporation of citizen science participation;
- (2)
- Many of the impacts from exotic pests are due to the fact that many such pests escape their natural enemies in their native habitats and many hosts in invaded regions never developed resistance and adaptations (if they ever can) [71]. Therefore, more efforts are needed to identify natural enemies that can be used in biological control;
- (3)
- For many exotic pests, basic research is urgently needed to investigate the species’ invasiveness, life history, genetics, dispersal mechanisms, and mutualism mechanisms [72];
- (4)
- The impact of invasive species research can be extended through large-scale citizen science activities and public education on exotic pests, including those targeted at inventorying species and monitoring their effects, among other efforts [73];
- (5)
- The introduction and spread of exotic pests can be interrupted by improving and implementing rules and regulations and strengthening quarantine law enforcement;
- (6)
- Closer collaborations in data and information sharing around the world should be performed [74].
- (1)
- Whether and/or to what level may an infested forest ecosystem recover if the exotic pest can be eradicated? Are there any concrete successful exam-ples?
- (2)
- What exotic pests may be enhanced or hindered by projected climate change, and how?
- (3)
- How do we better deal with multiple stresses including pest infestation, fire, and drought, at the same time?
- (4)
- How can advances in new technologies such as remote sensing, genetics, ar-tificial intelligence (AI), and machine learning assist in prevention and pest management?
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Herrmann, V.; Cass, W.B.; Williams, A.B.; Paull, S.J.; Gonzalez-Akre, E.B.; Helcoski, R.; Tepley, A.J.; Bourg, N.A.; Cosma, C.T. Long-Term Impacts of Invasive Insects and Pathogens on Composition, Biomass, and Diversity of Forests in Virginia’s Blue Ridge Mountains. Ecosystems 2021, 24, 89–105. [Google Scholar] [CrossRef]
- Lovett, G.M.; Canham, C.D.; Arthur, M.A.; Weathers, K.C.; Fitzhugh, R.D. Forest ecosystem responses to exotic pests and pathogens in eastern North America. Bioscience 2006, 56, 395–405. [Google Scholar] [CrossRef]
- Gandhi, K.J.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar] [CrossRef]
- Potter, K.M.; Escanferla, M.E.; Jetton, R.M.; Man, G. Important insect and disease threats to United States tree species and geographic patterns of their potential impacts. Forests 2019, 10, 304. [Google Scholar] [CrossRef]
- Wood, J.R.; Dickie, I.A.; Moeller, H.V.; Peltzer, D.A.; Bonner, K.I.; Rattray, G.; Wilmshurst, J.M. Novel interactions between non-native mammals and fungi facilitate establishment of invasive pines. J. Ecol. 2015, 103, 121–129. [Google Scholar] [CrossRef]
- Guo, Q.; Riitters, K.H.; Potter, K.M. A subcontinental analysis of forest fragmentation effects on insect and disease Invasion. Forests 2018, 9, 744. [Google Scholar] [CrossRef]
- Guo, Q.; Fei, S.; Potter, K.M.; Liebhold, A.M.; Wen, J. Tree diversity regulates forest pest invasion. Proc. Natl. Acad. Sci. USA 2019, 116, 7382–7386. [Google Scholar] [CrossRef]
- Abella, S.R. Forest decline after a 15-year “perfect storm” of invasion by hemlock woolly adelgid, drought, and hurricanes. Biol. Invasions 2018, 20, 695–707. [Google Scholar] [CrossRef]
- Jia, S.; Wang, X.; Hao, Z.; Bagchi, R. The effects of natural enemies on herb diversity in a temperate forest depend on species traits and neighbouring tree composition. J. Ecol. 2022. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M. Invasive forest defoliator contributes to the impending downward trend of oak dominance in eastern North America. Forestry 2016, 89, 284–289. [Google Scholar] [CrossRef]
- Zhong, Z.W.; Li, X.; Wang, D.L. Research progresses of plant-herbivore interactions. Chin. J. Plant Ecol. 2021, 45, 1036–1048. [Google Scholar] [CrossRef]
- Crowley, K.F.; Lovett, G.M.; Arthur, M.A.; Weathers, K.C. Long-term effects of pest-induced tree species change on carbon and nitrogen cycling in northeastern US forests: A modeling analysis. Forest Ecol. Manag. 2016, 372, 269–290. [Google Scholar] [CrossRef]
- Wardle, D.A.; Peltzer, D.A. Impacts of invasive biota in forest ecosystems in an aboveground–belowground context. Biol. Invasions 2017, 19, 3301–3316. [Google Scholar] [CrossRef]
- Kristensen, J.A.; Metcalfe, D.B.; Rousk, J. The biogeochemical consequences of litter transformation by insect herbivory in the Subarctic: A microcosm simulation experiment. Biogeochemistry 2018, 138, 323–336. [Google Scholar] [CrossRef]
- Messing, R.H.; Wright, M.G. Biological control of invasive species: Solution or pollution? Front. Ecol. Environ. 2006, 4, 132–140. [Google Scholar] [CrossRef]
- De Clercq, P.; Mason, P.G.; Babendreier, D. Benefits and risks of exotic biological control agents. BioControl 2011, 56, 681–698. [Google Scholar] [CrossRef]
- Sniezko, R.A.; Koch, J. Breeding trees resistant to insects and diseases: Putting theory into application. Biol. Invasions 2017, 19, 3377–3400. [Google Scholar] [CrossRef]
- Wilson, C.M.; Schaeffer, R.N.; Hickin, M.L.; Rigsby, C.M.; Sommi, A.F.; Thornber, C.S.; Orians, C.M.; Preisser, E.L. Chronic impacts of invasive herbivores on a foundational forest species: A whole-tree perspective. Ecology 2018, 99, 1783–1791. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Aber, J.D.; Canham, C.D. Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can. J. For. Res. 1999, 29, 630–645. [Google Scholar] [CrossRef]
- Fei, S.; Morin, R.S.; Oswalt, C.M.; Liebhold, A.M. Biomass losses resulting from insect and disease invasions in US forests. Proc. Natl. Acad. Sci. USA 2019, 116, 17371–17376. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.M.; Gallardo, A.; Gómez-Aparicio, L. Pathogen-induced tree mortality interacts with predicted climate change to alter soil respiration and nutrient availability in Mediterranean systems. Biogeochemistry 2019, 142, 53–71. [Google Scholar] [CrossRef]
- Bergemann, S.E.; Kordesch, N.C.; VanSant-Glass, W.; Garbelotto, M. Implications of tanoak decline in forests impacted by Phytophthora ramorum: Girdling decreases the soil hyphal abundance of ectomycorrhizal fungi associated with Notholithocarpus densiflorus. Madroño 2013, 60, 95–106. [Google Scholar] [CrossRef]
- Bjelke, U.; Boberg, J.; Oliva, J.; Tattersdill, K.; McKie, B.G. Dieback of riparian alder caused by the Phytophthora alni complex: Projected consequences for stream ecosystems. Freshw. Biol. 2016, 61, 565–579. [Google Scholar] [CrossRef]
- Block, C.E.; Knoepp, J.D.; Elliott, K.J.; Fraterrigo, J.M. Impacts of hemlock loss on nitrogen retention vary with soil nitrogen availability in the southern Appalachian mountains. Ecosystems 2012, 15, 1108–1120. [Google Scholar] [CrossRef]
- Brantley, S.; Ford, C.R.; Vose, J.M. Future species composition will affect forest water use after loss of eastern hemlock from southern Appalachian forests. Ecol. Appl. 2013, 23, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.K.; Vilà, M.; Cabeza, M. Global meta-analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Global Ecol. Biogeogr. 2016, 25, 596–606. [Google Scholar] [CrossRef]
- De la Fuente, B.; Beck, P.S. Invasive species may disrupt protected area networks: Insights from the pine wood nematode spread in Portugal. Forests 2018, 9, 282. [Google Scholar] [CrossRef]
- Edburg, S.L.; Hicke, J.A.; Brooks, P.D.; Pendall, E.G.; Ewers, B.E.; Norton, U.; Gochis, D.; Gutmann, E.D.; Meddens, A.J. Cascading impacts of bark beetle-caused tree mortality on coupled biogeophysical and biogeochemical processes. Front. Ecol. Environ. 2012, 10, 416–424. [Google Scholar] [CrossRef]
- Ellison, A.M.; Orwig, D.A.; Fitzpatrick, M.C.; Preisser, E.L. The past, present, and future of the hemlock woolly adelgid (Adelges tsugae) and its ecological interactions with eastern hemlock (Tsuga canadensis) forests. Insects 2018, 9, 172. [Google Scholar] [CrossRef]
- Hogg, B.N.; Daane, K.M. Impacts of exotic spider spillover on resident arthropod communities in a natural habitat. Ecological Entomol. 2015, 40, 69–77. [Google Scholar] [CrossRef]
- Ignace, D.D.; Fassler, A.; Bellemare, J. Decline of a foundation tree species due to invasive insects will trigger net release of soil organic carbon. Ecosphere 2018, 9, e02391. [Google Scholar] [CrossRef]
- lM-Arnold, A.; Grüning, M.; Simon, J.; Reinhardt, A.-B.; Lamersdorf, N.; Thies, C. Forest defoliator pests alter carbon and nitrogen cycles. R. Soc. Open Sci. 2016, 3, 160361. [Google Scholar] [CrossRef] [PubMed]
- Knoepp, J.D.; Vose, J.M.; Clinton, B.D.; Hunter, M.D. Hemlock infestation and mortality: Impacts on nutrient pools and cycling in Appalachian forests. Soil Sci. Soc. Am. J. 2011, 75, 1935–1945. [Google Scholar] [CrossRef]
- Letheren, A.; Hill, S.; Salie, J.; Parkman, J.; Chen, J. A little bug with a big bite: Impact of hemlock woolly adelgid infestations on forest ecosystems in the eastern USA and potential control strategies. Int. J. Environ. Res. Public Health 2017, 14, 438. [Google Scholar] [CrossRef]
- Lovett, G.M.; Arthur, M.A.; Weathers, K.C.; Griffin, J.M. Effects of introduced insects and diseases on forest ecosystems in the Catskill Mountains of New York. Ann. New York Acad. Sci. 2013, 1298, 66–77. [Google Scholar] [CrossRef]
- Milligan, P.D.; Martin, T.A.; Pringle, E.G.; Riginos, C.; Mizell, G.M.; Palmer, T.M. A soil-nesting invasive ant disrupts carbon dynamics in saplings of a foundational ant–plant. J. Ecol. 2022, 110, 359–373. [Google Scholar] [CrossRef]
- Nisbet, D.; Kreutzweiser, D.; Sibley, P.; Scarr, T. Ecological risks posed by emerald ash borer to riparian forest habitats: A review and problem formulation with management implications. For. Ecol. Manag. 2015, 358, 165–173. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Tingley, M.W.; Orwig, D.A.; Field, R.; Motzkin, G. Avian response to removal of a forest dominant: Consequences of hemlock woolly adelgid infestations. J. Biogeogr. 2002, 29, 1505–1516. [Google Scholar] [CrossRef]
- Gandhi, K.J.; Smith, A.; Hartzler, D.M.; Herms, D.A. Indirect effects of emerald ash borer-induced ash mortality and canopy gap formation on epigaeic beetles. Environ. Entomol. 2014, 43, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Klooster, W.S.; Gandhi, K.J.; Long, L.C.; Perry, K.I.; Rice, K.B.; Herms, D.A. Ecological impacts of emerald ash borer in forests at the epicenter of the invasion in North America. Forests 2018, 9, 250. [Google Scholar] [CrossRef]
- Guo, Q.F.; Ren, H. Productivity as related to diversity and age in planted versus natural forests. Global Ecol. Biogeogr. 2014, 23, 1461–1471. [Google Scholar] [CrossRef]
- Adame, P.; Alberdi, I.; Canellas, I.; Hernandez, L.; Aguirre, A.; Ruano, A.; Moreno-Fernandez, D.; González, A.I.; Torres, M.B.; Montes, F. Drivers and spread of non-native pests in forests: The case of Gonipterus platensis in Spanish Eucalyptus plantations. For. Ecol. Manag. 2022, 510, 120104. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien invasive pathogens and pests harming trees, forests, and plantations: Pathways, global consequences and management. Forests 2021, 12, 1364. [Google Scholar] [CrossRef]
- Cai, W.; Yang, C.; Wang, X.; Wu, C.; Larrieu, L.; Lopez-Vaamonde, C.; Wen, Q.; Douglas, W.Y. The ecological impact of pest-induced tree dieback on insect biodiversity in Yunnan pine plantations, China. For. Ecol. Manag. 2021, 491, 119173. [Google Scholar] [CrossRef]
- Debnam, S.; Saez, A.; Aizen, M.A.; Callaway, R.M. Exotic insect pollinators and native pollination systems. Plant Ecol. 2021, 222, 1075–1088. [Google Scholar] [CrossRef]
- Hoven, B.M.; Gorchov, D.L.; Knight, K.S.; Peters, V.E. The effect of emerald ash borer-caused tree mortality on the invasive shrub Amur honeysuckle and their combined effects on tree and shrub seedlings. Biol. Invasions 2017, 19, 2813–2836. [Google Scholar] [CrossRef]
- Fortini, L.B.; Kaiser, L.R.; Keith, L.M.; Price, J.; Hughes, R.F.; Jacobi, J.D.; Friday, J. The evolving threat of Rapid ‘Ōhi ‘a Death (ROD) to Hawai ‘i’s native ecosystems and rare plant species. For. Ecol. Manag. 2019, 448, 376–385. [Google Scholar] [CrossRef]
- Hughes, M.A.; Juzwik, J.; Harrington, T.C.; Keith, L.M. Pathogenicity, symptom development, and colonization of Metrosideros polymorpha by Ceratocystis lukuohia. Plant Dis. 2020, 104, 2233–2241. [Google Scholar] [CrossRef]
- Yelenik, S.G.; Roy, K.; Stallman, J. Successful restoration of Metrosideros polymorpha (ʻōhiʻa) is possible in forest sites with active Rapid ʻŌhiʻa Death infections. Restor Ecol. 2020, 28, 1257–1261. [Google Scholar] [CrossRef]
- Peng, S.L.; Xiang, Y. The invasion of exotic plants and effects of ecosystems. Acta Ecol. Sin. 1999, 19, 560–569. [Google Scholar]
- Morillas, L.; Pangle, R.E.; Maurer, G.E.; Pockman, W.T.; Mcdowell, N.; Huang, C.W.; Krofcheck, D.J.; Fox, A.M.; Sinsabaugh, R.L.; Rahn, T.A. Tree mortality decreases water availability and ecosystem resilience to drought in piñon-juniper woodlands in the southwestern US. J. Geophys. Res. Biogeosciences 2017, 122, 3343–3361. [Google Scholar] [CrossRef]
- Fulton, S.; West, B. Forestry impacts on water quality. South. For. Resour. Assess. 2002, 21, 635. [Google Scholar]
- Warren, R.J.; McMillan, A.; King, J.R.; Chick, L.; Bradford, M.A. Forest invader replaces predation but not dispersal services by a keystone species. Biol. Invasions 2015, 17, 3153–3162. [Google Scholar] [CrossRef]
- Chang, C.-H.; Bartz, M.L.; Brown, G.; Callaham, M.A.; Cameron, E.K.; Dávalos, A.; Dobson, A.; Görres, J.H.; Herrick, B.M.; Ikeda, H. The second wave of earthworm invasions in North America: Biology, environmental impacts, management and control of invasive jumping worms. Biol. Invasions 2021, 23, 3291–3322. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Yorks, T.E.; Leopold, D.J.; Raynal, D.J. Effects of Tsuga canadensis mortality on soil water chemistry and understory vegetation: Possible consequences of an invasive insect herbivore. Can. J. For. Res. 2003, 33, 1525–1537. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil 2012, 352, 389–403. [Google Scholar] [CrossRef]
- Dietze, M.C.; Matthes, J.H. A general ecophysiological framework for modelling the impact of pests and pathogens on forest ecosystems. Ecol. Lett. 2014, 17, 1418–1426. [Google Scholar] [CrossRef]
- Rodriguez, J.; Thompson, V.; Rubido-Bara, M.; Cordero-Rivera, A.; Gonzalez, L. Herbivore accumulation on invasive alien plants increases the distribution range of generalist herbivorous insects and supports proliferation of non-native insect pests. Biol. Invasions 2019, 21, 1511–1527. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Barnes, I.; de Beer, Z.W.; Roux, J.; Wingfield, B.D.; Taerum, S.J. Novel associations between ophiostomatoid fungi, insects and tree hosts: Current status—future prospects. Biol. Invasions 2017, 19, 3215–3228. [Google Scholar] [CrossRef]
- Bentz, B.J.; Duncan, J.P.; Powell, J.A. Elevational shifts in thermal suitability for mountain pine beetle population growth in a changing climate. Forestry 2016, 89, 271–283. [Google Scholar] [CrossRef]
- Guo, Q. Intercontinental biotic invasions: What can we learn from native populations and habitats? Biol. Invasions 2006, 8, 1451–1459. [Google Scholar] [CrossRef]
- Li, Y.; Bateman, C.; Skelton, J.; Wang, B.; Black, A.; Huang, Y.-T.; Gonzalez, A.; Jusino, M.A.; Nolen, Z.J.; Freeman, S. Preinvasion Assessment of Exotic Bark Beetle-Vectored Fungi to Detect Tree-Killing Pathogens. Phytopathology 2022, 112, 261–270. [Google Scholar] [CrossRef]
- Koch, F.H.; Yemshanov, D.; Haight, R.G.; MacQuarrie, C.J.; Liu, N.; Venette, R.; Ryall, K. Optimal invasive species surveillance in the real world: Practical advances from research. Emerg. Top. Life Sci. 2020, 4, 513–520. [Google Scholar]
- Wang, C.J.; Wang, R.; Yu, C.M.; Dang, X.P.; Sun, W.G.; Li, Q.F.; Wang, X.T.; Wan, J.Z. Risk assessment of insect pest expansion in alpine ecosystems under climate change. Pest Manag. Sci. 2021, 77, 3165–3178. [Google Scholar] [CrossRef]
- Raffa, K.F.; Brockerhoff, E.G.; Grégoire, J.-C.; Hamelin, R.C.; Liebhold, A.M.; Santini, A.; Venette, R.C.; Wingfield, M.J. Approaches to Forecasting Damage by Invasive Forest Insects and Pathogens: A Cross-Assessment. Bioscience 2023, biac108. [Google Scholar] [CrossRef]
- Hudgins, E.J.; Liebhold, A.M.; Leung, B. Predicting the spread of all invasive forest pests in the United States. Ecol. Lett. 2017, 20, 426–435. [Google Scholar] [CrossRef]
- Nunez-Mir, G.C.; Liebhold, A.M.; Guo, Q.; Brockerhoff, E.G.; Jo, I.; Ordonez, K.; Fei, S. Biotic resistance to exotic invasions: Its role in forest ecosystems, confounding artifacts, and future directions. Biol. Invasions 2017, 1–13. [Google Scholar] [CrossRef]
- Ward, S.F.; Riggins, J.J. Drivers of invasion by laurel wilt of redbay and sassafras in the southeastern US. Landsc. Ecol. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.; Pocock, M.J.; Bonte, J.; Fernandez-Conradi, P.; Valdés-Correcher, E. Citizen Science and Monitoring Forest Pests: A Beneficial Alliance? Curr. For. Rep. 2023, 9, 15–32. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Campbell, F.T.; Gordon, D.R.; Guo, Q.; Havill, N.; Kinder, B.; MacKenzie, R.; Lance, D.R.; Pearson, D.E.; Sing, S.E. The Role of International Cooperation in Invasive Species Research. In Invasive Species in Forests and Rangelands of the United States; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer: New York, NY, USA, 2021; pp. 293–303. [Google Scholar]
- Mina, M.; Messier, C.; Duveneck, M.J.; Fortin, M.-J.; Aquilué, N. Managing for the unexpected: Building resilient forest landscapes to cope with global change. Glob. Change Biol. 2022, 28, 4323–4341. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol.Biot. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Gamez, S.; Raban, R.R.; Marshall, J.M.; Alphey, L.; Li, M.; Rasgon, J.L.; Akbari, O.S. Combating mosquito-borne diseases using genetic control technologies. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Potter, K.M.; Riitters, K.H.; Guo, Q. Non-native tree regeneration indicates regional and national risks from current invasions. Front. For. Glob. Change 2022, 5, 966407. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Guo, Q. Possible cryptic invasion through "back introduction"? Front. Ecol. Environ. 2005, 3, 470–471. [Google Scholar] [CrossRef]
- Kolbe, J.J.; Wegener, J.E.; Stuart, Y.E.; Milstead, U.; Boronow, K.E.; Harrison, A.S.; Losos, J.B. An incipient invasion of brown anole lizards (Anolis sagrei) into their own native range in the Cayman Islands: A case of cryptic back-introduction. Biol. Invasions 2017, 19, 1989–1998. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wu, Z.-Z.; Yu, C.-M.; Wang, X.-T.; Wang, R.; Wan, J.-Z. Habitat heterogeneity and topographic variation as the drivers of insect pest distributions in alpine landscapes. Acta Ecol. Sin. 2022. [Google Scholar] [CrossRef]

| Source | Forest Pests | Community | Ecosystem-Level Impacts | Study Type |
|---|---|---|---|---|
| Avila et al. [22] | Phytophthora cinnamomi | Quercus suber | Altered biogeochemical cycles, soil respiration, and nutrient availability. | Field |
| Anderson-Teixeira et al. [2] | All pests on 66 plots | Oaks forests, Hemlock forests, ash forests | Reduced biomass and carbon storage. | Field |
| Bergemann et al. [23] | Phytophthora ramorum | Notholithocarpus densiflorus forest | Reduction in the hyphal abundance of ectomycorrhizal fungi from soil thus affecting decomposition, nutrient acquisition, and ecosystem succession. | Field |
| Bjelke et al. [24] | Phytophthora alni | Alder trees (Alnus spp.) | Reduced soil nitrogen, shade, and river/stream bank stability, changes in food webs of both terrestrial and aquatic. | Field |
| Block et al. [25] | Hemlock woolly adelgid | Hemlock forests | Decrease N retention. | Field |
| Brantley et al. [26] | Hemlock woolly adelgid | Hemlock forests | Reduced annual forest transpiration (Et); species replaced by deciduous species may increase forest Et but reduce stream discharge. | Field |
| Cameron et al. [27] | Terrestrial invertebrate invaders | Terrestrial ecosystems (general) | Single invaders increased soil nitrogen pools, while multiple species did not. | Review |
| Crowley et al. [13] | Beech bark disease, hemlock woolly adelgid (Adelges tsugae), sudden oak death | Tree species replacement | NPP lower, net C loss (first 100 years), total N lower. | Simulation |
| De la Fuente and Beck [28] | Pine wood nematode | Coniferous forests | Disrupt the coherence and functionality of protected area networks. | Field |
| Edburg et al. [29] | Bark beetle | Lodgepole pine forests | Reduced plant C-uptake and GPP, increased decomposition and nutrient loss; effects are time (stage)-dependent. | Conceptual |
| Ellison et al. [30] | Hemlock woolly adelgid | Hemlock (T. canadensis) forests | Reset successional sequences, homogenized biological diversity at landscape scales, altered hydrological dynamics, and changed forest stands from carbon sinks into carbon sources. | Review |
| Hogg and Daane [31] | Cheiracanthium mildei L. (spider) | Oak woodland Vineyards | Cascading negative cross-trophic effects that ultimately reduce ecosystem service. | Field |
| Ignace et al. [32] | Hemlock woolly adelgid, elongate hemlock scale (Fiorinia externa) | Hemlock (T. canadenis) forests | Dramatic increases in soil respiration; decrease in soil organic layer mass and in the C:N of the remaining organic material; and decline in soil organic layer C storage. | Field |
| l-M-Arnold et al. [33] | Winter moth and mottled umber | Deciduous oak forests | Increased soil C and N levels but reduced C:N ratio. | Field |
| Jenkins et al. [20] | Hemlock woolly adelgid | Eastern hemlock (Tsuga canadensis) forests | Light availability to the understory and seedling regeneration both increased. Net N mineralization, nitrification, and N turnover increased. Inorganic N availability and nitrification rates increased dramatically, leading to nitrate leaching. | Field |
| Knoepp et al. [34] | Hemlock woolly adelgid | Hemlock (T. canadensis) forests | During the 4-year study, litterfall composition changed, hemlock plots had cooler spring soil temperatures, greater surface soil and forest floor total C than hardwood plots. | Field |
| Kristensen et al. [15] | Geometrid moth | Birch forests | Lower foliar C, higher soil C-accumulation, reduced C:N of mineralization. | Microcosm experiment |
| Letheren et al. [35] | Hemlock woolly adelgid | Hemlock (T. canadensis) forests | Negative impacts on the diversity and stability of ecosystems. | Review |
| Lovett et al. [36] | Spongy moth (Lymantria dispar), hemlock woolly adelgid, beech bark disease, Asian long-horned beetle | Oak forests, beech forests, hemlock forests, sugar maple forests, white ash forests | Reduction in productivity, disruption of nutrient cycles, and reduction in seed production. | Field |
| Milligan et al. [37] | Soil-nesting invasive ant (Pheidole megacephala) | Acacia drepanolobium saplings | Reduced carbon fixation and storage. | Field |
| Nisbet et al. [38] | Emerald ash borer | Ash trees (riparian forests) | Reductions in high-quality leaf litter, large canopy openings. | Review and synthesis |
| Seidl et al. [39] | Five detrimental alien pests | Forests in Europe | Projected to significantly reduce the long-term C storage potential of European forests. | Simulation/modeling |
| Wilson et al. [19] | Hemlock woolly adelgid, hemlock scale (Fiorinia externa) | Hemlock (T. canadensis) forests | Lower above/belowground biomass ratios, more needle loss, impacted the concentrations of primary metabolites, increased free amino acids local, reduction in starch, and manipulation of nitrogen pools. | Field |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Potter, K.M.; Ren, H.; Zhang, P. Impacts of Exotic Pests on Forest Ecosystems: An Update. Forests 2023, 14, 605. https://doi.org/10.3390/f14030605
Guo Q, Potter KM, Ren H, Zhang P. Impacts of Exotic Pests on Forest Ecosystems: An Update. Forests. 2023; 14(3):605. https://doi.org/10.3390/f14030605
Chicago/Turabian StyleGuo, Qinfeng, Kevin M. Potter, Hai Ren, and Peixia Zhang. 2023. "Impacts of Exotic Pests on Forest Ecosystems: An Update" Forests 14, no. 3: 605. https://doi.org/10.3390/f14030605
APA StyleGuo, Q., Potter, K. M., Ren, H., & Zhang, P. (2023). Impacts of Exotic Pests on Forest Ecosystems: An Update. Forests, 14(3), 605. https://doi.org/10.3390/f14030605






