Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sample Collection
2.3. Chemical and Physical Measurements
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Soil C, N, and P Concentrations and Their Stoichiometric Ratios
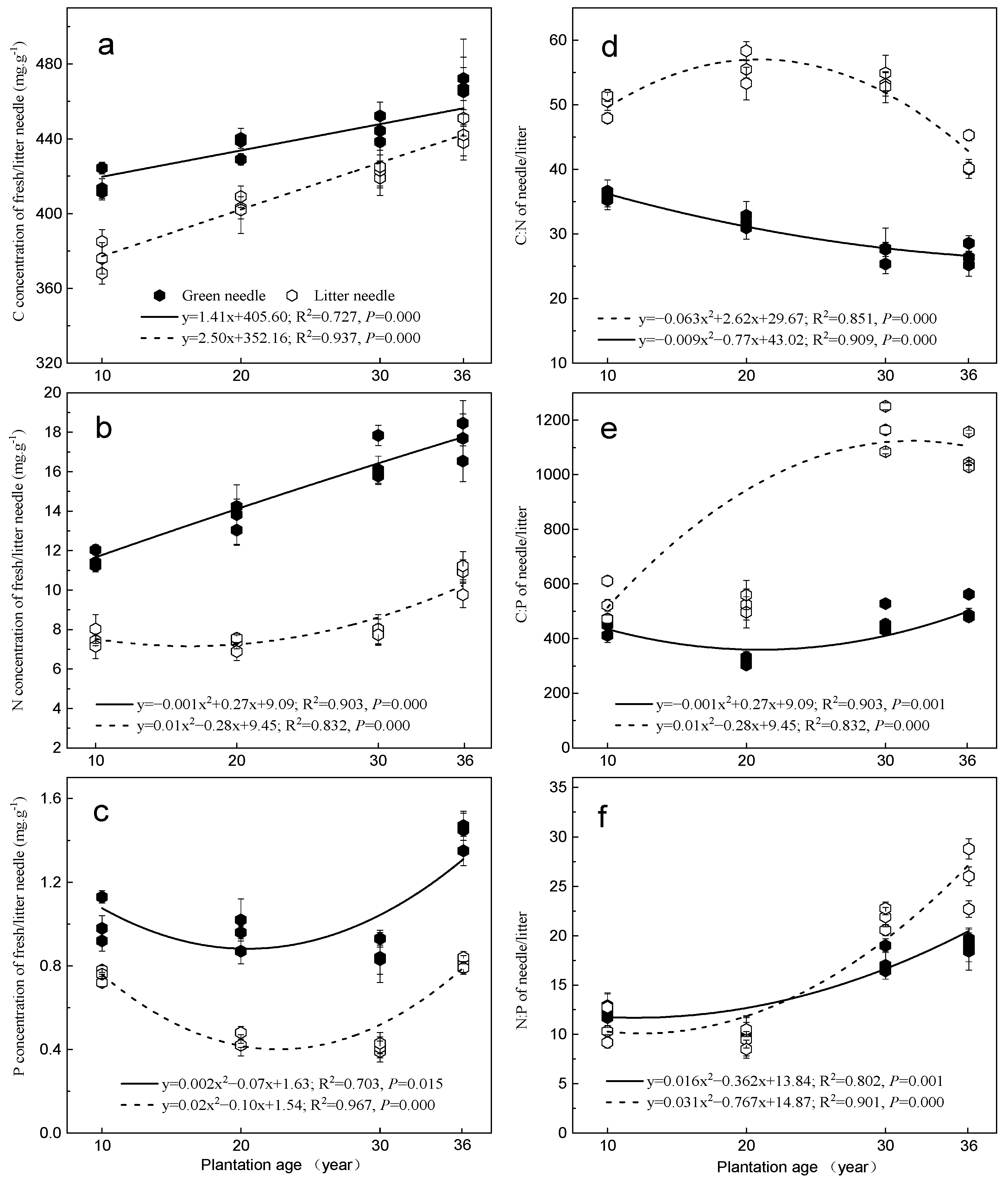
3.2. Needle C, N, and P Concentrations and Their Stoichiometric Ratios
3.3. Needle NuRE and Their Relationships with Soil Stoichiometry
3.4. Pattern of Needle Stoichiometric Homeostasis
4. Discussion
4.1. Changes in Soil C, N, and P Concentrations along a Chronosequence
4.2. Changes in Nutrient Limitation and Nutrient Resorption Efficiency along a Chronosequence
4.3. Interrelationship of Needle Nutrient Utilization, Stoichiometry, and Homeostasis
4.4. Inspiration for P. massoniana Plantation Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.; Xu, W.; Zhou, G.; Bai, Y.; Li, J.; Tang, X.; Chen, D.; Liu, Q.; Ma, W.; Xiong, G.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef]
- Chang, Y.; Zhong, Q.; Yang, H.; Xu, C.; Hua, W.; Li, B. Patterns and driving factors of leaf C, N, and P stoichiometry in two forest types with different stand ages in a mid-subtropical zone. For. Ecosyst. 2022, 9, 100005. [Google Scholar] [CrossRef]
- Erb, M.; Lu, J. Soil abiotic factors influence interactions between belowground herbivores and plant roots. J. Exp. Bot. 2013, 64, 1295–1303. [Google Scholar] [CrossRef]
- Chen, R.; Ran, J.; Hu, W.; Dong, L.; Ji, M.; Jia, X.; Lu, J.; Gong, H.; Aqeel, M.; Yao, S.; et al. Effects of biotic and abiotic factors on forest biomass fractions. Natl. Sci. Rev. 2021, 8, nwab025. [Google Scholar] [CrossRef]
- Fisher, J.B.; Malhi, Y.; Torres, I.C.; Metcalfe, D.B.; van de Weg, M.J.; Meir, P.; Silva-Espejo, J.E.; Huasco, W.H. Nutrient limitation in rainforests and cloud forests along a 3,000–m elevation gradient in the Peruvian Andes. Oecologia 2012, 172, 889–902. [Google Scholar] [CrossRef]
- Zhang, P.; Lü, X.T.; Li, M.H.; Wu, T.; Jin, G. N limitation increases along a temperate forest succession: Evidences from leaf stoichiometry and nutrient resorption. J. Plant Ecol. 2022, 15, 1021–1035. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.Å.M.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth––Modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta–analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Gundale, M.J.; Futter, M.; Ring, E.; Nordin, A.; Näsholm, T.; Laudon, H. Nitrogen dynamics in managed boreal forests: Recent advances and future research directions. Ambio 2016, 45, 175–187. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Gillooly, J.F. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 2009, 12, 369–384. [Google Scholar] [CrossRef]
- Zechmeister–Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Fink, P.; Goto, A.; Hood, J.M.; Jonas, J.; Kato, S. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, L.; Zhang, H.; Guo, Z.; Wang, G.G.; Smithd, W.K.; Wu, T. Does stoichiometric homeostasis differ among tree organs and with tree age? Forest Ecol. Manag. 2019, 453, 117637. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hättenschwiler, S. An experimental test of the hypothesis of non–homeostatic consumer stoichiometry in a plant litter–microbe system. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.; Guo, F.; Ma, X. Influence of long–term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Côté, B.; Fyles, J.W.; Djalilvand, H. Increasing N and P resorption efficiency and proficiency in northern deciduous hardwoods with decreasing foliar N and P concentrations. Ann. For. Sci. 2002, 59, 275–281. [Google Scholar] [CrossRef]
- Krishna, M.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant–soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef]
- Wang, K.; Wang, G.G.; Song, L.; Zhang, R.; Yan, T.; Li, Y. Linkages between nutrient resorption and ecological stoichiometry and homeostasis along a chronosequence of Mongolian pine plantations. Front. Plant Sci. 2021, 12, 692683. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Annapurna, C.; Raghubanshi, A.; Singh, J. Effect of leaf habit and soil type on nutrient resorption and conservation in woody species of a dry tropical environment. Can. J. Bot. 2001, 79, 1066–1075. [Google Scholar]
- Gerdol, R.; Iacumin, P.; Brancaleoni, L. Differential effects of soil chemistry on the foliar resorption of nitrogen and phosphorus across altitudinal gradients. Funct. Ecol. 2019, 33, 1351–1361. [Google Scholar] [CrossRef]
- Pan, J.; Guo, Q.; Li, H.; Luo, S.; Zhang, Y.; Yao, S.; Fan, X.; Sun, X.; Qi, Y. Dynamics of soil nutrients, microbial community structure, enzymatic activity, and their relationships along a chronosequence of Pinus massoniana plantations. Forests 2021, 12, 376. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Liang, H.; Huang, J.G.; Ma, Q.; Li, J.; Wang, Z.; Guo, X.; Zhu, H.; Jiang, S.; Zhou, P.; Yu, B.; et al. Contributions of competition and climate on radial growth of Pinus massoniana in subtropics of China. Agric. For. Meteorol. 2019, 274, 7–17. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, L.; Fang, Q.; Ding, G. Differences in soil physicochemical properties in different–aged Pinus massoniana plantations in Southwest China. Forests 2021, 12, 987. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Huang, G.; Zhao, S.; Dong, F.; Zhang, Y.; He, W.; Wang, P.; Yan, Z. Growth and nutrient stoichiometry responses to N and P fertilization of 8–year–old Masson pines (Pinus massoniana) in subtropical China. Plant Soil 2022, 477, 343–356. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Yin, H.; Liu, S.; Chen, G.; Fan, C.; Feng, M.; Li, X. The effects of crop tree management on the fine root traits of Pinus massoniana in Sichuan Province, China. Forests 2020, 11, 351. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Stone, W. A new colorimetric reagent for micro determination of ammonia. Proc. Soc. Exp. Biol. Med. 1956, 93, 589–591. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2003; pp. 53–62. [Google Scholar]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, W.; Gao, D.; Dai, Y.; Deng, J.; Yang, G. Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. Catena 2018, 161, 85–95. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Z.; Pan, X.; Chen, X.; Derrien, D.; Hu, F.; Liu, M.; Hättenschwiler, S. Carbon and nitrogen transfer from litter to soil is higher in slow than rapid decomposing plant litter: A synthesis of stable isotope studies. Soil Biol. Biochem. 2021, 156, 108196. [Google Scholar] [CrossRef]
- Ni, X.; Lin, C.; Chen, G.; Xie, J.; Yang, Z.; Liu, X.; Xiong, D.; Xu, C.; Yue, K.; Wu, F.; et al. Decline in nutrient inputs from litterfall following forest plantation in subtropical China. Forest Ecol. Manag. 2021, 496, 119445. [Google Scholar] [CrossRef]
- Deng, J.; Wang, S.; Ren, C.; Zhang, W.; Zhao, F.; Li, X.; Zhang, D.; Han, X.; Yang, G. Nitrogen and phosphorus resorption in relation to nutrition limitation along the chronosequence of black locust (Robinia pseudoacacia L.) plantation. Forests 2019, 10, 261. [Google Scholar] [CrossRef]
- Baker, T.R.; Burslem, D.F.; Swaine, M.D. Associations between tree growth, soil fertility and water availability at local and regional scales in Ghanaian tropical rain forest. J. Trop. Ecol. 2003, 19, 109–125. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.T.; Zhu, J.J.; Yang, K.; Yu, L.Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Waterman, J.M.; Hall, C.R.; Mikhael, M.; Cazzonelli, C.I.; Hartley, S.E.; Johnson, S.N. Short–term resistance that persists: Rapidly induced silicon anti–herbivore defence affects carbon–based plant defences. Funct. Ecol. 2021, 35, 82–92. [Google Scholar] [CrossRef]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Beroueg, A.; Lecompte, F.; Mollier, A.; Pagès, L. Genetic variation in root architectural traits in Lactuca and their roles in increasing phosphorus–use–efficiency in response to low phosphorus availability. Front. Plant Sci. 2021, 12, 658321. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago–Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar]
- Tong, R.; Zhou, B.; Jiang, L.; Ge, X.; Cao, Y. Spatial patterns of leaf carbon, nitrogen, and phosphorus stoichiometry and nutrient resorption in Chinese fir across subtropical China. Catena 2021, 201, 105221. [Google Scholar] [CrossRef]
- Sartori, K.; Violle, C.; Vile, D.; Vasseur, F.; de Villemereuil, P.; Bresson, J.; Gillespie, L.; Fletcher, L.R.; Sack, L.; Kazakou, E. Do leaf nitrogen resorption dynamics align with the slow-fast continuum? A test at the intraspecific level. Funct. Ecol. 2022, 36, 1315–1328. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Peng, X.; Huang, Z.; Liu, S.; Zhang, Q. The mechanism for exclusion of Pinus massoniana during the succession in subtropical forest ecosystems: Light competition or stoichiometric homoeostasis? Sci. Rep. 2015, 5, 10994. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Pan, Y.; Fang, F.; Tang, H. Patterns and internal stability of carbon, nitrogen, and phosphorus in soils and soil microbial biomass in terrestrial ecosystems in China: A data synthesis. Forests 2021, 12, 1544. [Google Scholar] [CrossRef]
- Ci, H.; Guo, C.; Tuo, B.; Zheng, L.T.; Xu, M.S.; Sai, B.L.; Yang, B.Y.; Yang, Y.C.; You, W.H.; Yan, E.R.; et al. Tree species with conservative foliar nutrient status and strong phosphorus homeostasis are regionally abundant in subtropical forests. J. Ecol. 2022, 110, 1497–1507. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.; van Logtestijn, R.S.; Aerts, R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: What is the link with other resource economics traits? New Phytol. 2010, 186, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wang, Q.; Li, H.; Ding, G.; Wen, X. Transcriptome–wide identification and expression profiles of Masson pine WRKY transcription factors in response to low phosphorus stress. Plant Mol. Biol. Rep. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wang, J.; Guo, Z.; Wang, G.G.; Zeng, D.; Wu, T. Tree stoichiometry and nutrient resorption along a chronosequence of Metasequoia glyptostroboides forests in coastal China. Forest Ecol. Manag. 2018, 430, 445–450. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Q.; Zhou, H.; Nong, Z.; Ye, S.; Deng, Q. Introduction of Dalbergia odorifera enhances nitrogen absorption on Eucalyptus through stimulating microbially mediated soil nitrogen–cycling. For. Ecosyst. 2021, 8, 59. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L. On the success and failure of mixed–species tree plantations: Lessons learned from a model system of Eucalyptus globulus and Acacia mearnsii. Forest Ecol. Manag. 2005, 209, 147–155. [Google Scholar] [CrossRef]
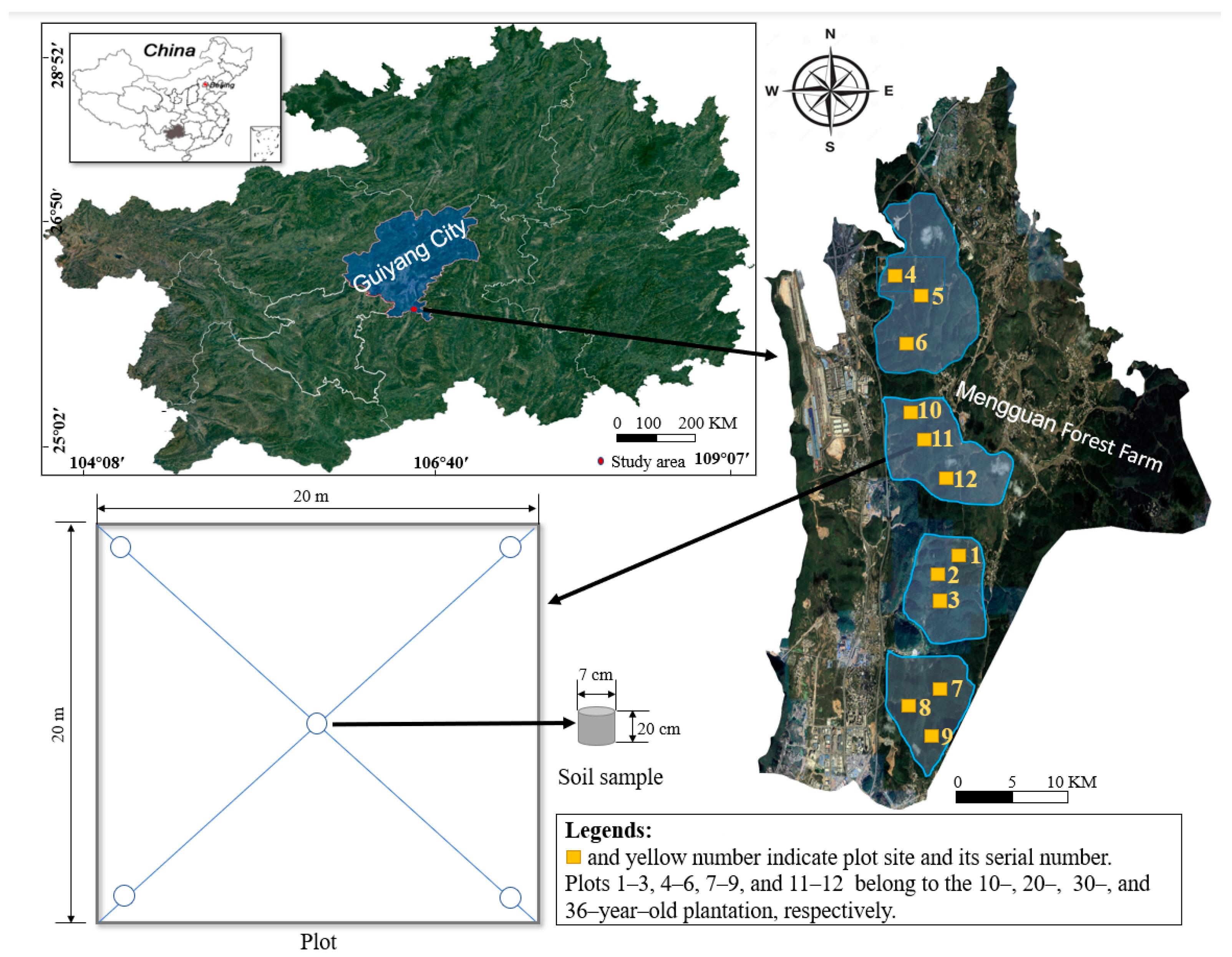


| Plantation Age | 10 Years Old | 20 Years Old | 30 Years Old | 36 Years Old |
|---|---|---|---|---|
| Altitude/m | 1194 ± 6.4 | 1175 ± 8.3 | 1206 ± 8.5 | 1214 ± 9.2 |
| Canopy density | 0.90 ± 0.07 | 0.85 ± 0.10 | 0.75 ± 0.09 | 0.80 ± 0.08 |
| Stand density/(trees·ha−1) | 4675 ± 256 | 2812 ± 135 | 1356 ± 103 | 1083 ± 94 |
| Mean diameter at breast height (DBH)/cm | 8.53 ± 1.56 | 12.66 ± 2.55 | 18.46 ± 3.01 | 21.26 ± 5.26 |
| Mean tree height/m | 7.43 ± 1.83 | 15.64 ± 2.74 | 18.94 ± 2.43 | 19.87 ± 3.26 |
| Nutrition Indexes | 10 Years Old | 20 Years Old | 30 Years Old | 36 Years Old |
|---|---|---|---|---|
| C (g·kg−1) | 9.98 ± 1.89 b | 9.48 ± 1.24 b | 11.58 ± 1.50 a | 11.85 ± 1.14 a |
| N (g·kg−1) | 1.95 ± 0.34 b | 1.51 ± 0.22 c | 2.32 ± 0.28 a | 2.37 ± 0.24 a |
| P (g·kg−1) | 0.38 ± 0.05 b | 0.49 ± 0.04 a | 0.24 ± 0.04 c | 0.21 ± 0.05 c |
| C:N | 5.87 ± 0.34 b | 6.63 ± 0.25 a | 5.87 ± 0.26 b | 5.45 ± 0.31 c |
| C:P | 28.88 ± 7.36 b | 20.09 ± 5.20 c | 48.09 ± 5.09 a | 43.89 ± 5.87 a |
| N:P | 5.86 ± 1.41 c | 2.24 ± 1.11 d | 9.45 ± 1.43 a | 7.93 ± 1.26 b |
| AN (mg·kg−1) | 12.96 ± 1.76 b | 11.04 ± 1.90 c | 13.62 ± 1.73 b | 15.35 ± 2.38 a |
| AP (mg·kg−1) | 7.14 ± 0.93 a | 1.41 ± 0.22 b | 1.79 ± 0.13 b | 1.59 ± 0.14 b |
| AN:AP | 4.55 ± 0.78 c | 8.81 ± 1.20 b | 8.52 ± 0.76 b | 10.03 ± 0.79 a |
| Factors | Items | Pearson Correlations | |
|---|---|---|---|
| NRE (p Value) | PRE (p Value) | ||
| Fresh needle | C | 0.866 ** (0.000) | |
| C:N | 0.921 ** (0.000) | ||
| Senesced needle | P | −0.790 ** (0.002) | |
| N | −0.615 * (0.033) | ||
| C:P | 0.834 ** (0.001) | ||
| N:P | 0.756 ** (0.004) | ||
| Soil | AP | −0.665 * (0.018) | −0.777 * (0.003) |
| AN:AP | 0.769 ** (0.003) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Li, H.; Sun, X.; An, Z.; Ding, G. Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations. Forests 2023, 14, 607. https://doi.org/10.3390/f14030607
Guo Q, Li H, Sun X, An Z, Ding G. Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations. Forests. 2023; 14(3):607. https://doi.org/10.3390/f14030607
Chicago/Turabian StyleGuo, Qiqiang, Huie Li, Xueguang Sun, Zhengfeng An, and Guijie Ding. 2023. "Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations" Forests 14, no. 3: 607. https://doi.org/10.3390/f14030607
APA StyleGuo, Q., Li, H., Sun, X., An, Z., & Ding, G. (2023). Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations. Forests, 14(3), 607. https://doi.org/10.3390/f14030607







