Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Source of Materials
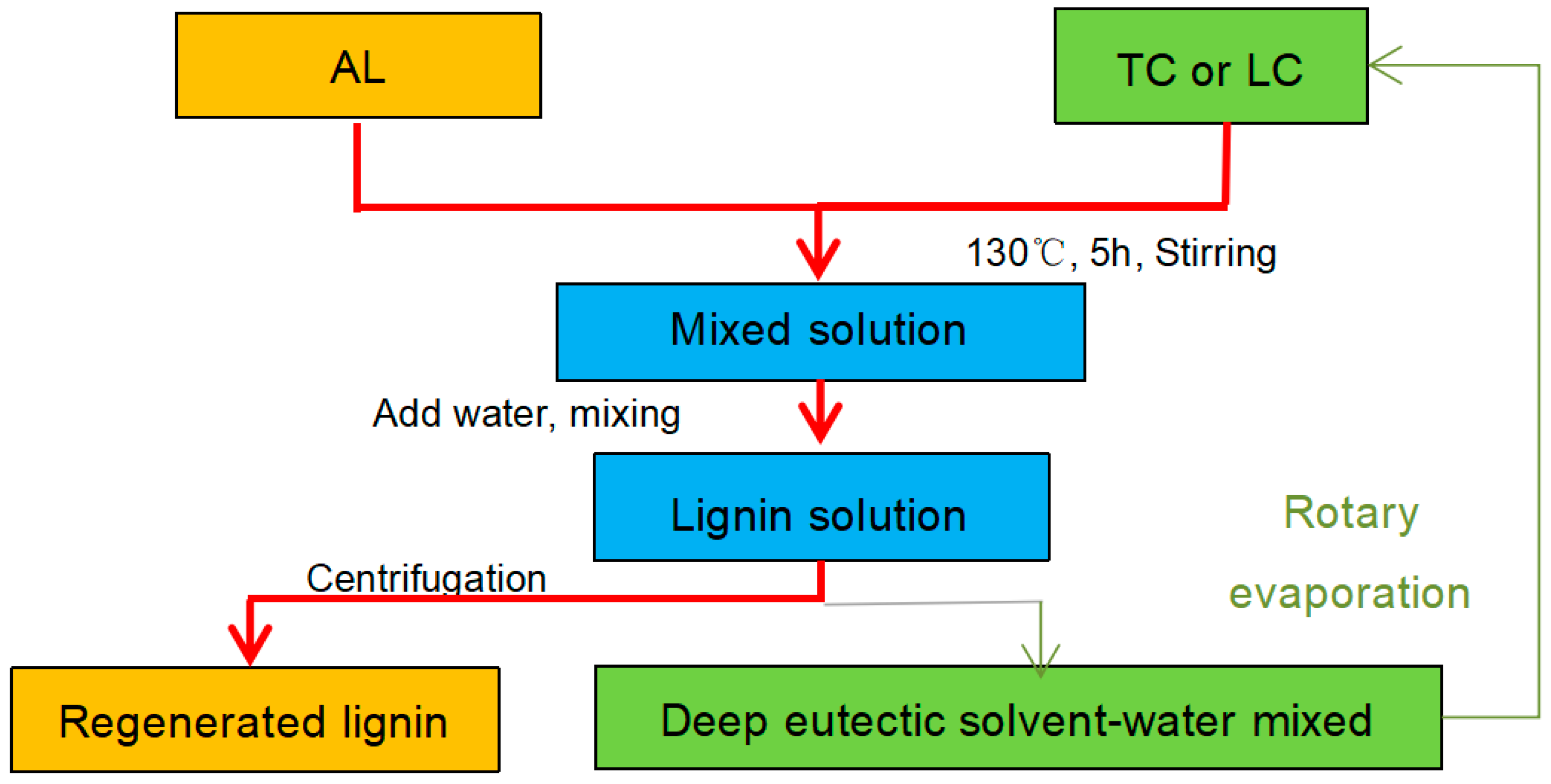
2.2. Modification of Alkaline Lignin
2.3. Characterization
2.4. Evaluation of Antioxidant Properties
2.5. Evaluation of UV Absorption Properties
3. Results & Discussion
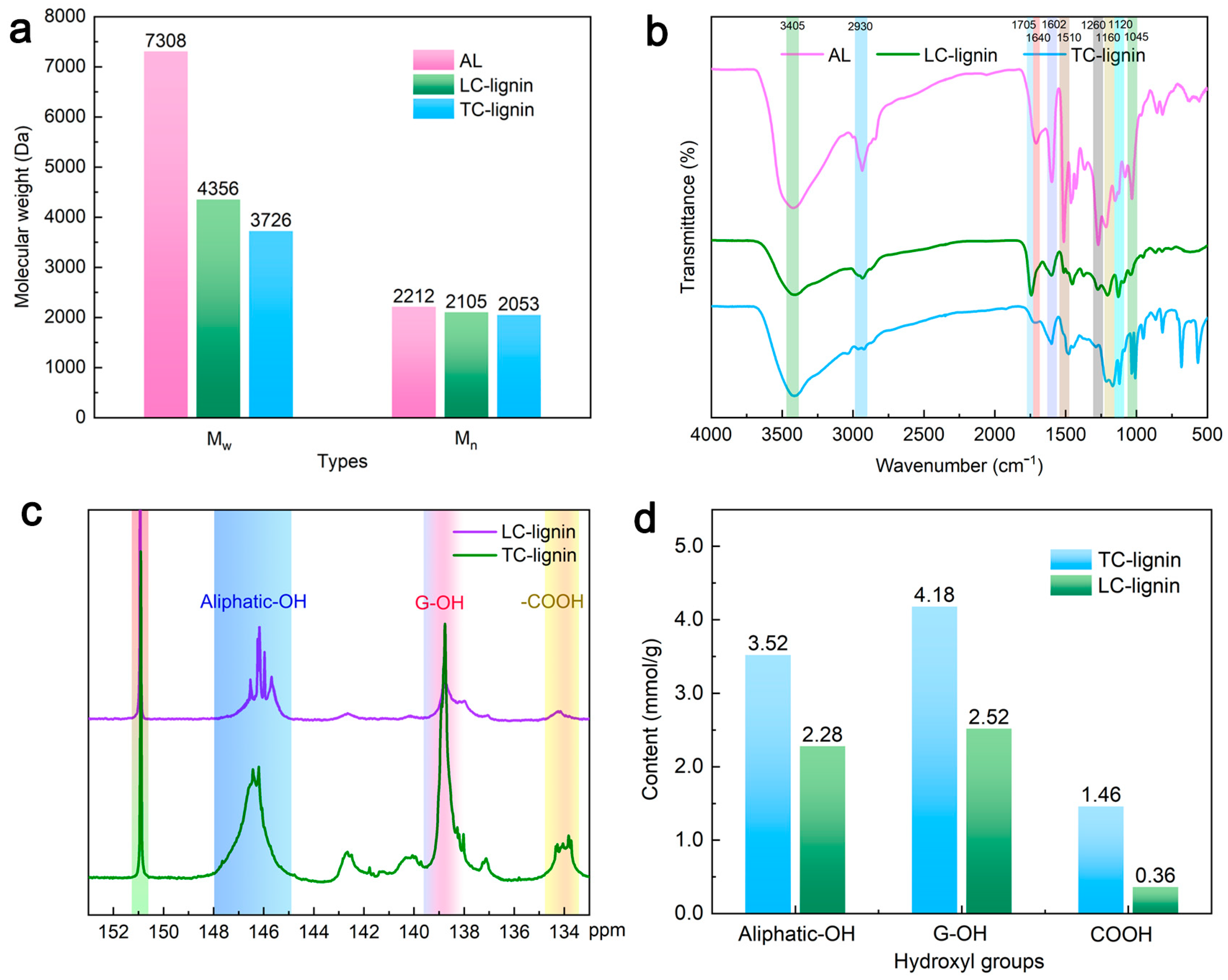
3.1. Molecular Weight Determination
3.2. FT-IR Analysis
3.3. 31P NMR Spectral Analysis
3.4. Antioxidant Activities against DPPH Radical
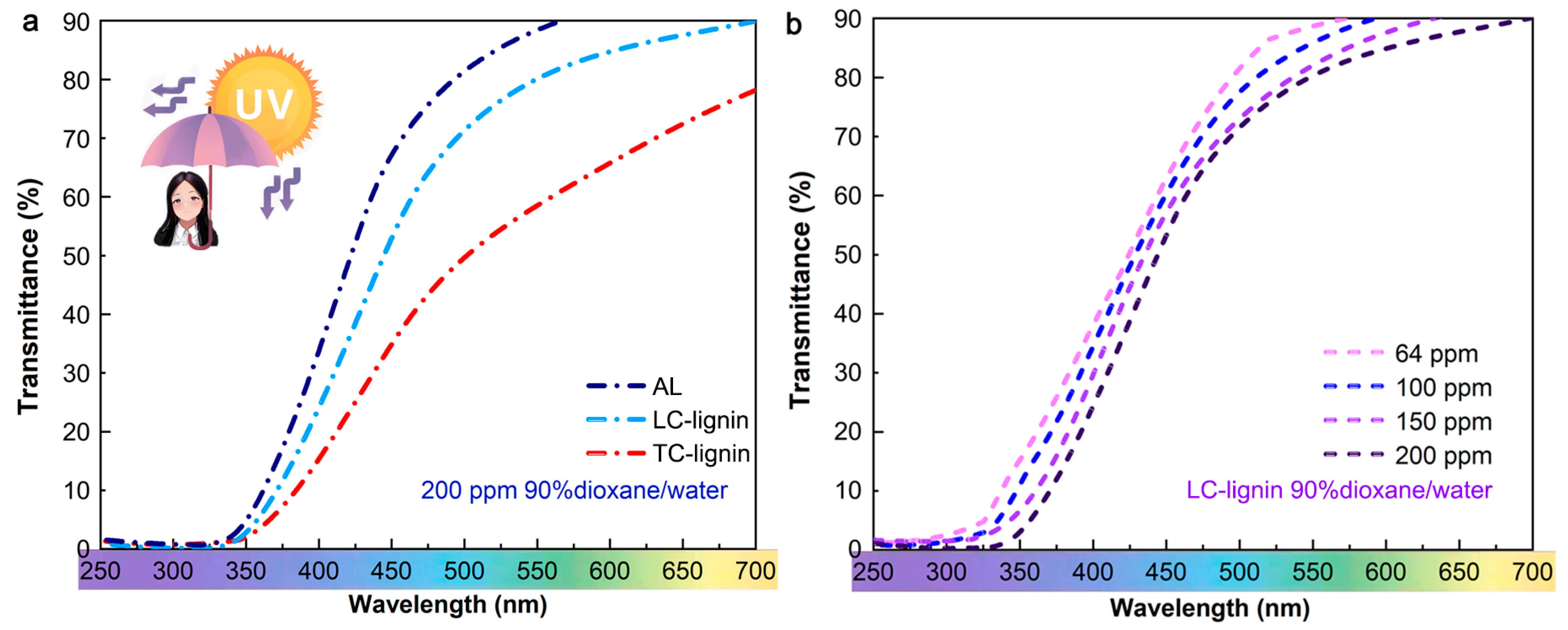
3.5. Ultraviolet Absorption Performance
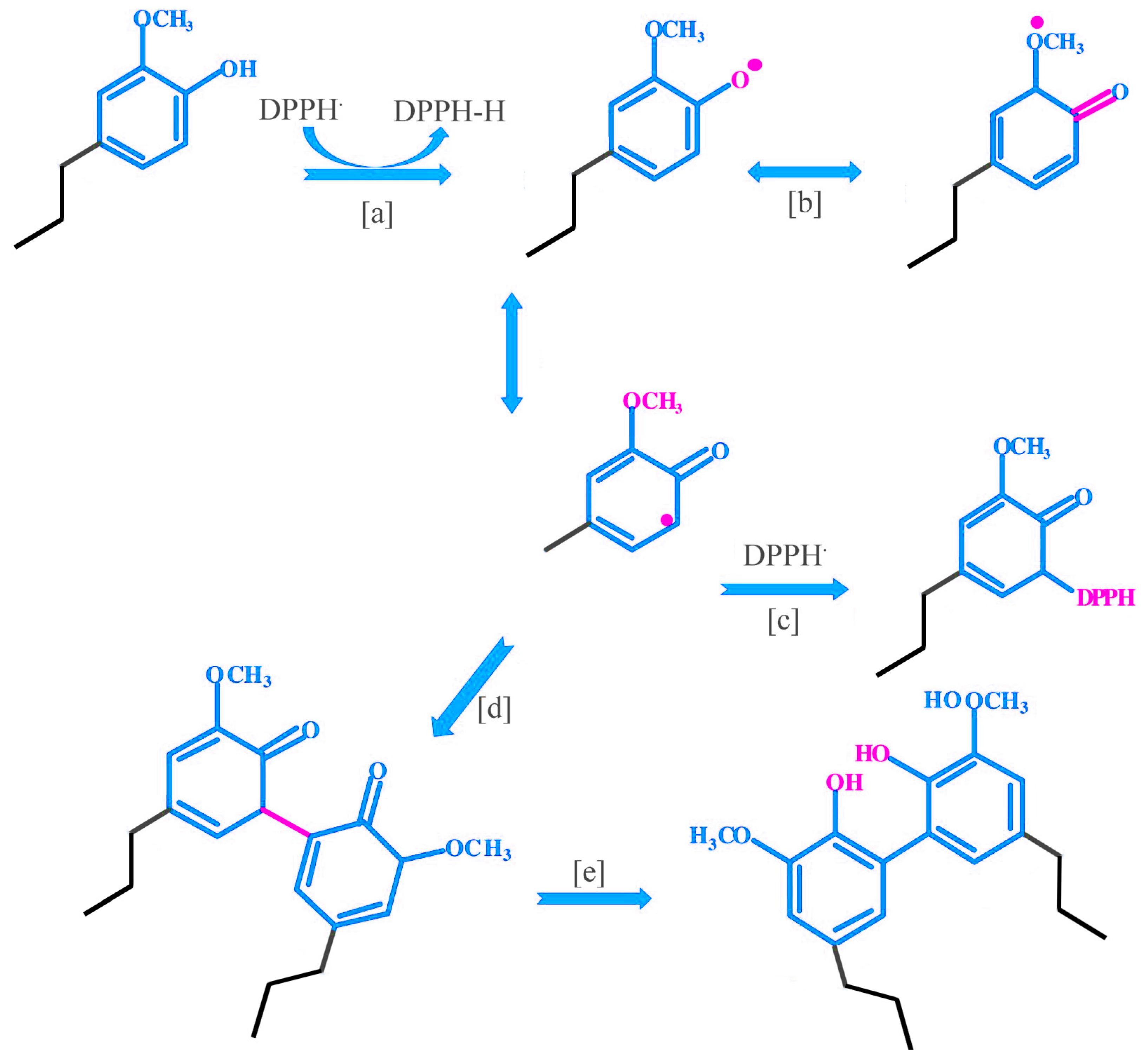
3.6. Possible Antioxidant Reaction Mechanism of Lignin Oligomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrescu, F.; Petrescu, R.; Buzea, E. New natural antioxidants. Indep. J. Manag. Prod. 2020, 11, 967. [Google Scholar] [CrossRef]
- Wang, Y.W.; Li, Y.N.; Lin, Q.B.; Wang, X.; Li, Z.H.; Wu, K.X. Functional and antioxidant properties of plastic bottle caps incorporated with BHA or BHT. Materials 2021, 14, 4545. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. J. Food Sci. 2017, 16, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Cherubim, D.J.D.; Martins, C.V.B.; Farina, L.O.; De Lucca, R.A.D. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Volperts, A.; Dobele, G.; Telysheva, G. Analytical pyrolysis—A tool for revealing of lignin structure-antioxidant activity relationship. J. Anal. Appl. Pyrol. 2015, 113, 360–369. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- An, L.L.; Wang, G.H.; Jia, H.Y.; Liu, C.Y.; Sui, W.J.; Si, C.L. Fractionation of enzymatic hydrolysis lignin by sequential extraction for enhancing antioxidant performance. Int. J. Biol. Macromol. 2017, 99, 674–681. [Google Scholar] [CrossRef]
- Lauberte, L.; Fabre, G.; Ponomarenko, J.; Dizhbite, T.; Evtuguin, D.V.; Telysheva, G.; Trouillas, P. Lignin Modification supported by DFT-based theoretical study as a way to produce competitive natural antioxidants. Molecules 2019, 24, 1794. [Google Scholar] [CrossRef]
- Aufischer, G.; Suss, R.; Kamm, B.; Paulik, C. Depolymerisation of kraft lignin to obtain high value-added products: Antioxidants and UV absorber. Holzforschung 2022, 76, 845–852. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, M.H.; Zu, Y.G.; Liu, W.J.; Yang, L.; Zhang, Y.; Zhao, X.H.; Zhang, X.N.; Zhang, X.N.; Li, W.G. Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem. 2012, 135, 63–67. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Ma, Z.H.; Wang, X.; Han, Y. Ethanol organosolv lignin from different agricultural residues: Toward basic structural units and antioxidant activity. Food Chem. 2022, 376, 131895. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.; Musajan, D.; He, M.Y.; Hou, G.B.; Li, Y.; Yimit, M. Study on extraction of cotton stalk lignin by different methods and its antioxidant property in polypropylene. Sep. Sci. Technol. 2022, 57, 263–273. [Google Scholar] [CrossRef]
- Lu, X.Y.; Gu, X.L.; Shi, Y.J. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Pan, X.J.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xiong, L.; Yoo, C.G.; Dong, C.Y.; Meng, X.Z.; Dai, J.; Ragauskas, A.; Yu, J.; Chen, X. Correlations of the physicochemical properties of organosolv lignins from Broussonetia papyrifera with their antioxidant activities. Sustain. Energ. Fuels 2020, 4, 5114–5119. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Zhao, H.F.; Guo, T.Y.; Wu, W.J.; Jin, Y.C. Structure-antioxidant activity relationship of active oxygen catalytic lignin and lignin-carbohydrate complex. Int. J. Biol. Macromol. 2019, 139, 21–29. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, Y.; Liu, L.; Fang, G.Z. Antioxidant activity of organosolv lignin degraded using SO42−/ZrO2 as catalyst. Bioresources 2015, 10, 6819–6829. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K. Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym. Sci. 2015, 293, 2585–2592. [Google Scholar] [CrossRef]
- Gil-Lalaguna, N.; Bautista, A.; Gonzalo, A.; Sanchez, J.L.; Arauzo, J. Obtaining biodiesel antioxidant additives by hydrothermal treatment of lignocellulosic bio-oil. Fuel Process. Technol. 2017, 166, 1–7. [Google Scholar] [CrossRef]
- Zhao, L.S.; Ouyang, X.P.; Ma, G.F.; Qian, Y.; Qiu, X.Q.; Ruan, T. Improving antioxidant activity of lignin by hydrogenolysis. Ind. Crops Prod. 2018, 125, 228–235. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhang, J.B.; Qn, L.; Ge, Y.Y. Enhancing antioxidant performance of lignin by enzymatic treatment with laccase. ACS Sustain. Chem. Eng. 2018, 6, 22591–22595. [Google Scholar] [CrossRef]
- Li, P.H.; Lu, Y.; Li, X.Y.; Ren, J.P.; Jiang, Z.W.; Jiang, B.; Wu, W.J. Comparison of the degradation performance of seven different choline chloride-based DES systems on alkaline lignin. Polymers 2022, 14, 5100. [Google Scholar] [CrossRef]
- Li, P.H.; Jiang, Z.W.; Yang, C.; Ren, J.P.; Jiang, B.; Wu, W.J. Degradation of alkaline lignin in the lactic acid-choline chloride system under mild conditions. J. Renew. Mater. 2023, 11, 2233–2248. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Argyropoulos, D.S. Correlations of the antioxidant properties of softwood kraft lignin fractions with the thermal stability of its blends with polyethylene. ACS Sustain. Chem. Eng. 2015, 3, 349–356. [Google Scholar] [CrossRef]
- Abd Latif, N.H.; Rahim, A.A.; Brosse, N.; Hussin, M.H. The structural characterization and antioxidant properties of oil palm fronds lignin incorporated with p-hydroxyacetophenone. Int. J. Biol. Macromol. 2019, 130, 947–957. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Martin-Sampedro, R.; Santos, J.I.; Wicklein, B.; Ibarra, D. Chemical, thermal and antioxidant properties of lignins solubilized during Soda/AQ pulping of orange and olive tree pruning residues. Molecules 2021, 26, 3819. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.W.; Li, D.Q.; You, T.T.; Zhang, X.; Xu, F.; Zhang, X.M.; Yang, Y.Q. New Lignin Streams Derived from Heteropoly Acids Enhanced Neutral Deep Eutectic Solvent Fractionation: Toward Structural Elucidation and Antioxidant Performance. ACS Sustain. Chem. Eng. 2020, 8, 12110–12119. [Google Scholar] [CrossRef]
- Cheng, X.C.; Guo, X.R.; Qin, Z.; Wang, X.D.; Liu, H.M.; Liu, Y.L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wang, S.Z.; Pan, Y.Q.; Ouyang, X.H.; Linhardt, R.J. Fermented cassava residue lignin prepared by sequential acid steam-explosion and hot-alkaline treatment and its antioxidant properties. Waste Biomass Valoriz. 2020, 11, 6115–6124. [Google Scholar] [CrossRef]
- Wei, X.X.; Liu, Y.; Luo, Y.D.; Shen, Z.; Wang, S.F.; Li, M.F.; Zhang, L.M. Effect of organosolv extraction on the structure and antioxidant activity of eucalyptus kraft lignin. Int. J. Biol. Macromol. 2021, 187, 462–470. [Google Scholar] [CrossRef]
- Zhang, H.N.; Ren, H.; Zhai, H.M. Analysis of phenolation potential of spruce kraft lignin and construction of its molecular structure model. Ind. Crops Prod. 2021, 167, 113506. [Google Scholar] [CrossRef]
- Qi, S.; Wang, G.H.; Sun, H.; Wang, L.L.; Liu, Q.M.; Ma, G.Z.; Parvez, A.M.; Si, C.L. Using lignin monomer as a novel capping agent for efficient acid-catalyzed depolymerization of high molecular weight lignin to improve its antioxidant activity. ACS Sustain. Chem. Eng. 2020, 8, 9104–9114. [Google Scholar] [CrossRef]
- Sun, S.L.; Liu, F.; Zhang, L.D.; Fan, X.L. One-step process based on the order of hydrothermal and alkaline treatment for producing lignin with high yield and antioxidant activity. Ind. Crops Prod. 2018, 119, 260–266. [Google Scholar] [CrossRef]
- Wei, Z.C.; Cai, C.Y.; Huang, Y.Z.; Wang, P.; Song, J.Y.; Deng, L.X.; Fu, Y. Strong biodegradable cellulose materials with improved crystallinity via hydrogen bonding tailoring strategy for UV blocking and antioxidant activity. Int. J. Biol. Macromol. 2020, 164, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone; Gigli, V.; Avitabile.; Saladino, R. Nano-Structured Lignin as green antioxidant and UV shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Jiang, Z.; Xu, X.; Jin, Y.; Wu, W. Mild depolymerization of alkaline lignin in a formic acid-choline chloride type DES system. Holzforschung 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Lu, Y.; Long, G.; Li, S.; Li, K.; Jiang, B.; Wu, W. Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties. Forests 2023, 14, 550. https://doi.org/10.3390/f14030550
Li P, Lu Y, Long G, Li S, Li K, Jiang B, Wu W. Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties. Forests. 2023; 14(3):550. https://doi.org/10.3390/f14030550
Chicago/Turabian StyleLi, Penghui, Yuan Lu, Guifang Long, Sixian Li, Kongyan Li, Bo Jiang, and Wenjuan Wu. 2023. "Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties" Forests 14, no. 3: 550. https://doi.org/10.3390/f14030550
APA StyleLi, P., Lu, Y., Long, G., Li, S., Li, K., Jiang, B., & Wu, W. (2023). Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties. Forests, 14(3), 550. https://doi.org/10.3390/f14030550







