Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Kraft Pulping
2.3. Black Liquor Characterization
2.4. Lignin Isolation
2.5. Determination of Lignin Properties
2.5.1. Determination of Volatile Material (VM) and Ash Content
2.5.2. Klason Lignin Content (LC)
2.5.3. Element Content Analysis (EA)
2.5.4. Total Phenolic Content (TPC) of Lignins
2.5.5. Carboxyl Group (CG) Content Determination
2.5.6. Ultraviolet–Visible (UV–Vis) Area and FT-IR Spectroscopy
2.5.7. Nitrobenzene Oxidation (NbOx)
2.5.8. Thermogravimetric Analysis (TGA)
2.5.9. Gel Permeation Chromatography (GPC) Analysis
3. Results and Discussion
4. Conclusions
- -
- Some lignin samples obtained from agro-treated raw materials can contain higher amounts of elements, such as K, P, and Si.
- -
- When using the kraft method for pulping processes, the obtained lignin may contain too much sulfur and its compounds.
- -
- Due to the high phenolic contents of the conifer lignins, they can be evaluated for pharmaceutical and insecticidal applications.
- -
- Because the ash content of the lignin samples for pine, poplar, OBT, and Indulin AT are between 1 and 3%, they can be used for high-value applications.
- -
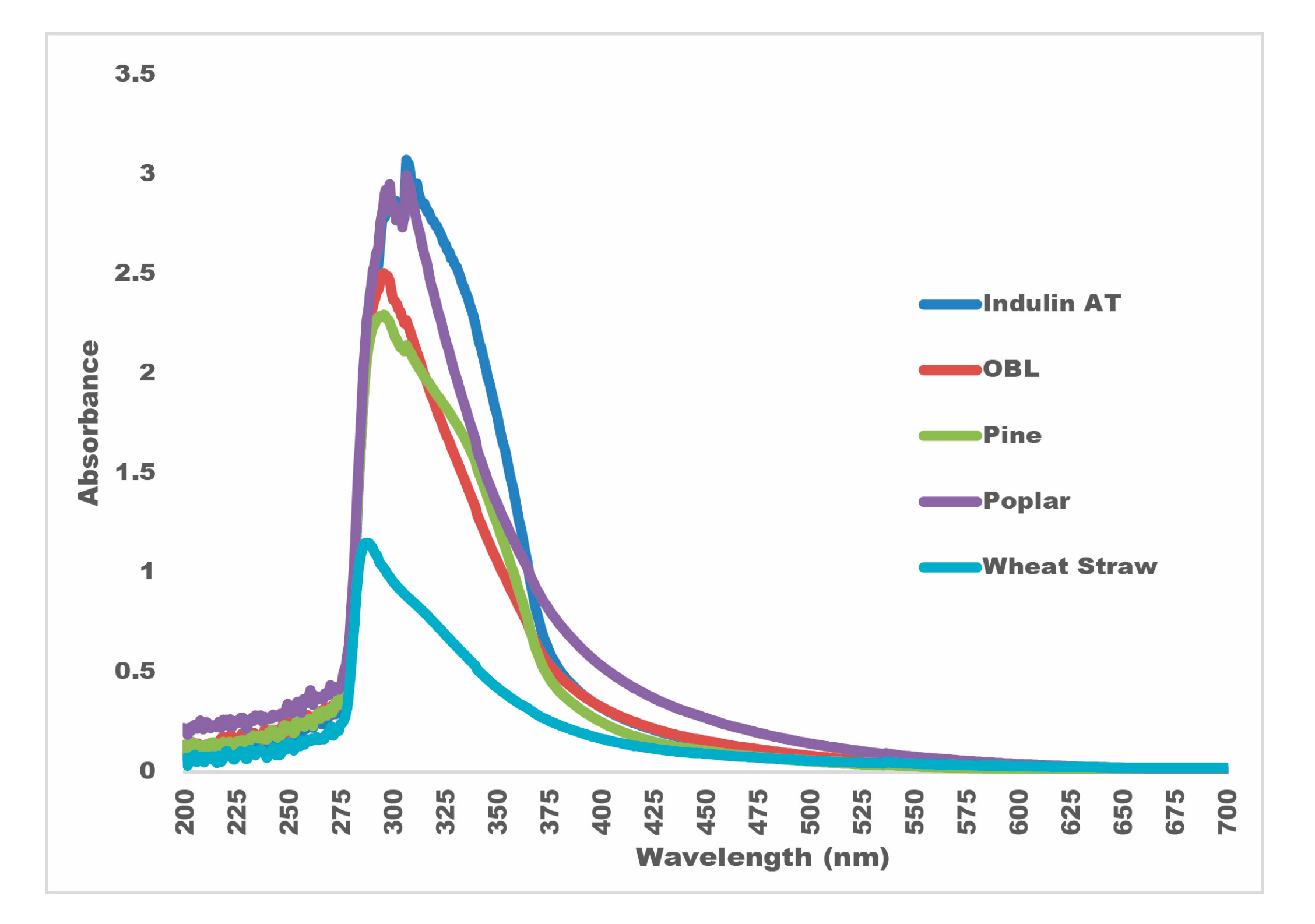
- Due to the UV-absorbance values between 288 and 307 nm, lignin samples have the potential to be used as UV-protectant materials or sunlight blockers.
- -
- Conifer lignin had the highest thermal degradation rate because of its lower ash and mineral composition and high moisture content. Indulin AT, OBL lignin, poplar, wheat straw, and pine lignin are the most resistant to extreme thermal conditions.
- -
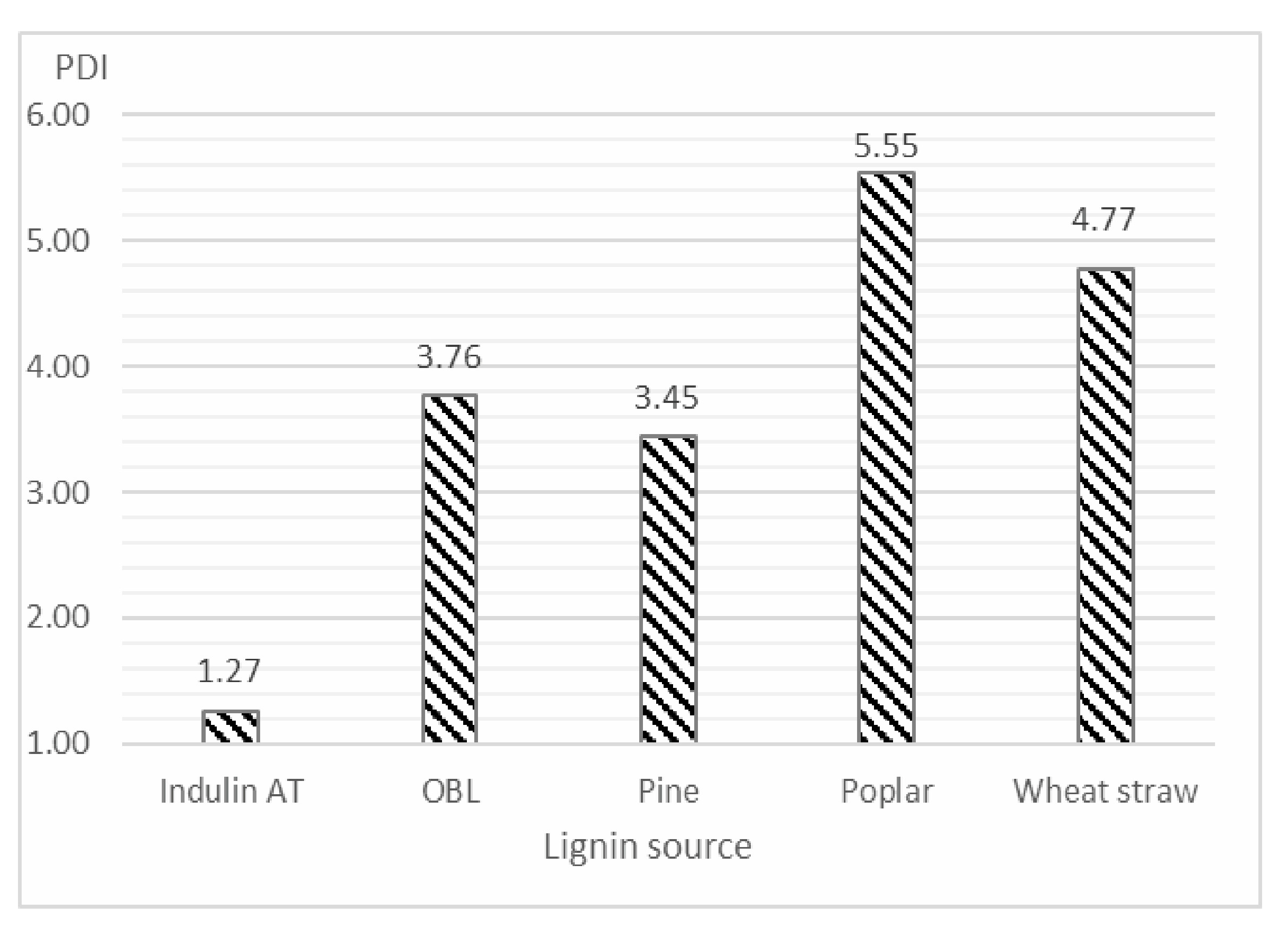
- Despite its negative properties, such as high polydispersity, high volatile matter content, and high ash and sulfur content, wheat straw lignin was found to have significant potential in terms of extrusion processability, with a higher syringyl lignin content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajpai, P. Biermann’s Handbook of Pulp and Paper: Volume 1: Raw Material and Pulp Making, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Zhou, X.-F.; Lu, X.-J. Structural characterization of kraft lignin for its green utilization. Wood Res. 2014, 59, 583–591. [Google Scholar]
- Setter, C.; Oliveira, T.J.P. Evaluation of the physical-mechanical and energy properties of coffee husk briquettes with kraft lignin during slow pyrolysis. Renew. Energy 2022, 189, 1007–1019. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Pola, L.; Collado, S.; Oulego, P.; Díaz, M. Kraft black liquor as a renewable source of value-added chemicals. Chem. Eng. J. 2022, 448, 137728. [Google Scholar] [CrossRef]
- Zhu, W.; Theliander, H. Precipitation of lignin from softwood black liquor: An investigation of the equilibrium and molecular properties of lignin. BioResources 2015, 10, 1696–1714. [Google Scholar] [CrossRef]
- Ammar, M.; Mechi, N.; Slimi, H.; Elaloui, E. Isolation and purification of alfa grass kraft lignin from industrial waste. Curr. Trends Biomed. Eng. Biosci 2017, 6, 31–35. [Google Scholar] [CrossRef]
- Yang, W.; Mu, H.; Huang, Y. Treatment of black liquor from the papermaking industry by acidification and reuse. J. Environ. Sci. 2003, 15, 697–700. [Google Scholar]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Haz, A.; Strizincova, P.; Majova, V.; Sskulcova, A.; Surina, I.; Jablonsky, M. Content of phenolic hydroxyl groups in lignin: Characterisation of 23 isolated non-wood lignin with various acids. Int. J. Recent Sci. Res. 2016, 7, 11547–11551. [Google Scholar]
- Lubis, M.A.; Labib, A.; Sudarmanto; Akbar, F.; Nuryawan, A.; Antov, P.; Kristak, L.; Papadopoulos, A.N.; Pizzi, A. Influence of lignin content and pressing time on plywood properties bonded with cold-setting adhesive based on Poly (Vinyl Alcohol), lignin, and hexamine. Polymers 2022, 14, 2111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seidi, F.; Huang, Y.; Xiao, H. Smart lignin-based polyurethane conjugated with corrosion inhibitor as bio-based anticorrosive sublayer coating. Ind. Crops Prod. 2022, 188, 115719. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, Y.H.; Sun, G.T.; Qiu, L.; Zhu, M.Q. Micromorphology control of the lignin-based activated carbon and the study on the pyrolysis and adsorption kinetics. Ind. Crops Prod. 2022, 175, 114266. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Sánchez, R.; Díaz-Carrasco, P.; Espinosa, E.; García-Domínguez, M.T.; Rodríguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef]
- Luo, J. Lignin-Based Carbon Fiber. Master’s Thesis, The University of Maine, Orono, ME, USA, 2010. [Google Scholar]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Özmen, N.; Çetin, N.S.; Karademir, A. Alkali sülfit ve organosolv ligninlerin karakterize edilmesi (Characterization of alkali sulfide and organosolv lignins). In Proceedings of the II. Ulusal Karadeniz Ormancılık Kongresi, Artvin, Turkey, 15–18 May 2002; pp. 1092–1101. [Google Scholar]
- Sharma, M.; Simões, A.; Alves, P.; Gando-Ferreira, L.M. Efficient recovery of lignin and hemicelluloses from kraft black liquor. KnE Mater. Sci. 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Li, H.; McDonald, A.G. Fractionation and characterization of industrial lignins. Ind. Crops Prod. 2014, 62, 67–76. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Hu, Z.; Du, X.; Liu, J.; Chang, H.M.; Jameel, H. Structural characterization of pine kraft lignin: BioChoice lignin vs. Indulin AT. J. Wood Chem. Technol. 2016, 36, 432–446. [Google Scholar] [CrossRef]
- Ateş, S.; Kırcı, H.; Tutus, A. Influence of cooking variables of Crimean Pine (Pinus nigra Arnold SBSP. Pallassiana) kraft pulp on the resulting paper sheets. Wood Res. 2008, 53, 81–90. [Google Scholar]
- İstek, A.; Özkan, İ. Effect of sodium borohydride on Populus tremula L. kraft pulping. Turk. J. Agric. For. 2008, 32, 131–136. [Google Scholar]
- Deniz, I.; Kirci, H.; Ates, S. Optimisation of wheat straw Triticum drum kraft pulping. Ind. Crops Prod. 2004, 19, 237–243. [Google Scholar] [CrossRef]
- SCAN Residual Alkali (Hydroxide Ion Content) SCAN N 33:94; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 1994.
- Technical Association of the Pulp and Paper Industry. Method T 625 cm-85. Analysis of Soda and Sulphate Black Liquor. In TAPPI Test Methods; TAPPI Press: Atlanta, GA, USA, 1985. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. Method T 211 om-93. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. In TAPPI Test Methods; TAPPI Press: Atlanta, GA, USA, 1993. [Google Scholar]
- Toledano, A.; Erdocia, X.; Serrano, L.; Labidi, J. Influence of extraction treatment on olive tree (Olea europaea) pruning lignin structure. Environ. Prog. Sustain. Energy 2013, 32, 1187–1194. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry. Method T 222 om-88, Acid-Insoluble Lignin in Wood and Pulp. In TAPPI Test Methods; TAPPI Press: Atlanta, GA, USA, 1988. [Google Scholar]
- García, A.; Toledano, A.; Andrés, M.Á.; Labidi, J. Study of the antioxidant capacity of Miscanthus sinensis lignins. Process Biochem. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1544, pp. 193–211. [Google Scholar]
- Asikkala, J.; Tamminen, T.; Argyropoulos, D.S. Accurate and reproducible determination of lignin molar mass by acetobromination. J. Agric. Food Chem. 2012, 60, 8968–8973. [Google Scholar] [CrossRef]
- Unodc the Global Afghan Opium Trade a Threat Assessment. Available online: https://www.unodc.org/documents/data-and-analysis/Studies/Global_Afghan_Opium_Trade_2011-web.pdf (accessed on 10 July 2021).
- Chen, Y.; Stark, N.M.; Cai, Z.; Frihart, C.R.; Lorenz, L.F.; Ibach, R.E. Chemical modification of kraft lignin: Effect on chemical and thermal properties. BioResources 2014, 9, 5488–5500. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Zou, Y. The esterification of sodium lignosulfonate with maleic anhydride in water solution. Int. J. Polym. Anal. 2015, 20, 69–81. [Google Scholar] [CrossRef]
- Koumba-Yoya, G.; Stevanovic, T. Study of organosolv lignins as adhesives in wood panel production. Polymers 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Fukushima, K.; Kakehi, A. Formation and chemical structures of acid-soluble lignin I: Sulfuric acid treatment time and acid-soluble lignin content of hardwood. J. Wood Sci. 2001, 47, 69–72. [Google Scholar] [CrossRef]
- Waltersson, J. The Metal Binding Properties of Kraft Lignin. In Degree Project Report; Innventia: Stockholm, Sweden, 2009. [Google Scholar]
- Domínguez-Robles, J.; Espinosa, E.; Savy, D.; Rosal, A.; Rodríguez Pascual, A. Biorefinery process combining Specel® process and selective lignin precipitation using mineral acids. BioResources 2016, 11, 7061–7077. [Google Scholar] [CrossRef]
- Mondal, A.K.; Qin, C.; Ragauskas, A.J.; Ni, Y.; Huang, F. Preparation and characterization of various kraft lignins and impact on Their Pyrolysis Behaviors. Ind. Eng. Chem. Res. 2020, 59, 3310–3320. [Google Scholar] [CrossRef]
- Klett, A.S.; Chappell, P.V.; Thies, M.C. Recovering ultraclean lignins of controlled molecular weight from Kraft black-liquor lignins. Chem. Commun. 2015, 51, 12855–12858. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; NorNadiah, M.Y.; Azian, H. Comparison studies between soda lignin and soda-anthraquinone lignin in terms of physico-chemical properties and structural features. J. Appl. Sci. 2006, 6, 292–296. [Google Scholar]
- Souto, F.; Calado, V.; Pereira, N. Lignin-based carbon fiber: A current overview. Mater. Res. Express 2018, 5, 072001. [Google Scholar] [CrossRef]
- Schmidt, J.A. Electronic spectroscopy of lignins. In Lignin and Lignans: Advances in Chemistry; Heitner, C., Dimmel, D., Schmidt, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 49–102. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Stamford, CT, USA, 2014. [Google Scholar]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV light blocker—A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Characterization of lignins isolated from industrial residues and their beneficial uses. BioResources 2016, 11, 8435–8456. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the physicochemical properties and thermal stability of Organosolv and Kraft lignins from hardwood and softwood biomass for their potential valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Brodin, I.; Sjöholm, E.; Gellerstedt, G. Kraft lignin as feedstock for chemical products: The effects of membrane filtration. Holzforschung 2009, 63, 290–297. [Google Scholar] [CrossRef]
- Schmidl, G.W. Molecular Weight Characterization and Rheology of Lignin for Carbon Fibers. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1992. [Google Scholar]
- Sun, R.; Lawther, J.M.; Banks, W.B.; Xiao, B. Effect of extraction procedure on the molecular weight of wheat straw lignins. Ind. Crops Prod. 1997, 6, 97–106. [Google Scholar] [CrossRef]
- Pan, X.J.; Sano, Y. Comparison of acetic acid lignin with milled wood and alkaline lignins from wheat straw. Holzforschung 2000, 54, 61–65. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Santos, T.M.; Rigual, V.; Oliet, M.; Alonso, M.V.; Rodriguez, F. Thermal stability, degradation kinetics, and molecular weight of organosolv lignins from Pinus radiata. Ind. Crops Prod. 2018, 111, 889–898. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Thermal properties of isolated and in situ lignin. In Lignin and Lignans: Advances in Chemistry; Heitner, C., Dimmel, D., Schmidt, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 301–320. [Google Scholar]
- Melro, E.; Filipe, A.; Sousa, D.; Valente, A.J.M.; Romano, A.; Antunes, F.E.; Medronho, B. Dissolution of kraft lignin in alkaline solutions. Int. J. Biol. Macromol. 2020, 148, 688–695. [Google Scholar] [CrossRef]


| Unit | Pine | Poplar | Wheat Straw | |
|---|---|---|---|---|
| Active alkali | % | 18 | 16 | 14 |
| Sulfidity | % | 30 | 26 | 20 |
| Raw Material to Liquor Ratio | kg/L | 1/4 | 1/4 | 1/4 |
| Cooking Temperature | °C | 170 | 170 | 160 |
| Total Cooking Time | min | 180 | 150 | 95 |
| Reference | - | [28] | [29] | [30] |
| Unit | Pine | Poplar | Wheat Straw | OBL | |
|---|---|---|---|---|---|
| Density | g/mL | 1.11 | 1.08 | 1.08 | 1.17 |
| Initial pH | - | 13.16 | 13.05 | 13.04 | 12.90 |
| TDS | % | 19.92 (0.44) | 16.67 (0.51) | 15.00 (0.22) | 50.00 (2.71) |
| IC | % | 26.24 (3.63) | 29.04 (2.71) | 27.48 (0.95) | 33.49 (2.36) |
| OC | % | 73.76 | 70.96 | 72.52 | 66.51 |
| Residual alkali | g/L | 7.17 | 6.41 | 5.27 | 13.25 |
| Lignin Source | Indulin AT | OBL | Pine | Poplar | Wheat Straw |
|---|---|---|---|---|---|
| Ash (%) | 2.02 (0.07) | 1.53 (0.38) | 1.90 (0.51) | 1.78 (0.75) | 3.41 (0.84) |
| VM (%) | 12.43 (1.26) | 14.65 (2.53) | 15.60 (4.00) | 18.35 (3.85) | 20.19 (3.74) |
| Klason lignin (%) | 85.60 (3.34) | 88.11 (2.19) | 88.11 (6.46) | 82.57 (4.450) | 85.88 (4.35) |
| Al (ppm) | 87.82 (0.80) | 33.61 (0.18) | 12.58 (0.05) | 7.64 (0.35) | 31.10 (0.27) |
| Na (ppm) | 1165.38 (1.04) | 1551.58 (9.38) | 578.67 (1.09) | 1168.46 (6.65) | 4214.68 (27.93) |
| Mg (ppm) | 162.62 (2.55) | 20.53 (0.12) | 11.36 (0.10) | 26.89 (0.13) | 38.25 (0.15) |
| Ca (ppm) | 12.55 (0.06) | 5.03 (0.01) | 3.18 (0.01) | 5.20 (0.04) | 12.29 (0.09) |
| K (ppm) | 137.66 (1.24) | 134.13 (3.97) | 16.01 (0.97) | 27.39 (1.03) | 598.20 (1.56) |
| Fe (ppm) | 68.98 (0.64) | 52.68 (0.16) | 8.88 (0.02) | 13.67 (0.57) | 55.89 (0.56) |
| P (ppm) | 29.03 (4.58) | 12.16 (0.28) | 8.37 (0.15) | 9.49 (0.08) | 238.06 (1.24) |
| Si (ppm) | 16.66 (0.40) | 4.80 (0.16) | - | - | 22.25 (0.32) |
| S (ppm) | 12,297.28 (60.70) | 20,397.88 (153.15) | 9862.00 (97.37) | 14,106.36 (81.05) | 24,567.28 (39.14) |
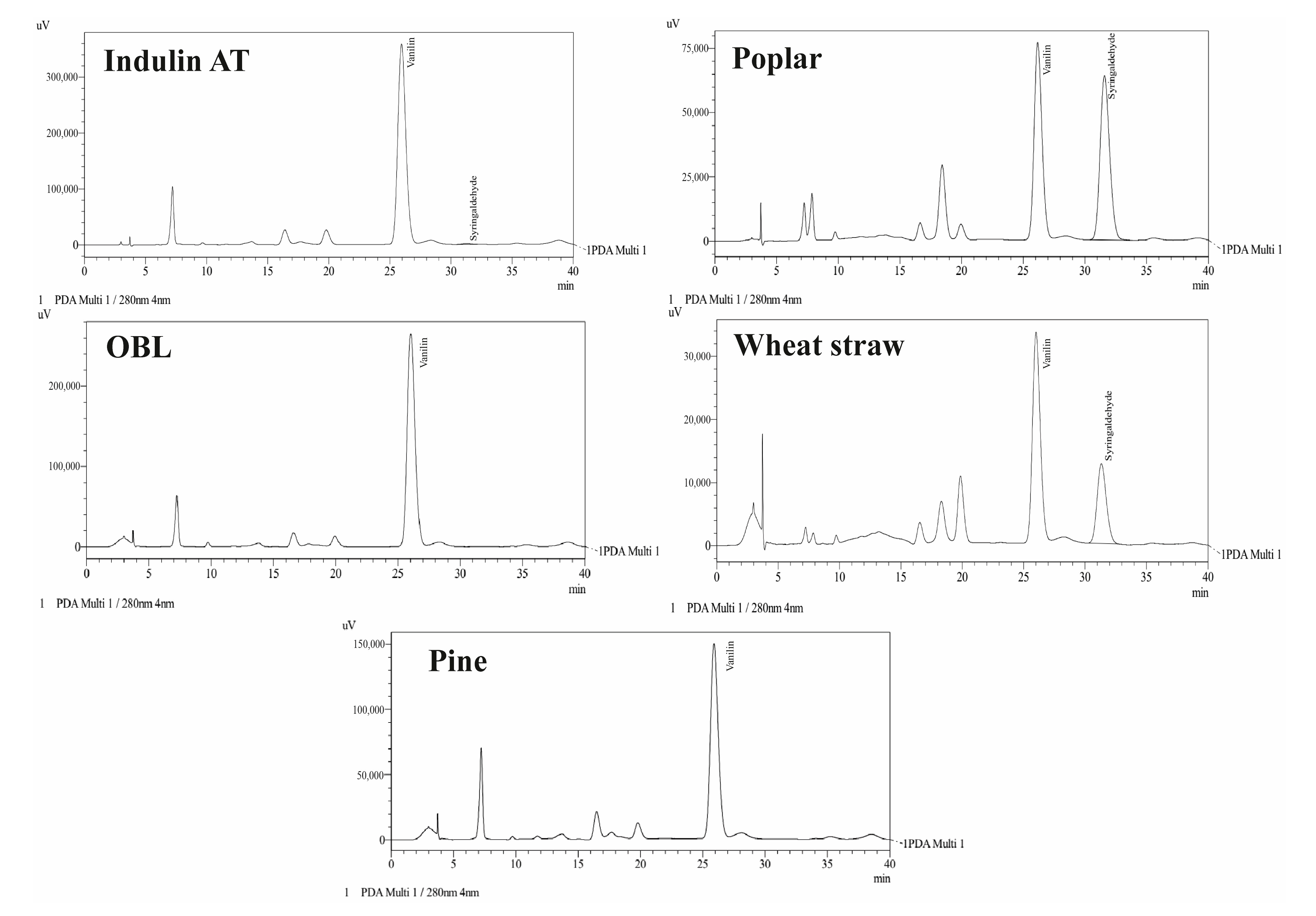
| Lignin Source | Phenolic (mg GAE/L) | COOH (%) | Vanillin (mmol/L) | Syringaldehyde (mmol/L) |
|---|---|---|---|---|
| Indulin AT | 555.25 | 4.16 | 1.250 | 0.108 |
| OBL | 525.25 | 8.66 | 0.870 | - |
| Pine | 826.91 | 9.78 | 0.500 | - |
| Poplar | 541.91 | 9.11 | 0.277 | 0.531 |
| Wheat straw | 439.41 | 10.68 | 0.137 | 0.181 |
| Lignin Source | Mn (Da) | Mw (Da) | |
|---|---|---|---|
| Indulin AT | 1718 | 2176 | |
| OBL | 1381 | 5196 | |
| Pine | 1854 | 6395 | |
| Poplar | 732 | 4061 | |
| Wheat straw | 607 | 2892 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olgun, Ç.; Ateş, S. Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests 2023, 14, 882. https://doi.org/10.3390/f14050882
Olgun Ç, Ateş S. Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests. 2023; 14(5):882. https://doi.org/10.3390/f14050882
Chicago/Turabian StyleOlgun, Çağrı, and Saim Ateş. 2023. "Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources" Forests 14, no. 5: 882. https://doi.org/10.3390/f14050882
APA StyleOlgun, Ç., & Ateş, S. (2023). Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests, 14(5), 882. https://doi.org/10.3390/f14050882







