Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
2.2.1. Moisture Content Determination
2.2.2. Determination of Net Photosynthetic Rate (Pn), Transpiration Rate (Tr)
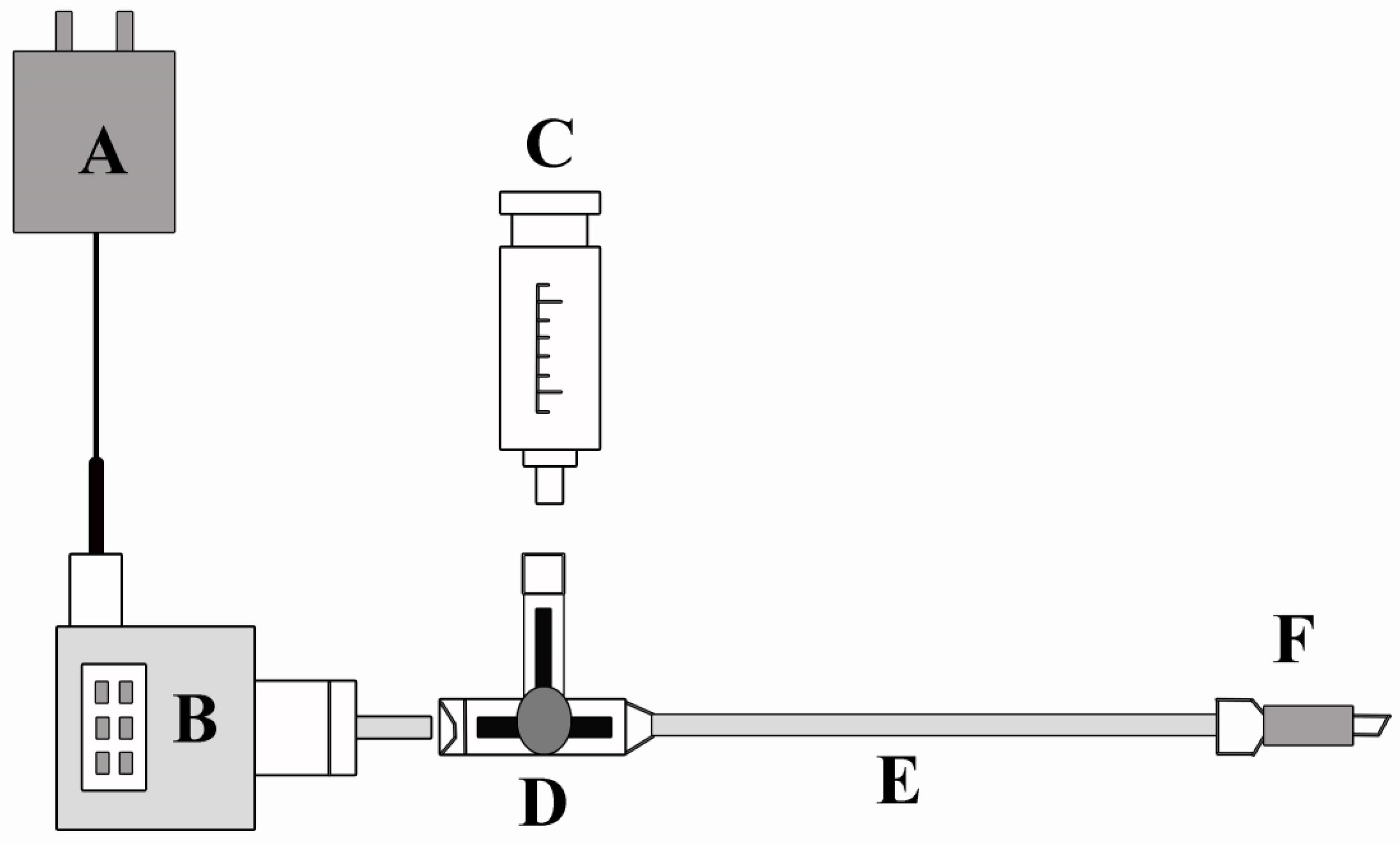
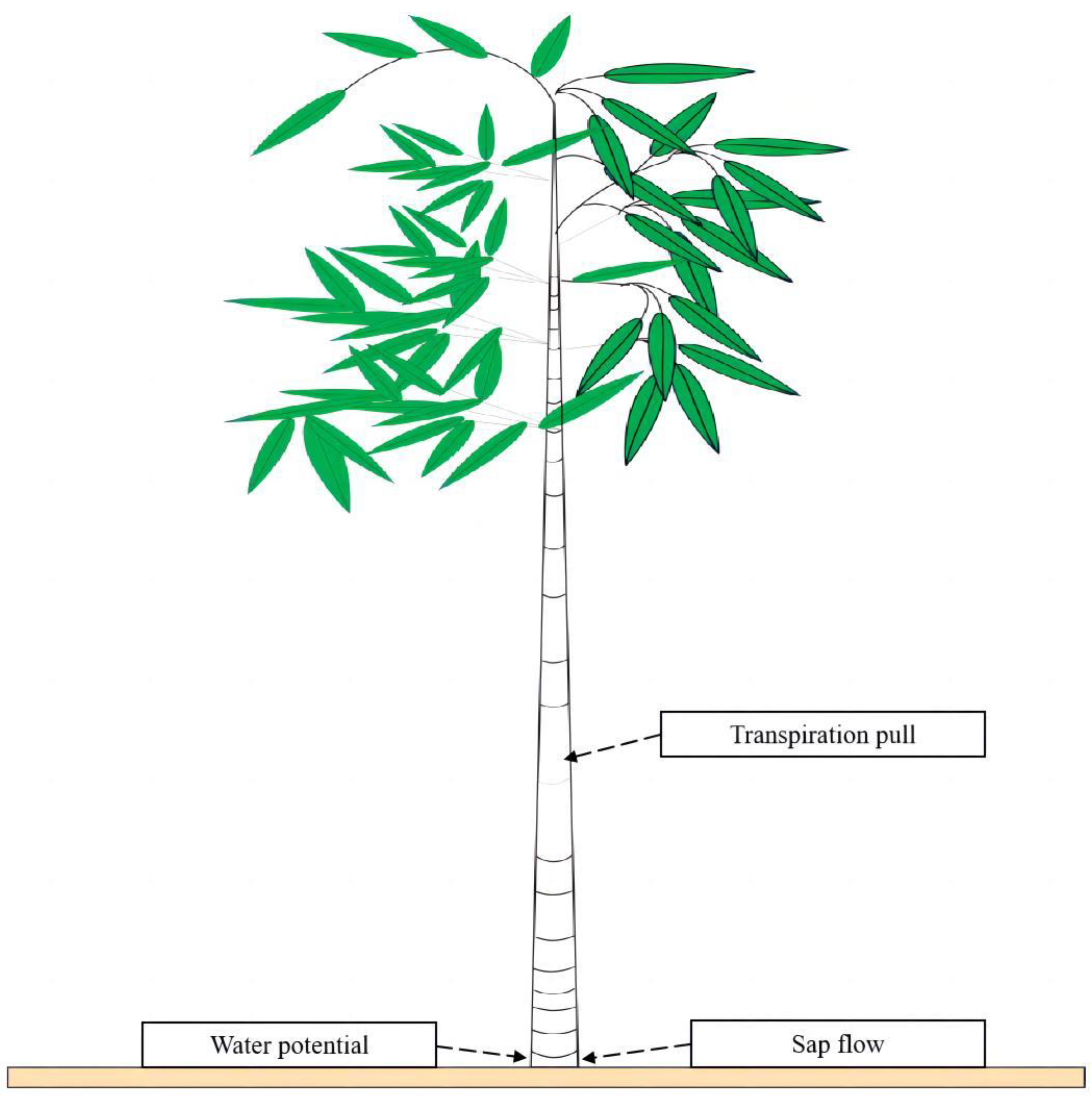
2.2.3. Determination of Transpiration Pull and Water Potential and Sap Flow
2.2.4. Determination of Soluble Sugars, Starch, and NSC Content
2.2.5. Statistical Analysis
3. Results
3.1. Phenological Observation of N. affinis
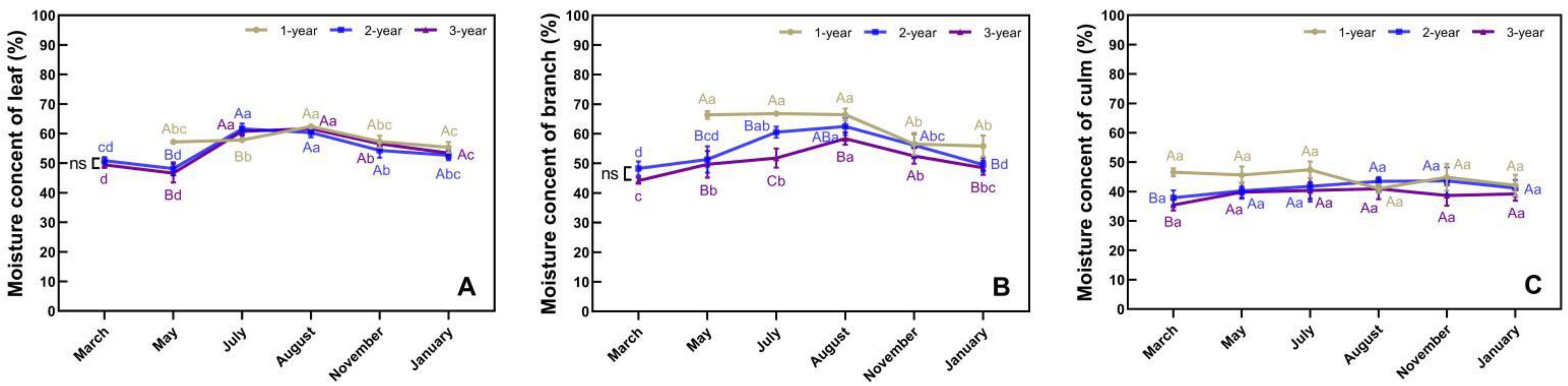
3.2. Phenological Changes in Moisture Content in Different Organs of N. affinis
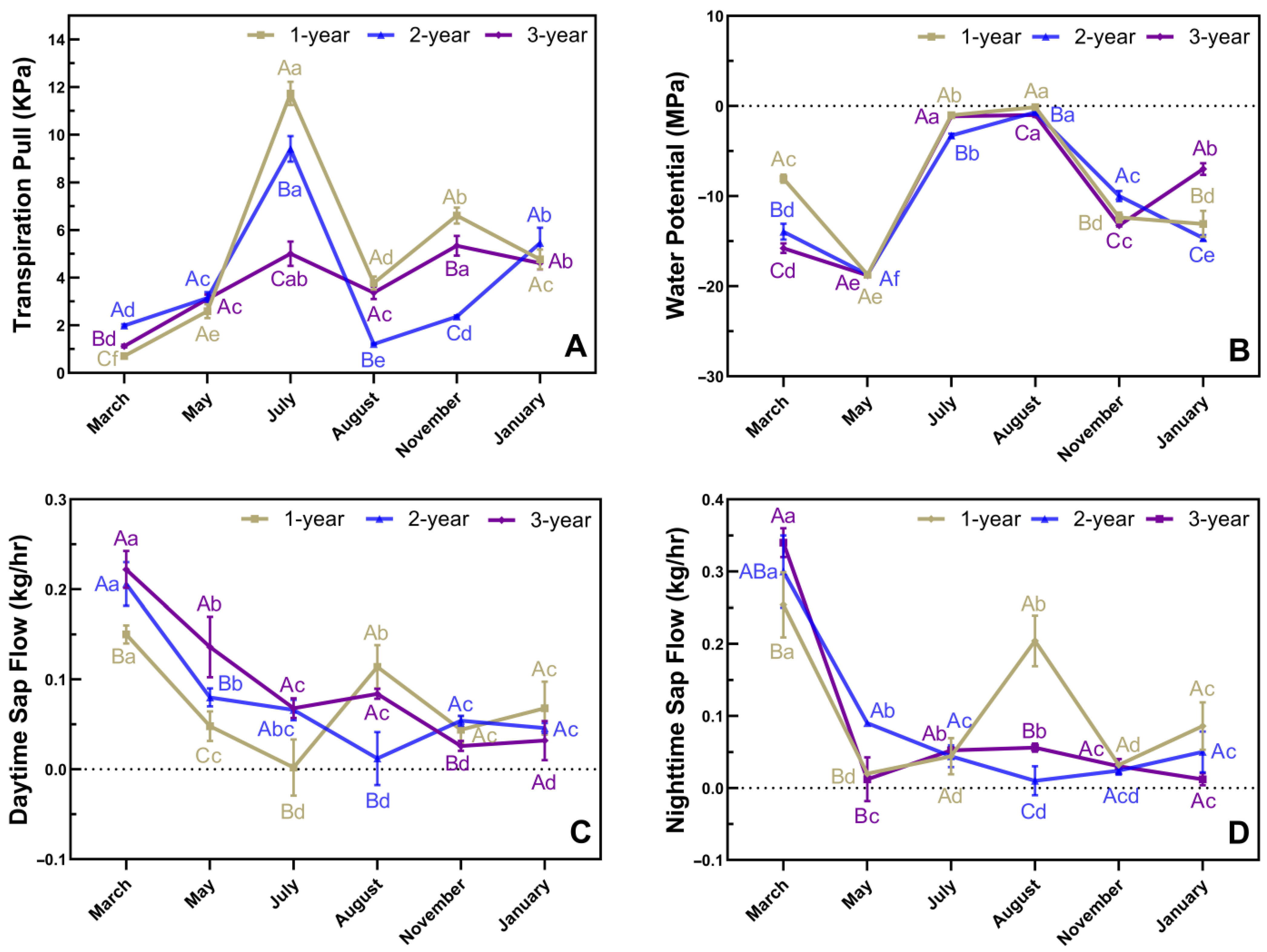
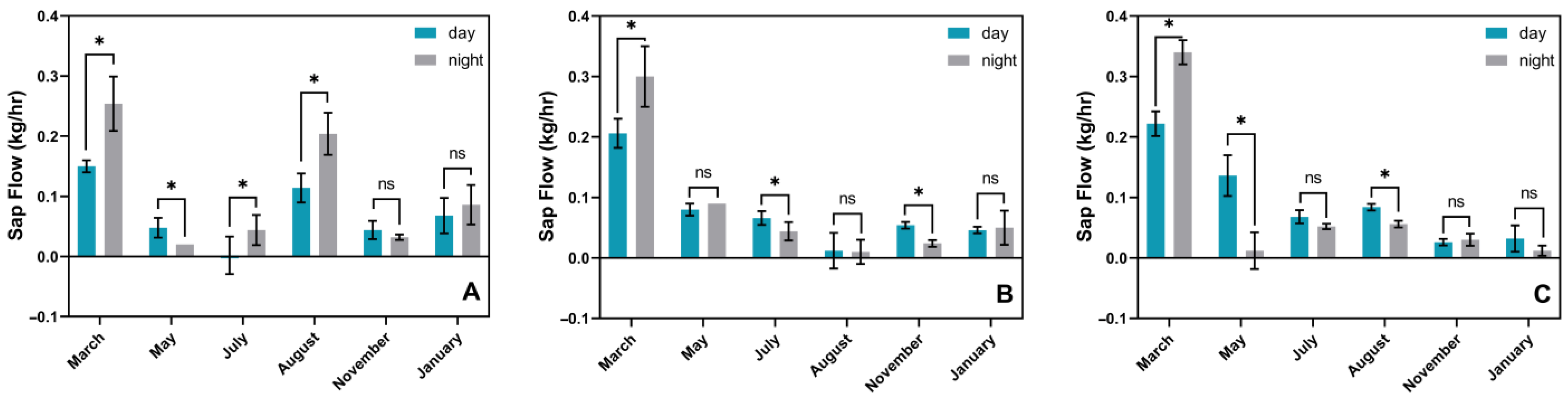
3.3. Phenological Changes in In Situ Transpiration Pull, In Situ Water Potential, and Sap Flow Rates in Culms of N. affinis of Different Ages
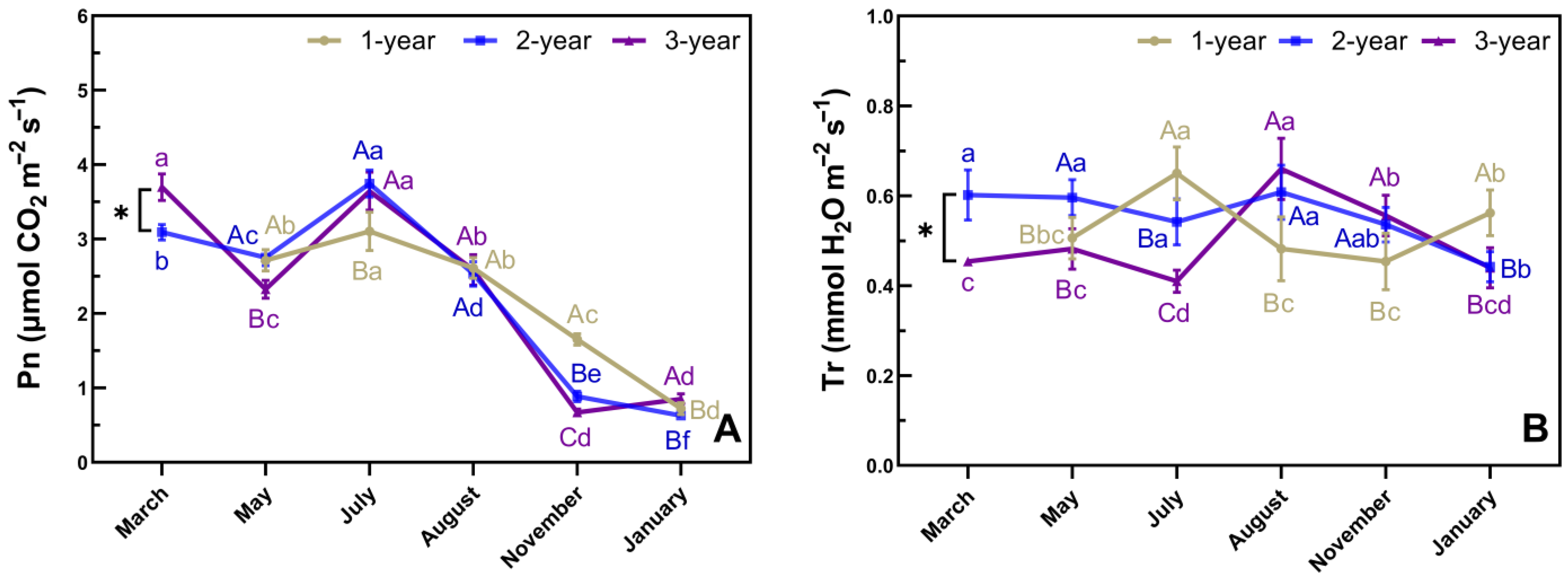
3.4. Phenological Changes in Pn and Tr
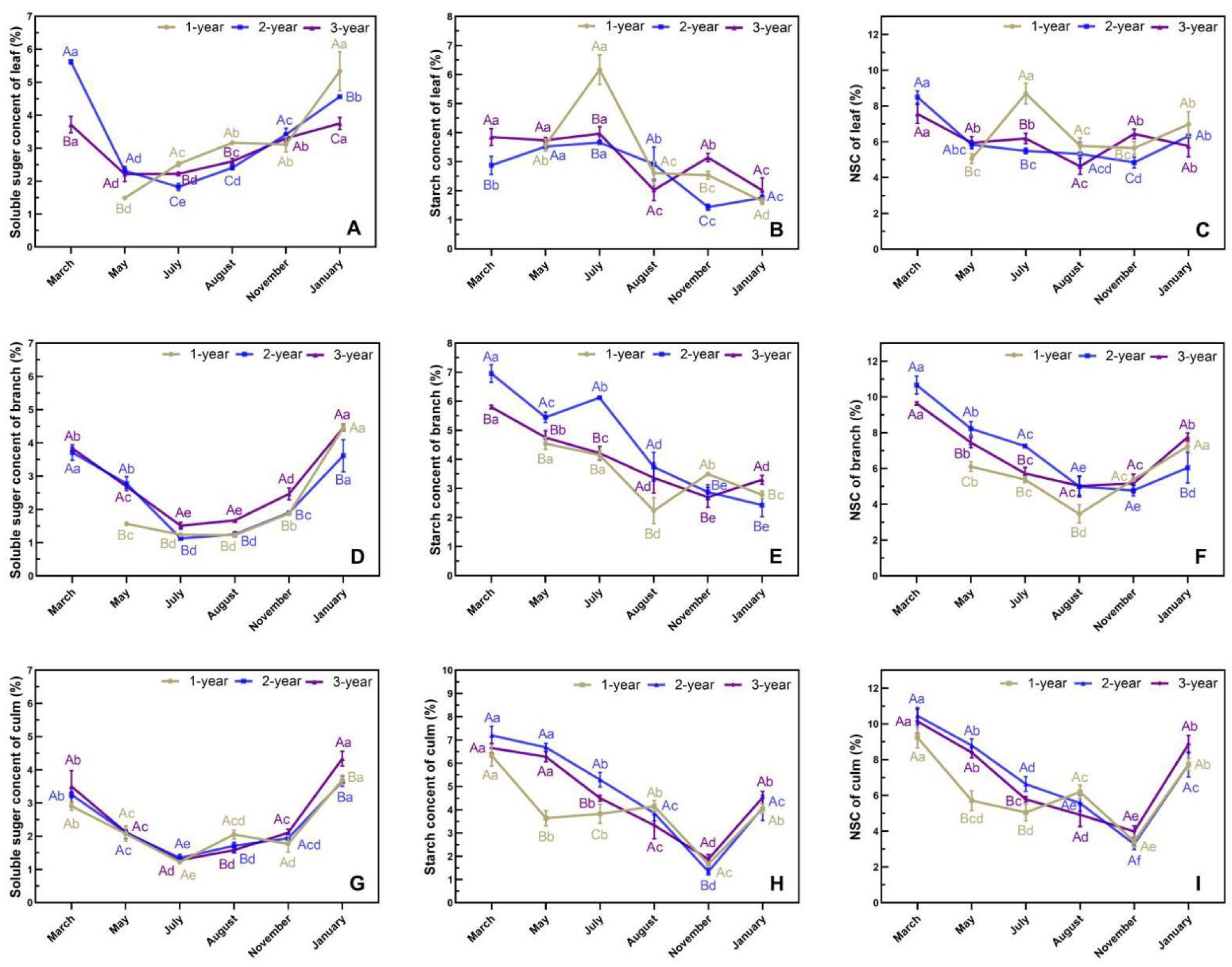
3.5. Phenological Changes in Photoassimilates in N. affinis
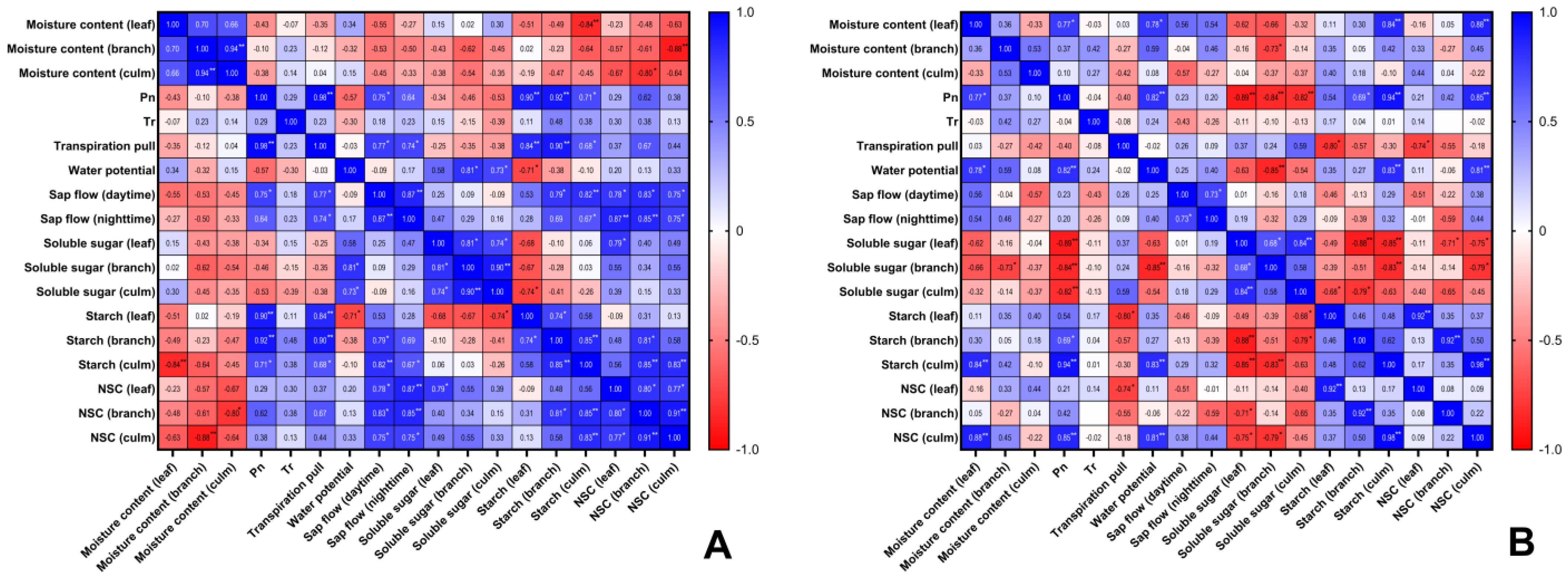
3.6. Correlation Analysis
4. Discussion
4.1. Water Status and Photoassimilate Accumulation in N. affinis before and after Branch and Leaf Extension
4.2. Water Status and Photoassimilate Accumulation in N. affinis during Shoot Emergence
4.3. Water Status and Photoassimilate Accumulation in N. affinis during the Dormancy Stage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, S.T.; Wu, J.H. Green-color conservation of ma bamboo (Dendrocalamus latiflorus) treated with chromium-based reagents. J. Wood Sci. 2000, 46, 40–44. [Google Scholar] [CrossRef]
- Jiang, Z. Bamboo and Rattan in the World; China Forestry Pub. House Press: Beijing, China, 2007. [Google Scholar]
- Zhao, J.; Su, W.; Fan, S.; Cai, C.; Zhu, X.; Liu, G. Characteristics of soil ammonia volatilization in fertilized moso bamboo (Phyllostachys edulis) forests. Sci. Silvae Sin. 2016, 52, 55–62. [Google Scholar]
- Mera, F.A.T.; Xu, C. Plantation management and bamboo resource economics in China. Cienc. Tecnol. 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Zhang, X. Development prospects of bamboo and rattan in the world. World For. Res. 2003, 16, 26–30. [Google Scholar]
- Chen, X.; Zhang, X.; Zhang, Y.; Booth, T.; He, X. Changes of carbon stocks in bamboo stands in China during 100 years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Fei, B.; Yu, Y. Bioconversion of bamboo to bioethanol using the two-stage organosolv and alkali pretreatment. BioResources 2012, 7, 5691–5699. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Zhang, Q.; Jiang, S. Present situation and the countermeasure analysis of bamboo timber processing industry in China. J. For. Eng. 2016, 1, 2–7. [Google Scholar]
- Jia, J.Q. Studies on the Regulation of Gibberellin and Auxin on Lignin and Cellulose Synthesis of Neosinocalamus affinis. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2007. [Google Scholar]
- Qi, J.; Hu, Y.; Xie, J.; Huang, X.; Luo, H.; Chen, S. Anatomical properties of three-year old Neosinocalamus affinis stalk at different heights. J. Northwest AF Univ. 2014, 42, 187–192. [Google Scholar]
- Wang, H.B.; Ding, W.P.; Gan, P.Y.; Li, Q.L. Effect of enriched nutrients on ash content and sand content in wheat flour. Cereal Feed. Ind. 2007, 9, 9–10. [Google Scholar]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Würth, M.K.; Pelaez-Riedl, S.; Wright, S.J.; Körner, C. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, W.; Yan, X. Non-structural carbohydrate levels of three co-occurring understory plants and their responses to forest thinning by gap creation in a dense pine plantation. J. For. Res. 2015, 26, 391–396. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Scofield, G.N.; Ruuska, S.A.; Aoki, N.; Lewis, D.C.; Tabe, L.M.; Jenkins, C.L. Starch storage in the stems of wheat plants: Localization and temporal changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Low root reserve accumulation during drought may lead to winter mortality in poplar seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant. Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Kramer, P. Physiology of Woody Plants; Elsevier Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wong, B.L.; Baggett, K.; Rye, A. Seasonal patterns of reserve and soluble carbohydrates in mature sugar maple (Acer saccharum). Can. J. Bot. 2003, 81, 780–788. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Sources and sinks in woody plants carbohydrate. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Lin, S.; Ji, X.; Zhan, H. Seasonal changes of endogenous soluble sugar and starch in different developmental stages of Fargesia yunnanensis. J. Wood Sci. 2016, 62, 1–11. [Google Scholar] [CrossRef]
- Shry, C.; Reiley, H.E. Introductory Horticulture; Cengage Learning Press: Stamford, CT, USA, 2016. [Google Scholar]
- Pan, R.; Buitrago, S.; Feng, Z.; Abou-Elwafa, S.F.; Xu, L.; Li, C.; Zhang, W. HvbZIP21, a novel transcription factor from wild barley confers drought tolerance by modulating ROS scavenging. Front. Plant Sci. 2022, 13, Art-878459. [Google Scholar] [CrossRef]
- Uga, Y.; Kitomi, Y.; Ishikawa, S.; Yano, M. Genetic improvement for root growth angle to enhance crop production. Breed. Sci. 2015, 65, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.X.; Shen, Y.K.; Li, D.Y.; Wang, Z.W.; Huang, Q.M.; Yang, D.D.; Gao, A.X. Bamboo in sub-tropical eastern China. In Primary Productivity of Grass Ecosystems of the Tropics and Subtropics; Chapman and Hall: London, UK, 1992; pp. 159–188. [Google Scholar]
- Rui, L.; During, H.J.; Werger, M.J.; Zhong, Z.C. Positioning of new shoots relative to adult shoots in groves of giant bamboo. Phyllostachys. Pubescens. Flora. 1998, 193, 315–321. [Google Scholar] [CrossRef]
- Baret, M.; DesRochers, A. Root connections can trigger physiological responses to defoliation in nondefoliated aspen suckers. Botany 2011, 89, 753–761. [Google Scholar] [CrossRef]
- Lau, R.R.; Young, D.R. Influence of physiological integration on survivorship and water relations in a clonal herb. Ecology 1988, 69, 215–219. [Google Scholar] [CrossRef]
- Caraco, T.; Kelly, C.K. On the adaptive value of physiological integraton in colonal plants. Ecology 1991, 72, 81–93. [Google Scholar] [CrossRef]
- Kroon, H.; Fransen, B.; van Rheenen, J.W.; van Dijk, A.; Kreulen, R. High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 1996, 106, 73–84. [Google Scholar] [CrossRef]
- Fang, D.; Mei, T.; Röll, A.; Hölscher, D. Water transfer between bamboo culms in the period of sprouting. Front. Plant Sci. 2019, 10, 786. [Google Scholar] [CrossRef]
- Xiong, D.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. 2020, 101, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, S.; Lin, F.; Hao, Z.; Jiang, P.; Dai, G. Effects of soil water and nitrogen on growth and photosynthetic response of Manchurian ash (Fraxinus mandshurica) seedlings in northeastern China. PLoS ONE 2012, 7, e30754. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of photosynthetic capacity to soil moisture gradient in perennial rhizome grass and perennial bunchgrass. BMC Plant Biol. 2011, 11, 1–11. [Google Scholar] [CrossRef]
- Zhan, H.; He, W.; Li, M.; Yu, L.; Li, J.; Wang, C.; Wang, S. The dynamics of non-structural carbohydrates in different types of bamboo in response to their phenological variations: Implications for managing bamboo plantations. Forests 2022, 13, 1218. [Google Scholar] [CrossRef]
- Dixon, M.A.; Tyree, M.T. A new stem hygrometer, corrected for temperature gradients and calibrated against the pressure bomb. Plant Cell Environ. 1984, 7, 693–697. [Google Scholar] [CrossRef]
- Marshall, D.C. Measurement of sap flow in conifers by heat transport. Plant Physiol. 1958, 33, 385. [Google Scholar] [CrossRef] [PubMed]
- Glassop, D.; Roessner, U.; Bacic, A.; Bonnett, G.D. Changes in the sugarcane metabolome with stem development. Are they related to sucrose accumulation? Plant Cell Physiol. 2007, 48. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Fan, J. Study on Photosynthetic Physiological Ecology of Main Forest-Forming Species in the Eastern Part of Northeast of China. Doctor’s Thesis, Northeast Forestry University, Harbin, China, 2002. [Google Scholar]
- Driesen, E.; De Proft, M.; Saeys, W. Soil Moisture levels affect the anatomy and mechanical properties of basil stems (Ocimum basilicum L.). Plants 2021, 10, 1320. [Google Scholar] [CrossRef]
- Mei, T.; Fang, D.; Röll, A.; Niu, F.; Hölscher, D. Water use patterns of four tropical bamboo species assessed with sap flux measurements. Front. Plant Sci. 2016, 6, 1202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.Z.; Fu, M.Y.; Xie, J.Z.; Yang, X.S.; Li, Z.C. Ecological functions of bamboo forest: Research and application. J. For. Res. 2005, 16, 143–147. [Google Scholar]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Cai, D.; Gao, Y.; Zhang, H.; Wang, Y.; Lin, C.; Ma, L.; Gu, L. Comprehensive profiling of rhizome-associated alternative splicing and alternative polyadenylation in moso bamboo (Phyllostachys edulis). Plant J. 2017, 91, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Vesala, T.; Sevanto, S.; Grönholm, T.; Salmon, Y.; Nikinmaa, E.; Hari, P.; Hölttä, T. Effect of leaf water potential on internal humidity and CO2 dissolution: Reverse transpiration and improved water use efficiency under negative pressure. Front. Plant Sci. 2017, 8, 54. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, P.; Zhang, Z.; Zhu, L.; Hu, Y.; Ouyang, L.; Ni, G.; Ye, Q. Culm age and rhizome affects night-time water recharge in the bamboo Phyllostachys pubescens. Front. Plant Sci. 2017, 8, 1928. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, Y.L.; Lu, J.; Shao, H.B. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Nilsson, O. Winter dormancy in trees. Curr. Biol. 2022, 32, R630–R634. [Google Scholar] [CrossRef]

| January | March | May | July | August | November | |
|---|---|---|---|---|---|---|
| Atmospheric temperature (°C) | 9.3 | 16.5 | 21.6 | 21.3 | 21.2 | 12.4 |
| Precipitation (mm) | 1.8 | 0.5 | 46.5 | 225.2 | 168.2 | 27.2 |
| Daily sunshine duration (h) | 8.1 | 10.2 | 11.4 | 10.3 | 9.6 | 6.6 |
| Relative humidity (%) | 63.8 | 42.8 | 56.7 | 74.4 | 75.6 | 73.3 |
| Sample Timing | Phenological Stage | Phenology of Bamboo Clump |
|---|---|---|
| March 2021 Spring/dry season | Before bud sprout | Few nodal buds sprouted on the new culms generated last year. |
| May 2021 Spring/dry season | Branching and leafing | Most new culms were in the branching and leafing stage, and the shoot buds were also pregnant at the culm base of the new culms and the 2-year culms. |
| July 2021 Summer/rainy season | Before shooting | Shoot bud development continued underground, and a few shoots emerged out of the ground. |
| August 2021 Summer/rainy season | Shooting and height growth | Most shoots emerged out of the ground and completed their height growth in the subsequent three months. |
| November 2021 Autumn/dry season | After culms have achieved maximum height | Height growth of new culms completed. The culm sheath of the middle and bottom parts of the new culms dropped off. The bamboo clump entered the dormancy stage. |
| January 2022 Winter/dry season | Dormancy | New culms ceased growing and the bamboo clumps entered dormancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Li, J.; Zhan, H.; Yu, L.; Wang, C.; Wang, S. Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology. Forests 2023, 14, 531. https://doi.org/10.3390/f14030531
Shi W, Li J, Zhan H, Yu L, Wang C, Wang S. Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology. Forests. 2023; 14(3):531. https://doi.org/10.3390/f14030531
Chicago/Turabian StyleShi, Wanli, Juan Li, Hui Zhan, Lixia Yu, Changming Wang, and Shuguang Wang. 2023. "Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology" Forests 14, no. 3: 531. https://doi.org/10.3390/f14030531
APA StyleShi, W., Li, J., Zhan, H., Yu, L., Wang, C., & Wang, S. (2023). Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology. Forests, 14(3), 531. https://doi.org/10.3390/f14030531





