Seasonal Patterns and Species Variability in the Leaf Traits of Dominant Plants in the Tropical Rainforests of Hainan Island, China
Abstract
1. Introduction
2. Materials and Methods
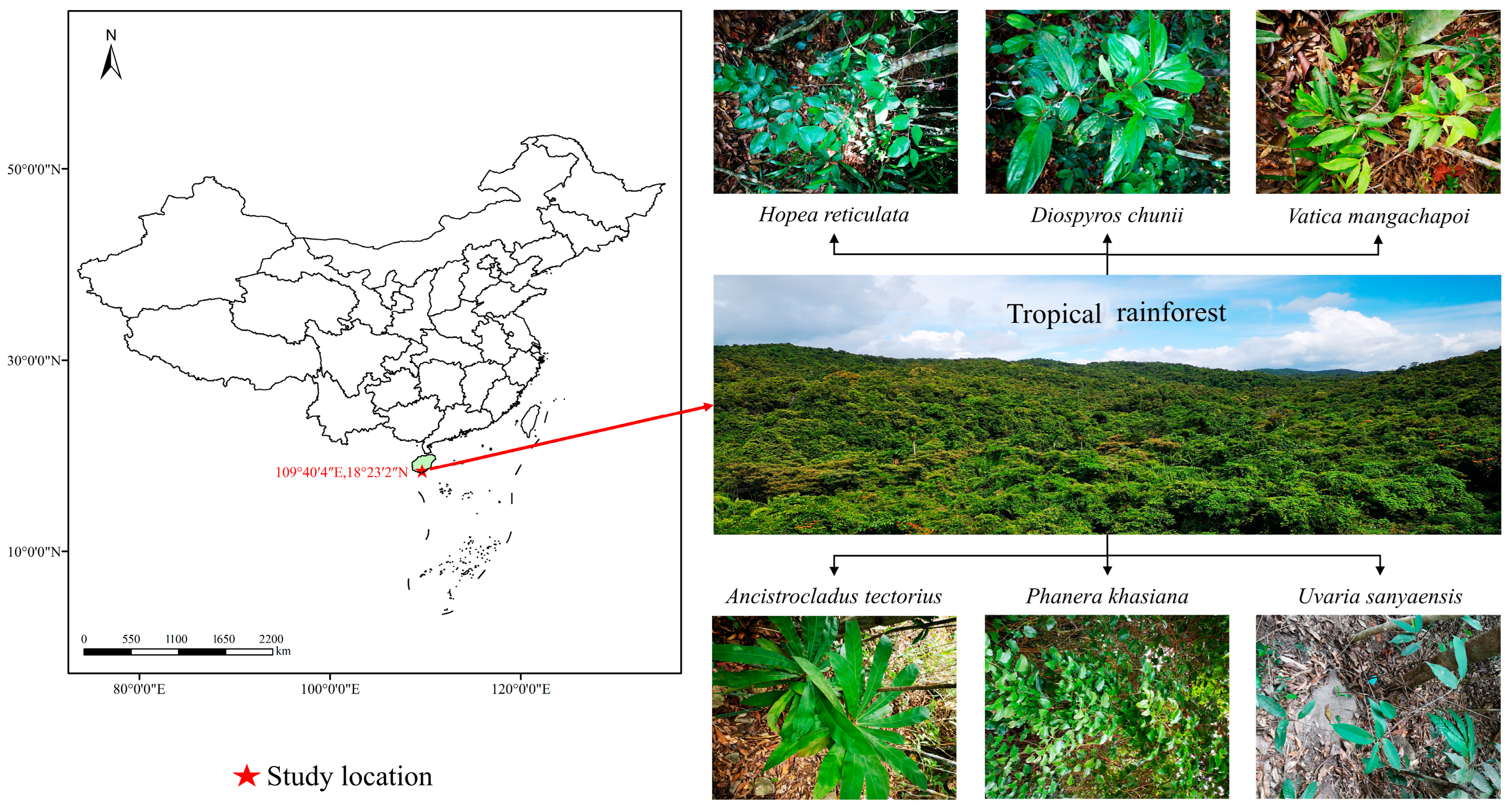
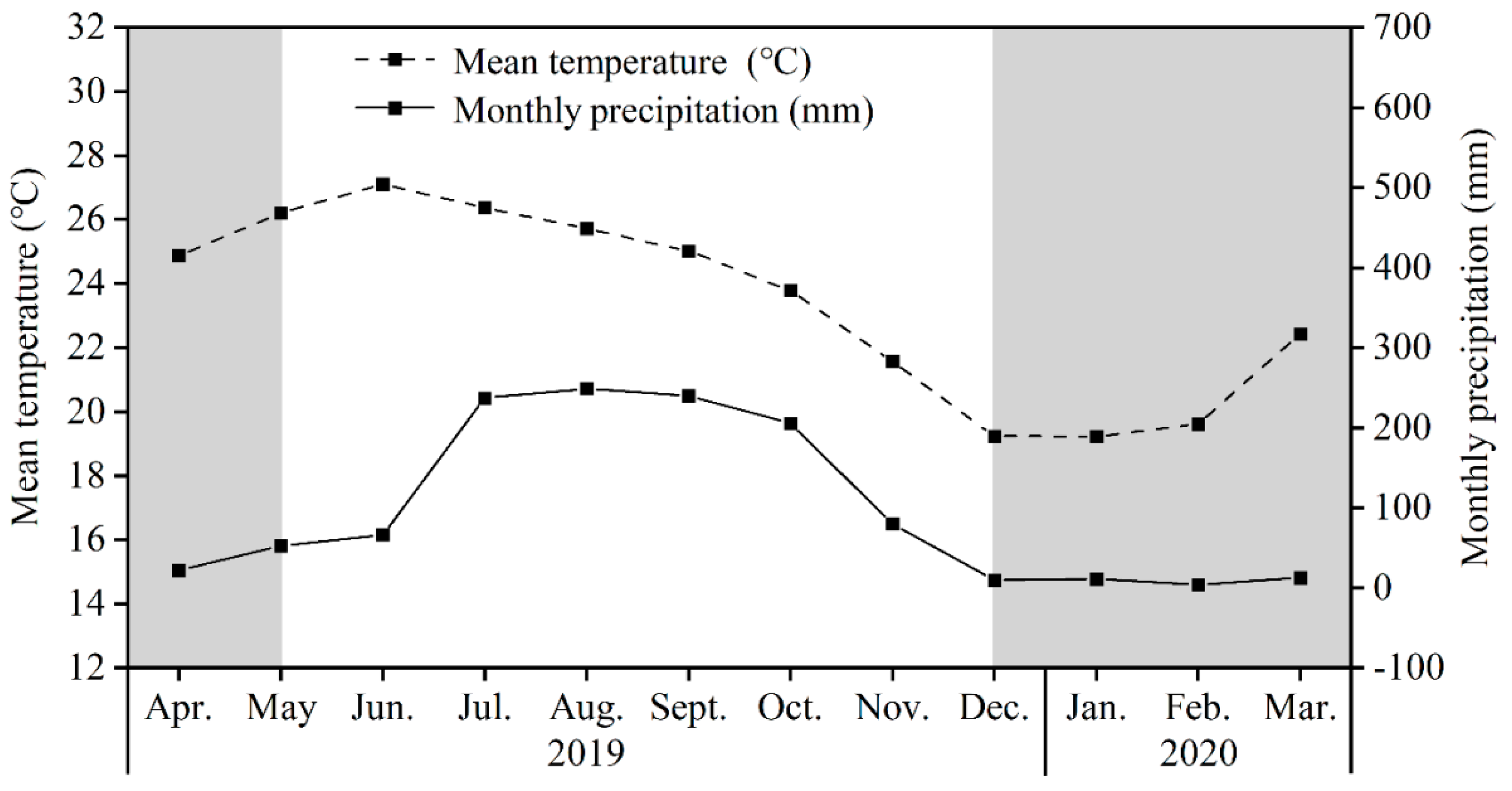
2.1. Study Site
2.2. Materials
2.3. Experimental Design and Sampling
2.4. Leaf Traits Measurements
2.4.1. Gas Exchange Parameter Measurements
2.4.2. Morphological Determination
2.4.3. Anatomical Measurements
2.5. Statistical Analyses
3. Results
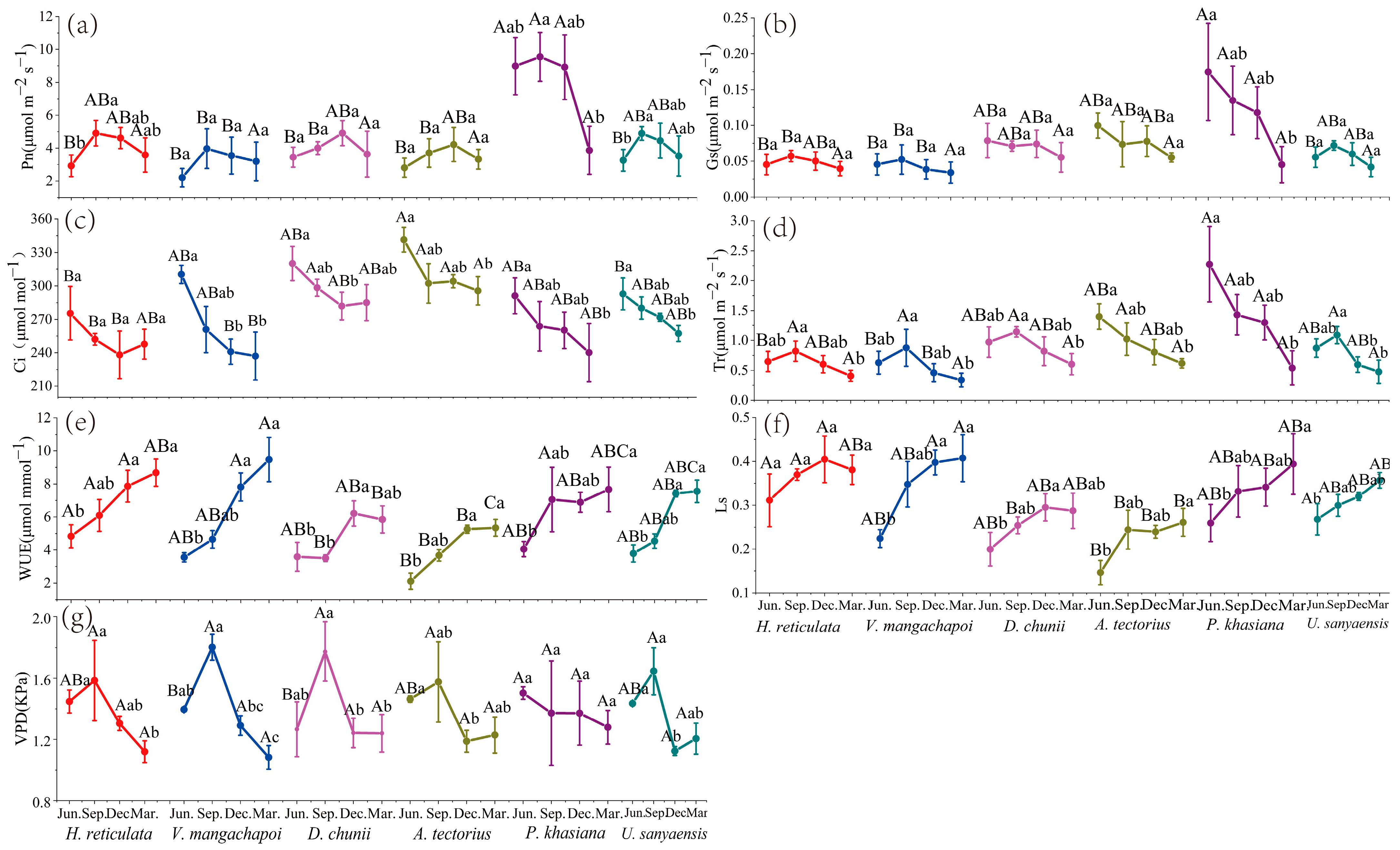
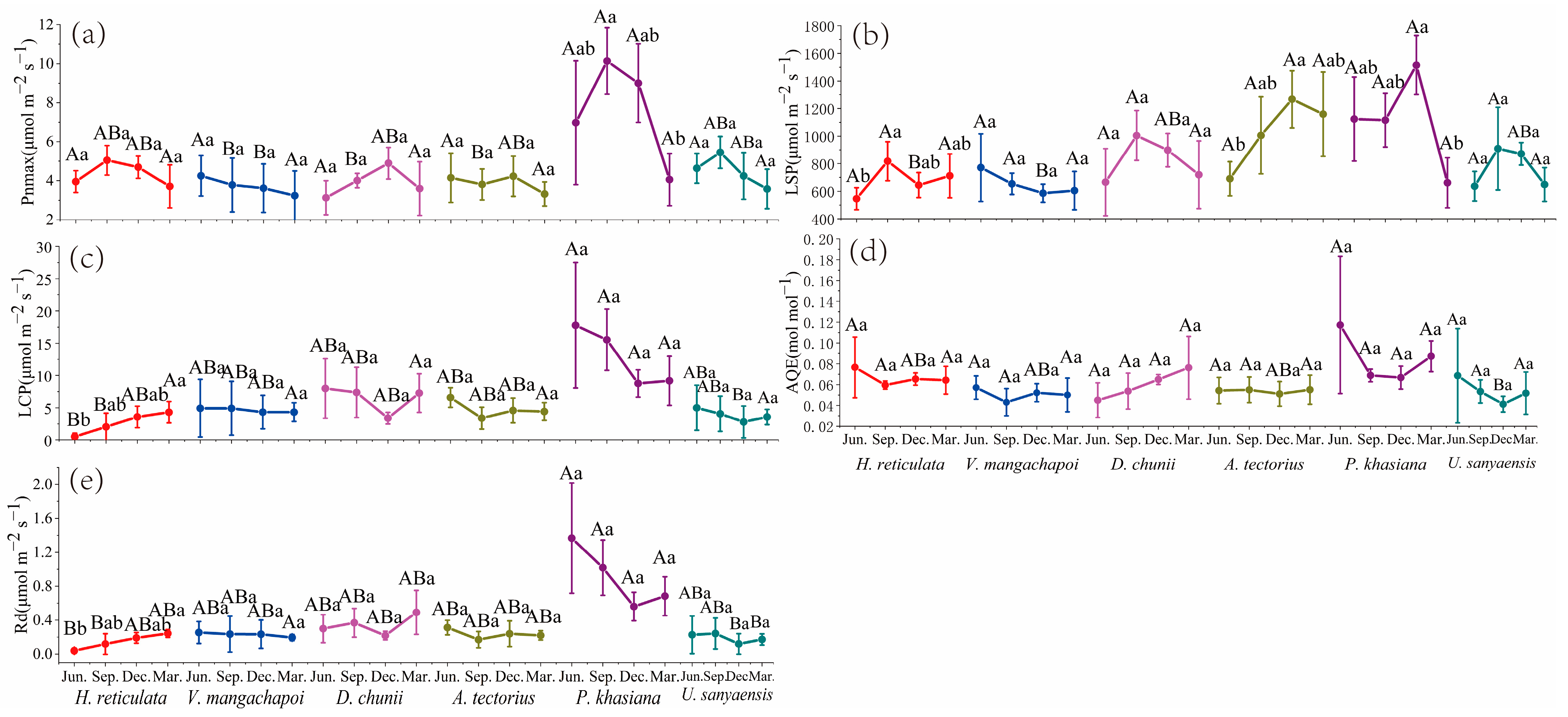
3.1. Gas Exchange Parameters Analysis
3.1.1. Instantaneous Gas Exchange Parameter Analysis
3.1.2. Light Response Curve Analysis
3.2. Leaf Anatomical Structure Analysis
3.3. Leaf Morphology
3.4. Phenotypic Plasticity of Leaf Traits
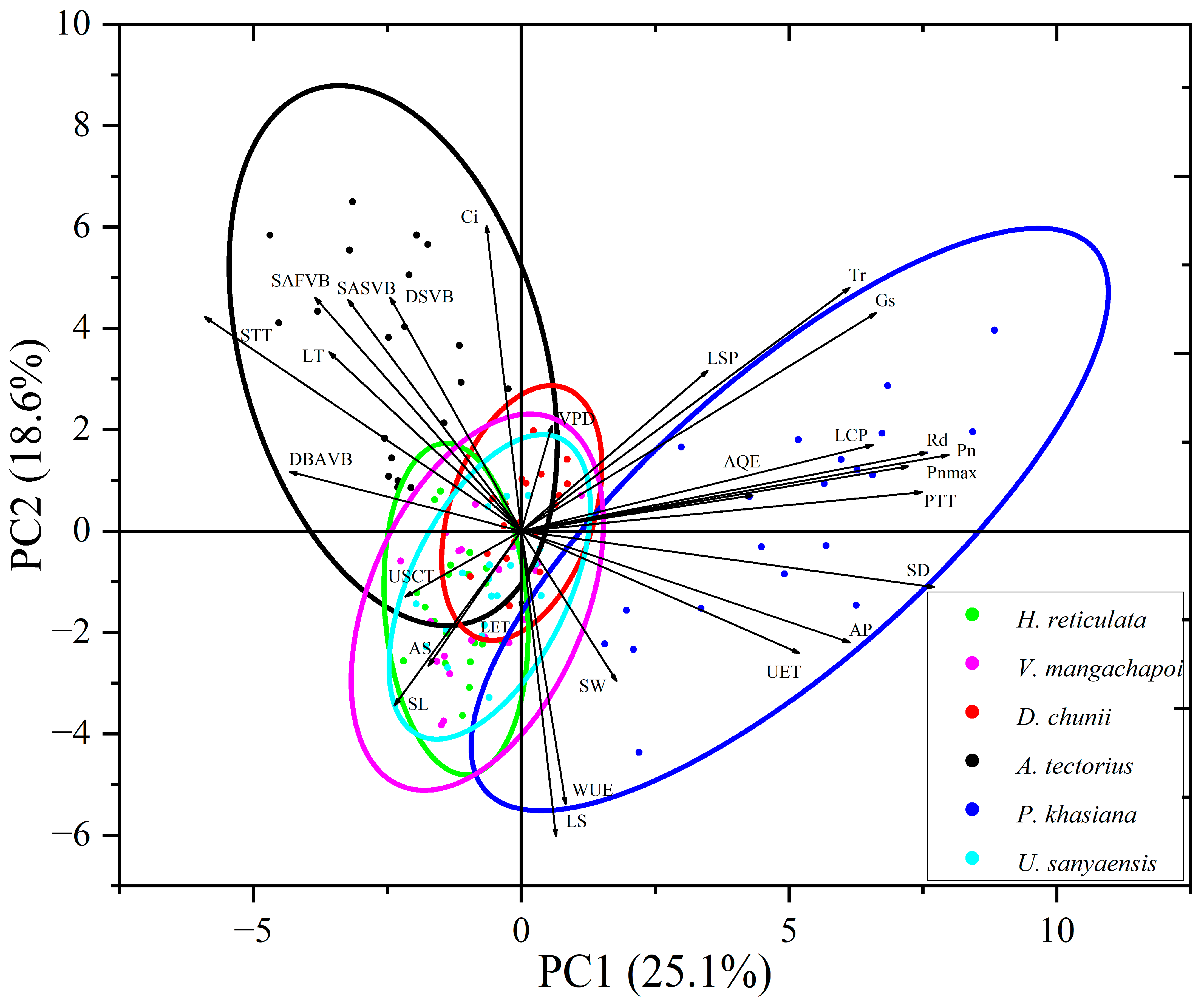
3.5. Principal Component Analysis of Leaf Traits
4. Discussion
4.1. Seasonal Variation of Leaf Functional Traits
4.2. Differences in Seasonal Drought Adaptation Strategies among Dominant Species
4.3. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Yang, Y.; Saatchi, S.S.; Xu, L.; Yu, Y.; Choi, S.; Phillips, N.; Kennedy, R.; Keller, M.; Knyazikhin, Y.; Myneni, R.B. Post-Drought Decline of the Amazon Carbon Sink. Nat. Commun. 2018, 9, 3172. [Google Scholar] [CrossRef]
- Petrík, P.; Zavadilová, I.; Šigut, L.; Kowalska, N.; Petek-Petrik, A.; Szatniewska, J.; Jocher, G.; Pavelka, M. Impact of Environmental Conditions and Seasonality on Ecosystem Transpiration and Evapotranspiration Partitioning (T/ET Ratio) of Pure European Beech Forest. Water 2022, 14, 3015. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, W.; Schäfer, K.V.R.; Lei, P.; Deng, X.; Forrester, D.I.; Fang, X.; Zeng, Y.; Ouyang, S.; Chen, L.; et al. Photosynthetic and Hydraulic Traits Influence Forest Resistance and Resilience to Drought Stress across Different Biomes. Sci. Total Environ. 2022, 828, 154517. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Konings, A.G.; Trugman, A.T.; Yu, K.; Bowling, D.R.; Gabbitas, R.; Karp, D.S.; Pacala, S.; Sperry, J.S.; Sulman, B.N.; et al. Hydraulic Diversity of Forests Regulates Ecosystem Resilience during Drought. Nature 2018, 561, 538–541. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Cernusak, L.A.; Eamus, D.; Ellsworth, D.S.; Colin Prentice, I.; Wright, I.J.; Boer, M.M.; Bradford, M.G.; Cale, P.; Cleverly, J.; et al. A Continental-scale Assessment of Variability in Leaf Traits: Within Species, across Sites and between Seasons. Funct. Ecol. 2018, 32, 1492–1506. [Google Scholar] [CrossRef]
- Hua, L.; Yu, F.; Qiu, Q.; He, Q.; Su, Y.; Liu, X.; Li, J. Relationships between Diurnal and Seasonal Variation of Photosynthetic Characteristics of Eucalyptus Plantation and Environmental Factors under Dry-Season Irrigation with Fertilization. Agric. Water Manag. 2021, 248, 106737. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Gou, X.; Fonti, P.; Xia, J.; Cao, Z.; Liu, J.; Wang, Y.; Zhang, J. Seasonal Variations in Leaf-Level Photosynthesis and Water Use Efficiency of Three Isohydric to Anisohydric Conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308–309, 108581. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; ur Rehman, H.; Bilal, H.M. Synergistic Consequences of Salinity and Potassium Deficiency in Quinoa: Linking with Stomatal Patterning, Ionic Relations and Oxidative Metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yee, E.G.; Callahan, H.S.; Griffin, K.L.; Palmer, M.I.; Lee, S. Seasonal Patterns of Native Plant Cover and Leaf Trait Variation on New York City Green Roofs. Urban Ecosyst. 2022, 25, 229–240. [Google Scholar] [CrossRef]
- Inoue, Y.; Ichie, T.; Kenzo, T.; Yoneyama, A.; Kumagai, T.; Nakashizuka, T. Effects of Rainfall Exclusion on Leaf Gas Exchange Traits and Osmotic Adjustment in Mature Canopy Trees of Dryobalanops Aromatica (Dipterocarpaceae) in a Malaysian Tropical Rain Forest. Tree Physiol. 2017, 37, 1301–1311. [Google Scholar] [CrossRef]
- Smith, M.N.; Stark, S.C.; Taylor, T.C.; Ferreira, M.L.; de Oliveira, E.; Restrepo-Coupe, N.; Chen, S.; Woodcock, T.; dos Santos, D.B.; Alves, L.F.; et al. Seasonal and Drought-related Changes in Leaf Area Profiles Depend on Height and Light Environment in an Amazon Forest. New Phytol. 2019, 222, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-F.; Li, X.; Su, Y.-H.; Lu, L.; Huang, C.-L. Seasonal Fluctuations and Temperature Dependence in Photosynthetic Parameters and Stomatal Conductance at the Leaf Scale of Populus Euphratica Oliv. Tree Physiol. 2011, 31, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, B.; Zorrilla-Fontanesi, Y.; van Wesemael, J.; Mduma, H.; Brown, A.; Carpentier, S.; Swennen, R. Effect of Seasonal Drought on the Agronomic Performance of Four Banana Genotypes (Musa spp.) in the East African Highlands. Agronomy 2021, 11, 4. [Google Scholar] [CrossRef]
- Wu, J.; Serbin, S.P.; Ely, K.S.; Wolfe, B.T.; Dickman, L.T.; Grossiord, C.; Michaletz, S.T.; Collins, A.D.; Detto, M.; McDowell, N.G.; et al. The Response of Stomatal Conductance to Seasonal Drought in Tropical Forests. Glob. Chang. Biol. 2020, 26, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Lyu, S.; Wen, X. Limitation of Soil Moisture on the Response of Transpiration to Vapor Pressure Deficit in a Subtropical Coniferous Plantation Subjected to Seasonal Drought. J. Hydrol. 2020, 591, 125301. [Google Scholar] [CrossRef]
- Wolfe, B.T.; Sperry, J.S.; Kursar, T.A. Does Leaf Shedding Protect Stems From Cavi-Tation During Seasonal Droughts? A Test of the Hydraulic Fuse Hypothesis. New Phytol. 2016, 212, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.; Dent, D.; Braden, D.; Ashton, M.S.; Berlyn, G.P.; Hall, J.S. Seasonal Variability of Photosynthetic Characteristics Influences Growth of Eight Tropical Tree Species at Two Sites With Contrasting Precipitation in Panama. For. Ecol. Manag. 2011, 261, 1643–1653. [Google Scholar] [CrossRef]
- Lu, X.; Zang, R.; Xu, Y.; Yu, S.; Zhao, H. Effects of Above- and below-Ground Interactions of Plants on Growth of Tree Seedlings in Low-Elevation Tropical Rainforests on Hainan Island, China. Forests 2021, 12, 905. [Google Scholar] [CrossRef]
- Xu, R.; Hu, X.; Liu, G.; Guo, W.; Liang, C.; Kong, X. Differences of Leaf Functional Traits Between Two Climbing Bamboo Species in Tropical Lowland Rainforest of Hainan Island. Sci. Silvae Sin. 2021, 57, 155–166. [Google Scholar] [CrossRef]
- Nolan, R.H.; Resco de Dios, V.; Boer, M.M.; Caccamo, G.; Goulden, M.L.; Bradstock, R.A. Predicting Dead Fine Fuel Moisture at Regional Scales Using Vapour Pressure Deficit from MODIS and Gridded Weather Data. Remote Sens. Environ. 2016, 174, 100–108. [Google Scholar] [CrossRef]
- Ye, Z.P. A Review on Modeling of Responses of Photosynthesis to Light and CO2. Chin. J. Plant Ecol. 2010, 34, 727–740. [Google Scholar]
- Polley, H.W.; Yang, C.; Wilsey, B.J.; Fay, P.A. Temporal Stability of Grassland Metacommunities Is Regulated More by Community Functional Traits than Species Diversity. Ecosphere 2020, 11, e03178. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Valladares, F.; Martinez-ferri, E.; Balaguer, L.; Perez-corona, E.; Manrique, E. Low Leaf-level Response to Light and Nutrients in Mediterranean Evergreen Oaks: A Conservative Resource-use Strategy? New Phytol. 2000, 148, 79–91. [Google Scholar] [CrossRef]
- Kripa, M.K.; Nivas, A.H.; Lele, N.; Thangaradjou, T.; Kumar, A.S.; Mankad, A.U.; Murthy, T.V.R. Seasonal Dynamics and Light Use Efficiency of Major Mangrove Species Over Indian Region. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 267–275. [Google Scholar] [CrossRef]
- Kitajima, K.; Mulkey, S.S.; Wright, S.J. Seasonal Leaf Phenotypes in the Canopy of a Tropical Dry Forest: Photosynthetic Characteristics and Associated Traits. Oecologia 1997, 109, 490–498. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Holbrook, N.M.; Cao, K.-F. Seasonal Dynamics in Photosynthesis of Woody Plants at the Northern Limit of Asian Tropics: Potential Role of Fog in Maintaining Tropical Rainforests and Agriculture in Southwest China. Tree Physiol. 2014, 34, 1069–1078. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D. Seasonal Trends in Photosynthetic Parameters and Stomatal Conductance of Blue Oak (Quercus Douglasii) under Prolonged Summer Drought and High Temperature. Tree Physiol. 2003, 23, 865–877. [Google Scholar] [CrossRef]
- Osuna, J.L.; Baldocchi, D.D.; Kobayashi, H.; Dawson, T.E. Seasonal Trends in Photosynthesis and Electron Transport during the Mediterranean Summer Drought in Leaves of Deciduous Oaks. Tree Physiol. 2015, 35, 485–500. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy Shifts in Leaf Physiology, Structure and Nutrient Content between Species of High- and Low-Rainfall and High- and Low-Nutrient Habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kloeppel, B.D.; Kubiske, M.E. Ecophysiological and Morphological Responses to Shade and Drought in Two Contrasting Ecotypes of Prunus Serotina. Tree Physiol. 1992, 10, 343–355. [Google Scholar] [CrossRef]
- Gratani, L.; Bombelli, A. Correlation between Leaf Age and Other Leaf Traits in Three Mediterranean Maquis Shrub Species: Quercus Ilex, Phillyrea Latifolia and Cistus Incanus. Environ. Exp. Bot. 2000, 43, 141–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Luan, J.-W.; Wang, Y.; Yang, H.; Liu, S.-R. Effects of Simulated Drought and Phosphorus Addition on Nitrogen Mineralization in Tropical Lowland Rain Forests. Chin. J. Plant Ecol. 2022, 46, 102–113. [Google Scholar] [CrossRef]
- Olsen, J.T.; Caudle, K.L.; Johnson, L.C.; Baer, S.G.; Maricle, B.R. Environmental and Genetic Variation in Leaf Anatomy among Populations of Andropogon Gerardii (Poaceae) along a Precipitation Gradient. Am. J. Bot. 2013, 100, 1957–1968. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Xiang, W.; Wen, Y.; Huang, D.-L.; Cao, K.-F.; Zhu, S.-D. Correlations between Leaf Economics, Mechanical Resistance and Drought Tolerance across 41 Cycad Species. Ann. Bot. 2022, 130, 345–354. [Google Scholar] [CrossRef]
- Song, Z.; Ni, X.; Yao, J.; Wang, F. Progress in Studying Heteromorphic Leaves in Populus Euphratica: Leaf Morphology, Anatomical Structure, Development Regulation and Their Ecological Adaptation to Arid Environments. Plant Signal. Behav. 2021, 16, 1870842. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; van der Heijden, G.M. Lianas Have a Seasonal Growth Advantage over Co-occurring Trees. Ecology 2019, 100, e02655. [Google Scholar] [CrossRef]
- Szatniewska, J.; Zavadilova, I.; Nezval, O.; Krejza, J.; Petrik, P.; Čater, M.; Stojanović, M. Species-Specific Growth and Transpiration Response to Changing Environmental Conditions in Floodplain Forest. For. Ecol. Manag. 2022, 516, 120248. [Google Scholar] [CrossRef]
- Coomes, D.A.; Kunstler, G.; Canham, C.D.; Wright, E. A Greater Range of Shade-Tolerance Niches in Nutrient-Rich Forests: An Explanation for Positive Richness-Productivity Relationships? J. Ecol. 2009, 97, 705–717. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and Life History of Tropical Dry Forest Tree Species: Coordination of Species’ Drought and Shade Tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | Description | Unit | Measurement (Time; Sample no.) |
|---|---|---|---|
| Pn | Net photosynthetic rate | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| Gs | Stomatal conductance | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| Ci | Intercellular carbon dioxide concentration | μmol mol−1 | Jun., Sep., Dec., Mar.; 5 |
| Tr | Transpiration rate | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| WUE | Water use efficiency | μmol mmol−1 | Jun., Sep., Dec., Mar.; 5 |
| Ls | Stomatal limitation | / | Jun., Sep., Dec., Mar.; 5 |
| VPD | Vapor pressure deficit | KPa | Jun., Sep., Dec., Mar.; 5 |
| AQE | Apparent quantum efficiency | mol mol−1 | Jun., Sep., Dec., Mar.; 5 |
| Pnmax | Maximum photosynthetic rates | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| LSP | Light saturation point | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| LCP | Light compensation point | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| Rd | Dark respiration efficiency | μmol m−2 s−1 | Jun., Sep., Dec., Mar.; 5 |
| LT | leaf thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| USCT | Upper stratum corneum thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| UET | Upper epidermal thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| LET | Lower epidermal thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| PTT | Palisade tissue thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| STT | Spongy tissue thickness | μm | Jun., Sep., Dec., Mar.; 5 |
| SAFVB | Sectional area of first-vascular bundle | mm2 | Jun., Sep., Dec., Mar.; 5 |
| DSVB | Diameter of second-order vascular bundle | μm | Jun., Sep., Dec., Mar.; 5 |
| SASVB | Sectional area of second-order vascular bundle | mm2 | Jun., Sep., Dec., Mar.; 5 |
| DBAVB | Distance between adjacent vascular bundle | μm | Jun., Sep., Dec., Mar.; 5 |
| SL | Stomata length | μm | Jun., Sep., Dec., Mar.; 5 |
| SW | Stomata width | μm | Jun., Sep., Dec., Mar.; 5 |
| AS | Area of single stomata | μm2 | Jun., Sep., Dec., Mar.; 5 |
| SD | Stomata density | number mm−2 | Jun., Sep., Dec., Mar.; 5 |
| AP | Percent of stomata area | % | Jun., Sep., Dec., Mar.; 5 |
| LA | Leaf area | cm2 | Jun., Sep., Dec., Mar. 3 |
| LDMC | Leaf dry-matter content | g kg−1 | Jun., Sep., Dec., Mar.; 3 |
| SLA | Specific leaf area | m2 kg−1 | Jun., Sep., Dec., Mar.; 3 |
| Index | Hopea reticulata | Vatica mangachapoi | Diospyros chunii | Ancistrocladus tectorius | Phanera khasiana | Uvaria sanyaensis | |
|---|---|---|---|---|---|---|---|
| Photosynthetic physiology | Pn | 0.40 | 0.45 | 0.30 | 0.34 | 0.60 | 0.33 |
| Gs | 0.31 | 0.35 | 0.30 | 0.45 | 0.74 | 0.42 | |
| Ci | 0.14 | 0.24 | 0.12 | 0.13 | 0.18 | 0.12 | |
| Tr | 0.50 | 0.61 | 0.47 | 0.56 | 0.76 | 0.56 | |
| WUE | 0.44 | 0.62 | 0.44 | 0.60 | 0.47 | 0.50 | |
| Ls | 0.23 | 0.45 | 0.32 | 0.44 | 0.34 | 0.25 | |
| VPD | 0.29 | 0.40 | 0.30 | 0.25 | 0.15 | 0.32 | |
| AQE | 0.22 | 0.25 | 0.41 | 0.07 | 0.43 | 0.40 | |
| Pnmax | 0.26 | 0.24 | 0.36 | 0.21 | 0.60 | 0.34 | |
| LSP | 0.33 | 0.24 | 0.34 | 0.45 | 0.56 | 0.30 | |
| LCP | 0.88 | 0.12 | 0.58 | 0.49 | 0.51 | 0.44 | |
| Rd | 0.84 | 0.23 | 0.56 | 0.46 | 0.59 | 0.51 | |
| Average | 0.41 | 0.35 | 0.37 | 0.37 | 0.49 | 0.37 | |
| Morphology | LT | 0.06 | 0.08 | 0.10 | 0.27 | 0.18 | 0.07 |
| USCT | 0.24 | 0.35 | 0.30 | 0.29 | 0.37 | 0.16 | |
| UET | 0.14 | 0.12 | 0.06 | 0.20 | 0.16 | 0.11 | |
| LET | 0.08 | 0.12 | 0.12 | 0.11 | 0.24 | 0.14 | |
| PTT | 0.25 | 0.08 | 0.06 | 0.43 | 0.17 | 0.17 | |
| STT | 0.01 | 0.12 | 0.22 | 0.35 | 0.40 | 0.44 | |
| SAFVB | 0.44 | 0.26 | 0.58 | 0.85 | 0.42 | 0.39 | |
| DSVB | 0.16 | 0.13 | 0.35 | 0.38 | 0.13 | 0.06 | |
| SASVB | 0.24 | 0.29 | 0.59 | 0.62 | 0.25 | 0.31 | |
| DBAVB | 0.35 | 0.34 | 0.53 | 0.14 | 0.27 | 0.23 | |
| SL | 0.09 | 0.15 | 0.16 | 0.17 | 0.13 | 0.12 | |
| SW | 0.16 | 0.06 | 0.11 | 0.11 | 0.03 | 0.12 | |
| AS | 0.18 | 0.27 | 0.25 | 0.27 | 0.14 | 0.29 | |
| SD | 0.04 | 0.04 | 0.07 | 0.16 | 0.06 | 0.10 | |
| AP | 0.20 | 0.26 | 0.30 | 0.33 | 0.18 | 0.30 | |
| LA | 0.35 | 0.16 | 0.16 | 0.27 | 0.53 | 0.38 | |
| LDMC | 0.33 | 0.51 | 0.48 | 0.17 | 0.28 | 0.57 | |
| SLA | 0.39 | 0.60 | 0.41 | 0.54 | 0.53 | 0.63 | |
| Average | 0.21 | 0.22 | 0.27 | 0.32 | 0.25 | 0.26 | |
| Total Average | 0.31 | 0.29 | 0.32 | 0.35 | 0.37 | 0.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Qiu, Q.; Nong, J.; Fan, S.; Liu, G. Seasonal Patterns and Species Variability in the Leaf Traits of Dominant Plants in the Tropical Rainforests of Hainan Island, China. Forests 2023, 14, 522. https://doi.org/10.3390/f14030522
Xu R, Qiu Q, Nong J, Fan S, Liu G. Seasonal Patterns and Species Variability in the Leaf Traits of Dominant Plants in the Tropical Rainforests of Hainan Island, China. Forests. 2023; 14(3):522. https://doi.org/10.3390/f14030522
Chicago/Turabian StyleXu, Ruijing, Quan Qiu, Junqing Nong, Shaohui Fan, and Guanglu Liu. 2023. "Seasonal Patterns and Species Variability in the Leaf Traits of Dominant Plants in the Tropical Rainforests of Hainan Island, China" Forests 14, no. 3: 522. https://doi.org/10.3390/f14030522
APA StyleXu, R., Qiu, Q., Nong, J., Fan, S., & Liu, G. (2023). Seasonal Patterns and Species Variability in the Leaf Traits of Dominant Plants in the Tropical Rainforests of Hainan Island, China. Forests, 14(3), 522. https://doi.org/10.3390/f14030522






