Abstract
In global greening, biogenic volatile organic compound (BVOC) emissions and their influencing factors have been considered due to their significant roles in the biosphere and atmosphere. Many studies have reported relationships of BVOC emissions with environmental factors and plant ecophysiology. However, the direct and indirect effects of environmental factors on BVOC emissions remain unclear, and the causal relationships between plant ecophysiology and BVOC emissions are ambiguous. We measured the isoprene and monoterpene emissions from dominant greening plants using a dynamic enclosure system and quantified the interactions of environment–-plant and ecophysiology–BVOC emissions using a path analysis model. We found that isoprene emission was directly affected by photosynthetic rate, and indirectly affected by photosynthetically active radiation and air temperature (Tair). Monoterpene emissions were directly affected by atmospheric pressure, relative air humidity and specific leaf weight, and indirectly affected by Tair.
1. Introduction
Biogenic volatile organic compounds (BVOCs) are mainly derived from plants and dominate the global flux of VOCs ranging from 700–1000 Tg C yr−1 [1,2]. Isoprene (C5H8) and monoterpenes (C10H16) are significant BVOCs with global fluxes of 299.1–440.5 Tg C yr−1 and 63.2–82.7 Tg C yr−1, and exhibit high reactivity with atmospheric chemical lifetimes of minutes to hours [3,4]. They participate not only in biogeochemical processes in response to biotic and abiotic stresses, but also in atmospheric reactions as precursors of ozone (O3) and secondary organic aerosols (SOAs) [5,6]. In recent decades, vegetation greenness on earth has increased driven by climate changes and human activities [7]. According to MODIS data, the global leaf area increased by 5.4×106 km2 between 2000 and 2017 at a rate of 2.3% per decade, which was strikingly prominent in China and India driven by land-use management [8]. In China, forest area increased by 19% in a single decade due to the implementation of several programs to protect and expand forests, and it contributed 42% to the increase in leaf area [8]. In addition to improving ecosystem services, vegetation greening enhances the emissions of BVOCs and contributes to the formation of near-surface O3 and SOAs [9]. Wang et al. [10] indicated that the emissions of BVOCs in China increased at a rate of 1.09% yr−1 from 2001–2016 as a result of land cover change and replacement of crop- or grasslands with forestlands with a high BVOC emission potential. In terms of global greening, we should focus on the influencing factors of BVOC emissions to provide scientific guidance for plant selection in different environments with the aim of regulating air pollution.
Many studies have investigated the effects of environmental factors (such as temperature, light and humidity) on BVOC emissions and plant ecophysiological parameters (such as leaf water content, leaf dry mass, net photosynthetic rate, intercellular CO2 concentration, transpiration rate and stomatal conductance) in controlled chambers [11,12,13,14,15,16,17]. However, existing studies usually consider the total effects (including direct and indirect effects), thereby ignoring the identification of the direct effect of a certain environmental variable and the indirect effect mediated by another environmental variable or plant ecophysiological parameter. When the indirect effect is significant, the important roles of intermediate variables might be underestimated. Path analysis (PA) can separate the direct and indirect impacts on dependent variables to clarify the role of each independent variable [18]. Accordingly, we established a PA model to quantify the direct and indirect effects of environmental factors on plant ecophysiology and BVOC emissions.
Studies have reported relationships of BVOC emissions with plant ecology and physiology. Yuan et al. [19] and Chen et al. [20] regarded BVOC emissions as dependent variables in response to leaf structure and plant physiology in regression analyses, while Lahr et al. [21] treated BVOC emissions as independent variables to explain the effects on plant photosynthesis, considering the role of isoprene emission in improving the thermotolerance of photosystems. To date, the causal relationships between plant ecophysiology and BVOC emissions remain ambiguous, which has resulted in incomplete knowledge of the interaction processes among the environment, plant ecophysiology and BVOC emissions. Underlying causal interdependencies between variables can be observed through PA [22]. Pathways among environmental factors, plant ecophysiological parameters and BVOC emission rates were constructed to determine the causal structure between these variables to shed light on the action mechanisms of environment–plant and ecophysiology–BVOC emissions interaction.
In this study, we measured the isoprene and monoterpene emissions of six evergreen broad-leaved species (Dimocarpus longan, Litchi chinensis, Eucalyptus grandis, E. citriodora, Acacia confusa and Casuarina equisetifolia) and two needle-leaved species (Pinus massoniana and P. elliottii) in winter and summer to characterize the BVOC emissions of dominant greening plants in Xiamen, China. Environmental factors and plant ecophysiological parameters related to BVOC emissions were analysed to establish a PA model among environment, plant ecophysiology and BVOC emissions, with the aims of quantifying the direct and indirect effects on isoprene and monoterpene emissions and illuminating the interaction processes among environmental variables, plant ecophysiology and BVOC emissions.
2. Materials and Methods
2.1. Study Area and Tree Species
This study was conducted in Xiamen, China (24.42–24.90° N, 117.88–118.42° E), a coastal greening city characterized by a subtropical monsoon climate (Figure S1). In Xiamen, the average air temperature (Tair) and relative air humidity (RH) were 16 °C and 72% in December 2020 (winter), and 28 °C and 85% in June 2021 (summer). Xiamen exhibits a clear gradient of urbanization, which can be divided into urban, suburban and rural areas from the island towards the outer edge based on road density [23]. The fractional vegetation cover increased from 0.22 in 2005 to 0.31 in 2015, and the government initiated the Beautiful Xiamen project to enhance vegetation greenness [24,25]. According to a forest management planning inventory, eight dominant woody species were selected, including D. longan, L. chinensis, E. grandis, E. citriodora, A. confusa, C. equisetifolia, P. massoniana and P. elliottii.
2.2. BVOC Sampling and Analysis
BVOC emissions were sampled using a dynamic enclosure system in December 2020 and June 2021 (Table S1). For each species, 3 healthy and mature trees were selected as replicates along each urbanization gradient (urban, suburban and rural areas), and a blank sample (empty enclosure without a branch) was collected as the background value. On sunny days, a no-shade branch of each tree was enclosed in a 10 L Teflon bag with a transparency of above 95% for in situ sampling of BVOC emissions. The sampling bag was evacuated, clean air filtered through a drying tube (allochroic silicagel) and O3 scrubber (KI tube) was pumped into each sampling bag [26,27]. After the sampling bag was filled with clean air, an air sampler pump, drying tube, O3 scrubber, Teflon bag and adsorption tube containing Tenax GR and Carbopack B were sequentially connected via Teflon tubes to establish a closed-loop system to collect BVOCs (Figure S2) [27,28]. The flow rates of the inlet and outlet of the closed-loop system were 200 mL/min during the sampling, and the sampling time was 45 min for each adsorption tube [29].
The sampled BVOCs were analysed via a thermal desorption (TD) instrument (TurboMatrix 650 ATD, PerkinElmer Instruments, CT, USA) combined with a gas chromatography–mass spectrometry (GC-MS) instrument (QP2010, Shimadzu, Kyoto, Japan) [30]. In regard to TD, BVOCs in the adsorption tube were desorbed at 280 °C for 10 min, adsorbed in a cold trap (−30 °C), heated to 280 °C at a rate of 40 °C/s and maintained for 3 min. Samples from the TD instrument were transferred into the GC-MS instrument through carrier gas (He) with a flow rate of 1 mL/min and separated using a DB-624 capillary column (60 m length, 0.25 mm inner diameter and 1.40 μm film thickness). The GC oven temperature was controlled by a program. The initial temperature of 40 °C was maintained for 10 min, then increased to 180 °C at 5 °C/min and maintained for 5 min, and finally increased to 200 °C at 20 °C/min for 1 min. The MS ion source temperature was 200 °C, and the mass scanning range was 40–210 m/z.
BVOCs were identified and quantified via the external standard method according to the retention time, peak area, and standard mass spectra in the NIST 8 library [31]. Regarding monoterpenes, α-pinene (7785-26-4), β-myrcene (123-35-3), β-pinene (18172-67-3), 3-carene (498-15-7), α-terpinene (99-86-5), d-limonene (5989-27-5) and γ-terpinene (99-85-4) were quantified, which could be emitted by the selected tree species. Pure isoprene (78-79-5) and monoterpenes standards were prepared, respectively. A standard mixture was diluted with acetone and injected into an adsorption tube using a micro-syringe, and then the adsorption tube was analysed by TD-GC-MS as described above. A standard curve for each VOC was obtained at 6 levels (0.05, 0.10, 0.15, 0.20, 0.25 and 0.30 μg) with a correlation coefficient above 0.995. Three repetitions were performed at each gradient.
The BVOC emission rates of plant leaves were calculated according to the following equation [27]:
where E (μg g−1 h−1) is the BVOC emission rate, M (μg) is the BVOC concentration contained in the adsorption tube of the sampled branch, M0 (μg) is the BVOC concentration contained in the adsorption tube of the blank sample, m (g) is the dry weight of leaves in the sampling bag and t (h) is the sampling time.
E = (M − M0)/mt
The standard emission rates of isoprene and monoterpenes were calculated according to Model G93 [32]. In Model G93, the emission of isoprene was calculated by multiplying a standard isoprene emission factor with functions of a temperature dependence and a light dependence. Monoterpene emission rates were calculated by multiplying a standard emission factor with an exponential function depending only on temperature.
2.3. Measurements of the Environment and Plant Ecophysiology
The measured environmental factors included photosynthetically active radiation (PAR), atmospheric pressure (P), Tair and RH, and the plant ecophysiological factors included specific leaf weight (SLW: the dry mass per leaf area), photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and vapour pressure deficit based on the leaf temperature (Vpd).
In the course of BVOC sampling, an LI-6400 XT instrument (LICOR, Lincoln, Dearborn, MI, USA) with a 6 cm2 leaf chamber was used to record PAR, P, Tair, RH, Pn, Tr, Gs, Ci and Vpd at 10 min intervals. The flow rate of the sample cell was maintained at 500 μmol s−1. At each measurement, one or more leaves near the sampling bag were randomly selected to fill the leaf chamber. The mean values of multiple records represent the environmental conditions and leaf physiology during the measurement period. After the measurements, the branch in the sampling bag was harvested. The broadleaves or needles were separated from the branch and scanned with Adobe Photoshop CS6 for leaf area calculation in ImageJ (https://imagej.nih.gov/ij/index.html, accessed on 1 January 2022) [33]. Then, leaf dry weight was measured after oven-drying at 120 °C for 48 h. The SLW was calculated according to the following equation [34]:
where SLW (g m−2) is the specific leaf weight, m (g) and s (m2) are the dry weight and area of leaves in the sampling bag, respectively.
SLW = m/s
2.4. Statistical Analyses
Principal component analysis (PCA), based on the BVOC emission rates (isoprene, α-pinene, β-myrcene, β-pinene, 3-carene, α-terpinene, d-limonene and γ-terpinene) of the eight tree species (D. longan, L. chinensis, E. grandis, E. citriodora, A. confusa, C. equisetifolia, P. massoniana and P. elliottii), was conducted to determine the emission profiles of different tree species. The differences of BVOC emissions in different seasons (winter and summer) and from various vegetation types (broad- and needle-leaved trees) were determined by t-test method.
PA model, a typical structural equation model, explores the control mechanism of multiple independent variables on dependent variables via correlation coefficient and determination coefficient [35]. It allows one to assess or reject a multivariate hypothesis regarding the potential causal structure [22]. When the value of CMIN/DF (chi-square/degrees of freedom) is less than 3 and the comparative fit index (CFI) is greater than 0.95, this indicates that the PA model fits well [36].
The PA model of environment–plant and ecophysiology–BVOC emissions interaction was established using IBM SPSS Amos v24 to determine the key factors and their pathways. Pearson’s correlations among the isoprene and monoterpene emission rates and the environmental factors and plant ecophysiology were examined. Regression analyses were conducted to determine the relationships of the emission rates of isoprene and monoterpenes of the broad- and needle-leaved trees with environmental variables and plant ecophysiological parameters. The pathways in the PA model were established based on the Pearson’s correlations between the factors. The causal relationships between the variables were determined based on determination coefficients and scientific findings and inferences. The PA model provided a close fit, in which the CMIN/DF value was 2.78 and the CFI value was 0.96, and all pathways were significant (p ˂ 0.05).
3. Results
3.1. Seasonal Variations in Isoprene and Monoterpene Emission Rates
The emission rates of isoprene and monoterpenes have obvious seasonal differences. In our study, the isoprene emission rates were significantly higher in summer (1.88 ± 0.31 µg g−1 h−1) than in winter (1.06 ± 0.29 µg g−1 h−1) (p ˂ 0.05) (Figure 1A). Additionally, the monoterpene emission rates were significantly higher in summer (0.38 ± 0.06 µg g−1 h−1) than in winter (0.20 ± 0.03 µg g−1 h−1) (p ˂ 0.05) (Figure 1B).
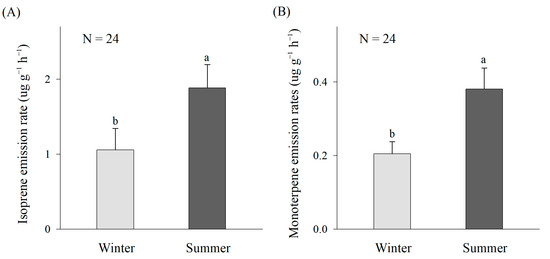
Figure 1.
Seasonal patterns of the isoprene (A) and monoterpene (B) emission rates. The data are the mean ± SE of 24 replicates. The different lowercase letters denote significant differences of BVOC emission rates in between winter and summer at p ˂ 0.05 level.
3.2. Differences of Isoprene and Monoterpene Emissions in Urban, Suburban and Rural Environments
The isoprene and monoterpene emission rates were higher in rural environments than in urban and suburban environments. In the suburban and rural environments, the emission rates of isoprene and monoterpenes were significantly higher in summer than in winter (p ˂ 0.05) (Figure 2). In winter, the isoprene emission rates were significantly higher in rural (1.49 ± 0.66 µg g−1 h−1) than in suburban environments (0.34 ± 0.10 µg g−1 h−1) (p ˂ 0.05) (Figure 2A), and the monoterpene emission rates were significantly different among rural (0.27 ± 0.07 µg g−1 h−1), urban (0.22 ± 0.06 µg g−1 h−1) and suburban environments (0.13 ± 0.04 µg g−1 h−1) (p ˂ 0.05) (Figure 2B). In summer, the isoprene emission rates were significantly higher in rural (2.28 ± 0.65 µg g−1 h−1) than in suburban (1.73 ± 0.47 µg g−1 h−1) and urban environments (1.63 ± 0.49 µg g−1 h−1) (p ˂ 0.05) (Figure 2A), and the monoterpene emission rates were significantly higher in rural (0.44 ± 0.12 µg g−1 h−1) than in urban environments (0.27 ± 0.07 µg g−1 h−1) (p ˂ 0.05) (Figure 2B).
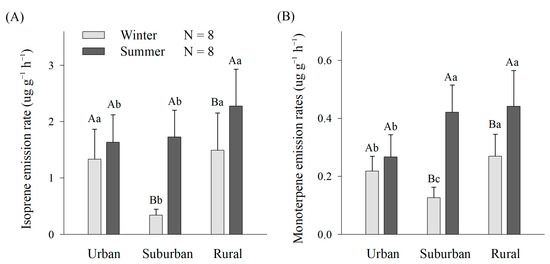
Figure 2.
Isoprene (A) and monoterpene (B) emission rates of dominant greening plants in urban, suburban and rural regions. The data are the mean ± SE of eight replicates. The different capital letters denote significant differences of BVOC emission rates in both winter and summer in a certain urbanization gradient at p ˂ 0.05. The different lowercase letters denote significant differences of BVOC emission rates among urban, suburban and rural regions in a certain season (p ˂ 0.05).
3.3. Differences of Isoprene and Monoterpene Emissions between Broadleaf and Needle
The isoprene emission rates of broad-leaved trees (1.95 ± 0.27 µg g−1 h−1) were significantly higher than those of needle-leaved trees (0.02 ± 0.00 µg g−1 h−1) (p ˂ 0.05) (Figure 3A). The monoterpene emission rates of needle-leaved trees (0.73 ± 0.09 µg g−1 h−1) were obviously higher than those of broad-leaved trees (0.15 ± 0.02 µg g−1 h−1) (p ˂ 0.05) (Figure 3B).
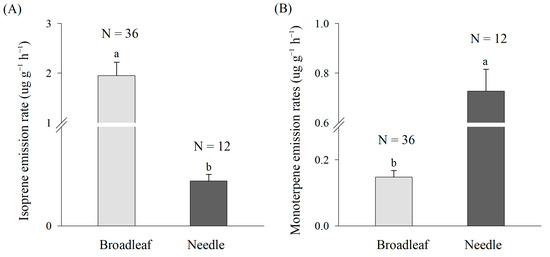
Figure 3.
Emission rates of isoprene (A) and monoterpenes (B) of broadleaf and needle. The data are the mean ± SE. The replicates were 36 for broadleaf and 12 for needle. The different lowercase letters denote significant differences of BVOC emission rates between broadleaf and needle at p ˂ 0.05 level.
The results of PCA indicated a clear separation of BVOC emission profiles among the selected tree species. The two major PCs explained 82.89% of the total variance, with PC1 accounting for 62.10% of the total variance, representing monoterpene emissions (α-pinene, β-myrcene, β-pinene, 3-carene, α-terpinene, d-limonene and γ-terpinene), and PC2 accounting for 20.79% of the total variance, representing isoprene emissions (Figure 3). The two Eucalyptus species (E. grandis and E. citriodora) exhibited high isoprene and high monoterpene emission rates (especially d-limonene). C. equisetifolia exhibited high isoprene and low monoterpene emission rates. The Pinus species (P. massoniana and P. elliottii) and L. chinensis exhibited high monoterpenes and low isoprene emission rates. D. longan and A. confusa attained low isoprene and low monoterpene emission rates (Figure 4).
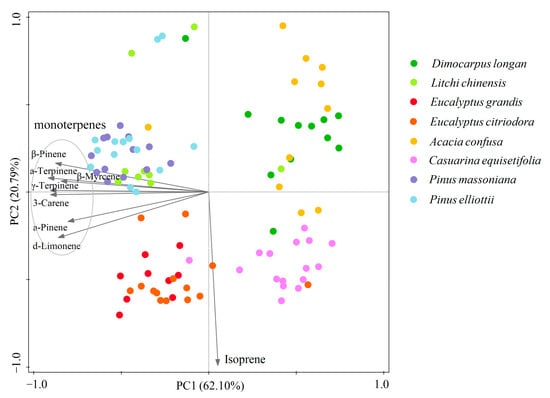
Figure 4.
Principal component analysis of the emission rates of isoprene and monoterpenes (α-pinene, β-myrcene, β-pinene, 3-carene, α-terpinene, d-limonene and γ-terpinene) of the eight woody trees.
3.4. PA Model of Environment–Plant and Ecophysiology–BVOC Emissions Interaction
According to the results of Pearson’s correlation analysis, the isoprene emission rate exhibited significant relationships with PAR, Tair, SLW, Pn, Tr, Gs and Ci (Figure 5). The monoterpene emission rate showed significant relationships with P, Tair, RH, SLW, Tr and Vpd (Figure 5). RH was negatively related with PAR (p ˂ 0.05) and Vpd (p ˂ 0.01). PAR exhibited significant relationships with Tair, Pn, Tr, Gs and Ci (p ˂ 0.01). Tair was negatively related with P, SLW and Ci and positively correlated with Tr and Vpd (p ˂ 0.01). P attained negative trends with Gs and Vpd (p ˂ 0.01). Pn and SLW attained a significant negative trend (p ˂ 0.01). Tr was positively correlated with Pn and Vpd (p ˂ 0.01). Gs showed positive correlations with Pn and Tr (p ˂ 0.01) (Figure 5).
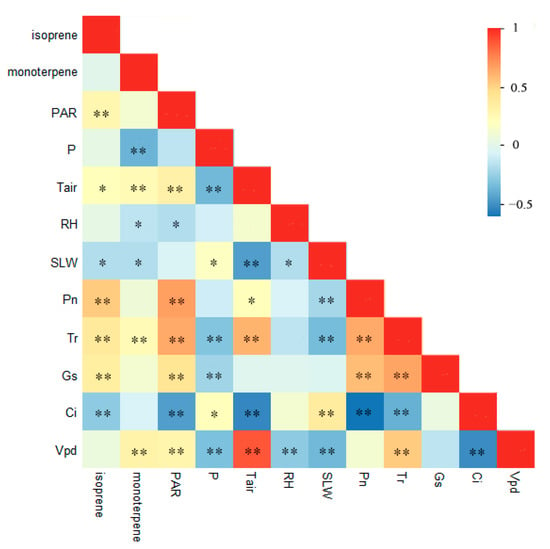
Figure 5.
Pearson’s correlations among the isoprene and monoterpene emission rates and the environmental factors and plant ecophysiology. * is significant at p ˂ 0.05; ** is significant at p ˂ 0.01.
The regression analysis showed that the isoprene emission rates of broad-leaved trees were not strongly related to environmental factors (Figure S3), while they were closely related to plant physiological parameters (Figure S4). The isoprene emission rates of broad-leaved trees exhibited positive linear trends with Pn, Tr and Gs (p ˂ 0.05), and the regression coefficients were 0.43, 0.41 and 0.39 in winter, respectively (Figure S4). The monoterpene emission rates of needle-leaved trees exhibited positive trends with PAR (R2 = 0.32, p ˂ 0.05, Figure S6A) and Tair (R2 = 0.25, p ˂ 0.05, Figure S6C), while they were not strongly related to plant ecophysiological parameters (Figure S5).
We established the pathways of PA model based on the Pearson’s correlations between the factors, and the causal relationships based on the regression coefficients and scientific findings. In the PA model, the environment and plant ecophysiology affected the BVOC emissions. The dominant factors and the pathways influencing the emission rates of isoprene and monoterpenes were identified. The standardized total effects of Pn, PAR, Ci, Vpd, Tr, Gs, SLW, Tair, RH and P on the isoprene emission rate were 0.51, 0.36, −0.31, −0.24, 0.20, 0.19, −0.09, 0.08, 0.02 and −0.01, respectively, indicating that the primary factor influencing isoprene emission rate was Pn (Table S2). Pn not only directly impacted the isoprene emission rate (the standardized direct effect was 0.50), but also indirectly affected by influencing the SLW (the standardized indirect effect was 0.01) (Figure 6). PAR indirectly affected the isoprene emission rate, mainly through six pathways, namely, from PAR to Ci→Pn or Pn or Gs→Tr→Pn or Tr→Pn or Gs→Pn or Gs→Vpd→Pn to isoprene, with standardized indirect effects of 0.13, 0.09, 0.04, 0.04, 0.03 and 0.01, respectively (Figure 6). Tair indirectly influenced the isoprene emission rate, mainly through four pathways, i.e., from Tair to Ci→Pn or Tr→Pn or Vpd→Tr→Pn to isoprene and from Tair to SLW to isoprene, with standardized indirect effects of 0.13, 0.10, 0.05 and 0.04, respectively (Figure 6).
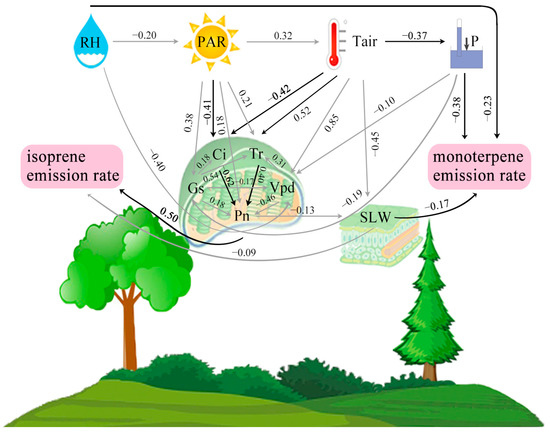
Figure 6.
PA model among the environmental factors (PAR: photosynthetically active radiation; P: atmospheric pressure; Tair: air temperature; RH: relative air humidity), plant eco-physiological factors (SLW: specific leaf weight; Pn: photosynthetic rate; Tr: transpiration rate; Gs: stomatal conductance; Ci: intercellular CO2 concentration; Vpd: vapour pressure deficit) and the emission rates of isoprene and monoterpenes. Standard coefficients are shown on each line. The black lines indicate the major pathways affecting the emission rates of isoprene and monoterpenes.
Regarding the monoterpene emission rate, the standardized total effects of P, RH, Tair, SLW, PAR, Pn, Ci, Vpd, Tr and Gs were −0.38, −0.25, 0.22, −0.17, 0.08, 0.02, −0.01, −0.01, 0.01 and 0.01, respectively, suggesting that the primary factors influencing the monoterpene emission rate were P, RH, Tair and SLW (Table S2). In regard to direct effects, the monoterpene emission rate was negatively impacted by P, RH and SLW with the standardized direct effect of −0.38, −0.23 and −0.17, respectively (Figure 6). Indirectly, Tair affected the monoterpene emission rate by influencing the P or SLW, with standardized indirect effects of 0.14 and 0.08, respectively (Figure 6).
4. Discussion
4.1. BVOC Emission Characteristics
The emission rates of isoprene and monoterpenes in the suburban and rural environments were higher in summer than in winter, due to the seasonal changes in Tair and PAR [37,38,39,40]. In our study, the seasonality of isoprene and monoterpene emissions was not obvious in the urban environment, possibly due to the geographical location of its coastal islands affected by marine air masses [41,42].
The biosynthesis and storage of isoprene and monoterpenes determine the emissions from broad- and needle-leaved trees. Isoprene is produced from dimethylallyl diphosphate under the catalysis of isoprene synthase via the methylerythritol 4-phosphate pathway in chloroplasts [4,43]. Broad-leaved trees generally contain more chloroplasts with a larger leaf area than those of coniferous trees, which is favourable for isoprene synthesis and emission. Monoterpene is produced from geranyl diphosphate under monoterpene synthase and is usually stored in palisade tissue, spongy tissue, oil glands and vascular bundles [44]. Most needle-leaved trees contain specific storage tissues and organs, which facilitates the storages and emissions of monoterpenes [45].
The PCA indicated that the considered plant species exhibited diverse emission patterns. Eucalyptus trees (E. grandis and E. citriodora) are the high BVOC emitters, with standard isoprene emission rates of 39.78 ± 16.04 µg g−1 h−1 and 19.13 ± 5.80 µg g−1 h−1, and standard monoterpene emission rates of 0.44 ± 0.16 µg g−1 h−1 and 0.32 ± 0.08 µg g−1 h−1. Compared with the mature trees in our study, the Eucalyptus trees which were 12 to 24 months old showed higher emission rates of isoprene and monoterpene [46]. C. equisetifolia was a emitter with high isoprene, whose standard isoprene emission rate with 9.57 ± 2.15 µg g−1 h−1 was higher than that measured by Zhao et al. [47] and Huang et al. [48] using a static enclosure sampling method. Pinus trees (P. massoniana and P. elliottii) are the emitters with high monoterpenes. The standard monoterpene emission rate of P. massoniana with 0.90 ± 0.23 µg g−1 h−1 was similar to that measured by Yang et al. [49] and Huang et al. [48]. In addition, BVOC emission capacity varies with leaf ontogeny [50,51,52]. Further, it is necessary to record the leaf growth stages to determine the effects of the leaf development on BVOC emissions.
4.2. Direct and Indirect Effects of Environmental Factors and Plant Ecophysiology on Isoprene Emissions
The Pn directly determines the isoprene emission rate. Isoprene is synthesized through dimethylallyl diphosphate, and isopentenyl pyrophosphate produced from pyruvate and glyceraldehyde 3-phosphate, then it is released into the environment through plasma membranes, cell walls and sometimes cuticles via passive diffusion and vesicle trafficking [53,54,55]. Bisphosphoglyceric acid and 3-phosphoglyceric acid may connect photosynthetic carbon metabolism, and the carbon substrate and energy for isoprene synthesis and emission could be provided by photosynthesis [56]. Plant VOC emissions account for up to 10% of the total carbon assimilated via photosynthetic processes [57]. As isoprene with a low molecular weight (68.12 Da) is immediately released without a stored pool, the emission process is terminated within minutes if Pn reaches zero [58]. Under a high Pn, isoprene emission rate can be enhanced to prevent the damage of membrane function and integrity from accumulation of volatiles [59,60,61].
Indirectly, the PAR affected the isoprene emission rate by influencing photosynthetic processes. Similar to many studies, PAR attained a positive linear relationship with Pn within the light saturation point [62,63]. With decreasing PAR, the role of photosynthesis could shift from inducing nonphotochemical quenching to photosynthetic limitation of electron flow, and Pn could decline [64,65]. In the PA model, PAR affected Pn by influencing Ci and Tr. Increased PAR could enhance ribulose bisphosphate (RuBP) carboxylase activation and decrease the Ci, then increase the Pn [56,66,67]. Transpiration is the evaporation process of water vapour on the leaf surface driven by solar radiation, and PAR positively affects Tr [68]. Water vapour diffuses out of leaves in the same way that CO2 enters leaf cells. With increasing photosynthetic electron transport capacity resulting from an enhanced Tr, the Pn increased [69].
The Tair indirectly determined the isoprene emission rate by influencing the plant ecophysiological variables. With increasing Tair, RuBP carboxylation and regeneration rates were enhanced, and the Ci decreased; then, the Pn increased [69]. With increasing temperature, evaporation of water vapour from the leaf surface accelerated, and the Tr increased; then, the Pn was consequently enhanced [69].
Primarily, the isoprene emission rate depended on photosynthesis directly, and relied on PAR and Tair indirectly. This is consistent with many studies on woody plants [19,26]. In Model G93, PAR and Tair were used to estimate the isoprene emission rate as emission activity factors [31]. However, the isoprene emission rate may be light-independent for Crassulacean acid metabolism plants, which only open their stomata in the dark to fix CO2 [70]. Under extremely high temperatures, RuBP could be inactivated by heat-sensitive Rubisco activase, and leaf stomata could close, which could slow photosynthetic processes and reduce the isoprene emission rate [71,72,73]. Further, it is necessary to explore BVOC emission processes under extreme conditions.
4.3. Interaction among Environmental Factors, Plant Ecophysiology and Monoterpene Emissions
Monoterpenes are stored in particular organs after synthesis; thus, their emissions involve volatilization from stored organs rather than being released directly like isoprene, which is related to transport resistance along volatile path and storage pool size [74,75,76].
Directly, the P had a negative effect on the monoterpene emission rate. With P increased, the press on leaf surface strengthen due to the collision of abundant air molecules, then the resistance of monoterpene release from leaves into atmosphere increased, and the monoterpene emission rate decreased [77,78]. Conversely, a low P resulted in a high monoterpene emission rate. Indirectly, the P determined the monoterpene emission rate via photosynthesis and SLW.
The RH imposed an inverted effect on the monoterpene emission rate. The lipophilic monoterpenes occurring within leaves entered the atmosphere by passing through hydrophobic cuticles or stomata. A high air humidity could induce hydration of the leaf epidermis [79]. According to diffusion-based models, most VOCs (except isoprene) could accumulate to very high concentrations in lipophilic layers if they passively diffused into adjacent hydrophilic layers [55]. Widhalm et al. [55] proposed that VOCs could be mediated by carrier or lipid transfer proteins across the aqueous environment to prevent repartition into the lipophilic layers. We speculated that hydrated leaf cuticles increased the transport resistance of lipophilic monoterpenes into the atmosphere, so the monoterpene emission rate declined. Moreover, leaf cuticles usually consist of cutin and biopolymers. Water could promote flexibility of the polymer matrix functioning as a plasticizer and increase the leaf cuticle elasticity, which could improve the pool sizes of monoterpenes and impact the emission rates [80]. A low RH or drought could enhance the monoterpene emission rate, consistent with the results of a meta-analysis study [81,82]. This result suggested that plants could respond to drought stress by releasing monoterpenes.
The SLW affected the monoterpene emission rate directly, which could reflect plant allocation strategies for available resources and adaptation to changing environments [83]. SLW varies with leaf composition, thickness or both as a complex of leaf area and dry weight [84]. Usually, low-SLW leaves are thin, with fewer layers of palisade parenchyma and spongy mesophyll, a small epidermal cell thickness and thin cuticle, which reduces the resistance of monoterpene across multiple organelle and allows monoterpene to be released at a high emission rate [85,86]. Conversely, high-SLW leaves exhibited a high resistance to monoterpene transport, resulting in low emission rates of monoterpenes. In addition, the size and distribution of the monoterpene pool probably varied with SLW and instantaneously affected the monoterpene emission rate [87,88]. However, it remains to be further studied how SLW affects monoterpene storage to regulate its emission rate.
Indirectly, the Tair determined the monoterpene emission rate by influencing P and SLW. The Tair was negatively related to P and SLW (Figure 5). Consistent with many studies, the P (99.32 ± 0.00 kPa) and SLW (66.64 ± 2.37 g m−2) in summer with higher Tair were lower than those in winter (100.62 ± 0.00 kPa and 163.38 ± 10.45 g m−2, respectively) [89,90,91]. Then, the low P and SLW increased the monoterpene emission rate. As a consequence, a high air temperature could increase the monoterpene emission rate.
The monoterpene emission rate was directly affected by P, RH and SLW, and indirectly determined by Tair. We only measured the instantaneous monoterpene emission rates in winter and summer; accordingly, it is difficult to determine how the transport resistance and pool size vary over time and affect the release of monoterpenes. After cessation of monoterpene biosynthesis, the emission from storage pools could be sustained for several hours to days at a low level, especially at low temperatures [92]. Research on Halimium halimifolium L. suggested that the monoterpene emission rate was high during the first 48 h of exposure in a 38 °C heat wave and then declined, probably due to the combined decrease in de novo synthesis and stored compounds [73]. Long-term acclimation effects may involve leaf anatomical and phenological changes, metabolic adjustments and gene activation. Considering the presence of monoterpene repositories in leaves, it is necessary to measure not only instantaneous monoterpene emissions but also the long-term response to environmental factors.
4.4. Implications of the PA Model on BVOC Emissions
In this study, we innovatively quantified the interactions among environment and plant ecophysiology and BVOC emissions by PA model. Environmental factors and plant ecophysiology jointly affect BVOC emissions. The isoprene emission rate positively depended on PAR and Tair primarily. The monoterpene emission rate was positively affected by Tair, and negatively affected by P and RH. With greening, the selection of suitable tree species based on the environment and plant characteristics could decrease the emissions of BVOCs and atmospheric pollutants they form. In high-temperature environments, such as heat-island-affected areas, plants with low BVOC emissions are recommended instead of high-BVOC emitters (e.g., E. grandis, E. citriodora). In humid environments (e.g., near rivers) or low-pressure areas (e.g., typhoon centres), plants with low monoterpene emissions, such as C. equisetifolia, are recommended.
The emission rates/factors of isoprene and monoterpenes for the dominant greening trees in Xiamen were reported for the first time. These results can be used for BVOC models to reduce the uncertainty associated with emission rate measurement. Environmental factors (such as temperature and light) have been successfully used to develop BVOC models (e.g., the Model of Emissions of Gases and Aerosols from Nature (MEGAN) and the biosphere emissions inventory system (BEIS)), while physiological parameters are rarely used in the BVOC models. The interaction processes among environmental factors and plant ecophysiological parameters and BVOC emissions could be captured by some models to ameliorate BVOC estimations. Importantly, an improved understanding of how environmental factors interact with plant ecophysiology and BVOC emissions is a crucial component of predicting feedbacks both biosphere and atmosphere.
5. Conclusions
We reported the BVOC emission characteristics and their interaction processes with environmental factors and plant ecophysiology in a case study of Xiamen, China. The BVOC emission rates were higher in summer than in winter. Isoprene was mainly emitted by broad-leaved trees, and monoterpenes were mostly discharged by needle-leaved trees. Eucalyptus plants (E. grandis and E. citriodora) and C. equisetifolia are high isoprene emitters. E. grandis, E. citriodora, P. massoniana, P. elliottii and L. chinensis were high-monoterpene emitters. The major factors influencing the isoprene emission rate were photosynthesis, PAR and Tair. The isoprene emission rate depended on Pn directly, and on PAR and Tair indirectly. The monoterpene emission rate was directly impacted by P, RH and SLW and indirectly impacted by Tair.
With global greening, the interactions of environment–plant and ecophysiology–BVOC emissions should be considered when selecting tree species. Our study could provide scientific guidance for plant configuration for cities in the same climate zone to mitigate the emissions of BVOCs and air pollutants. In addition, the results could be used for models to improve the estimation accuracy on BVOC emissions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14030523/s1, Figure S1: Locations of research area and sample sites; Figure S2. Schematic diagram of the dynamic enclosure system used for BVOC sampling; Figure S3. Relationships of the isoprene emission rates of broad-leaved trees (solid) and needle-leaved trees (hollow) with photosynthetically active radiation (PAR, (A)), atmospheric pressure (P, (B)), air temperature (Tair, (C)), and relative air humidity (RH, (D)); Figure S4. Correlations of the isoprene emission rates of broad-leaved trees (solid) and needle-leaved trees (hollow) in winter (circle) and summer (triangle) with SLW (specific leaf weight, (A)), Pn (photosynthetic rate, (B)), Tr (transpiration rate, (C)), Gs (stomatal conductance, (D)), Ci (intercellular CO2 concentration, (E)), and Vpd (vapour pressure deficit, (F)); Figure S5. Relationships of the monoterpene emission rates of broad-leaved trees (hollow) and needle-leaved trees (solid) with photosynthetically active radiation (PAR, A), atmospheric pressure (P, (B)), air temperature (Tair, (C)), and relative air humidity (RH, (D)); Figure S6. Correlations of the monoterpene emission rates of broad-leaved trees (hollow) and needle-leaved trees (solid) in winter (circle) and summer (triangle) with SLW (specific leaf weight, (A)), Pn (photosynthetic rate, (B)), Tr (transpiration rate, (C)), Gs (stomatal conductance, (D)), Ci (intercellular CO2 concentration, (E)), and Vpd (vapour pressure deficit, (F)); Table S1: Sampling date in this study and air temperature (Tair) and photosynthetically active radiation (PAR) during the sampling; Table S2. Standard effects of environmental factors and plant ecophysiology on the emission rates of isoprene and monoterpenes in the PA model.
Author Contributions
Conceptualization, C.D., H.L. and Y.R.; Data curation, C.D.; Formal analysis, C.D. and H.L.; Funding acquisition, Y.R.; Investigation, C.D., Z.W. and H.L.; Methodology, C.D.; Project administration, Y.R.; Resources, Z.W.; Software, C.D. and H.L.; Supervision, Y.R.; Validation, C.D.; Visualization, C.D.; Writing—original draft, C.D.; Writing—review and editing, Z.W. and Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research Program of China [2022YFF1303001], the National Science Foundation of China [grant number 31972951, 31670645, 42171100, 41801182, 41807502 and 42001210], the National Social Science Fund [grant number 17ZDA058], Fujian Provincial Department of S&T Project [grant number 2021I0041, 2021T3058 and 2018T3018], the Strategic Priority Research Program of the Chinese Academy of Sciences [grant number XDA23020502], the Ningbo Municipal Department of Science and Technology [grant number 2019C10056] and the Key Laboratory of Urban Environment and Health of Chinese Academy of Sciences [grant number KLUEH-C-201701].
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Messina, P.; Lathière, J.; Sindelarova, K.; Vuichard, N.; Granier, C.; Ghattas, J.; Cozic, A.; Hauglustaine, D.A. Global biogenic volatile organic compound emissions in the ORCHIDEE and MEGAN models and sensitivity to key parameters. Atmos. Chem. Phys. 2016, 16, 14169–14202. [Google Scholar] [CrossRef]
- Duan, C.; Liao, H.; Wang, K.; Ren, Y. The research hotspots and trends of volatile organic compound emissions from anthropogenic and natural sources: A systematic quantitative review. Environ. Res. 2022, 216, 114386. [Google Scholar] [CrossRef] [PubMed]
- Atkinsona, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Res. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Sindelarova, K.; Markova, J.; Simpson, D.; Huszar, P.; Karlicky, J.; Darras, S.; Granier, C. High-resolution biogenic global emission inventory for the time period 2000–2019 for air quality modelling. Earth Syst. Sci. Data 2022, 14, 251–270. [Google Scholar] [CrossRef]
- Wu, K.; Yang, X.; Chen, D.; Gu, S.; Lu, Y.; Jiang, Q.; Wang, K.; Ou, Y.; Qian, Y.; Shao, P.; et al. Estimation of biogenic VOC emissions and their corresponding impact on ozone and secondary organic aerosol formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Yang, W.; Cao, J.; Wu, Y.; Kong, F.; Li, L. Review on plant terpenoid emissions worldwide and in China. Sci. Total Environ. 2021, 787, 147454. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Wang, X.; Park, T.; Chen, C.; Lian, X.; He, Y.; Bjerke, J.; Chen, A.; Ciais, P.; Tømmervik, H.; et al. Characteristics, drivers and feedbacks of global greening. Earth Environ. 2019, 1, 14–27. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- Gu, S.; Guenther, A.; Faiola, C. Effects of anthropogenic and biogenic volatile organic compounds on Los Angeles air quality. Environ. Sci. Technol. 2021, 55, 12191–12201. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Guenther, A.; Yang, X.; Wang, L.; Xiao, T.; Li, J.; Feng, J.; Xu, Q.; Cheng, H. Land cover change dominates decadal trends of biogenic volatile organic compound (BVOC) emission in China. Atmos. Chem. Phys. 2020, preprint. [Google Scholar]
- Simon, E.; Kuhn, U.; Rottenberger, S.; Meixner, F.; Kesselmeier, J. Coupling isoprene and monoterpene emissions from Amazonian tree species with physiological and environmental parameters using a neural network approach. Plant Cell Environ. 2005, 28, 287–301. [Google Scholar] [CrossRef]
- Dindorf, T.; Kuhn, U.; Ganzeveld, L.; Schebeske, G.; Ciccioli, P.; Holzke, C.; Koble, R.; Seufert, G.; Kesselmeier, J. Significant light and temperature dependent monoterpene emissions from European beech (Fagus sylvatica L.) and their potential impact on the European volatile organic compound budget. J. Geophys. Res. 2006, 111, D16305. [Google Scholar] [CrossRef]
- Kleiber, A.; Duan, Q.; Jansen, K.; Verena Junker, L.; Kammerer, B.; Rennenberg, H.; Ensminger, I.; Gessler, A.; Kreuzwieser, J. Drought effects on root and needle terpenoid content of a coastal and an interior Douglas fir provenance. Tree Physiol. 2017, 37, 1648–1658. [Google Scholar] [CrossRef]
- Tiiva, P.; Haikio, E.; Kasurinen, A. Impact of warming, moderate nitrogen addition and bark herbivory on BVOC emissions and growth of Scots pine (Pinus sylvestris L.) seedlings. Tree Physiol. 2018, 38, 1461–1475. [Google Scholar] [CrossRef]
- Simin, T.; Tang, J.; Holst, T.; Rinnan, R. Volatile organic compound emission in tundra shrubs—Dependence on species characteristics and the near-surface environment. Environ. Exp. Bot. 2021, 184, 104387. [Google Scholar] [CrossRef]
- Pikkarainen, L.; Nissinen, K.; Ghimire, R.; Kivimaenpaa, M.; Ikonen, V.; Kilpelainen, A.; Virjamo, V.; Yu, H.; Kirsikka-Aho, S.; Salminen, T.; et al. Responses in growth and emissions of biogenic volatile organic compounds in Scots pine, Norway spruce and silver birch seedlings to different warming treatments in a controlled field experiment. Sci. Total Environ. 2022, 821, 153277. [Google Scholar] [CrossRef] [PubMed]
- Kivimäenpää, M.; Riikonen, J.; Valolahti, H.; Elina, H.; Holopainen, J.; Holopainen, T. Effects of elevated ozone and warming on terpenoid emissions and concentrations of Norway spruce depend on needle phenology and age. Tree Physiol. 2022, 42, 1570–1586. [Google Scholar] [CrossRef]
- Rinnan, R.; Iversen, L.; Tang, J.; Vedel-Petersen, I.; Schollert, M.; Schurgers, G. Separating direct and indirect effects of rising temperatures on biogenic volatile emissions in the Arctic. Proc. Natl. Acad. Sci. USA 2020, 117, 32476–32483. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Z.; Kännaste, A.; Guo, M.; Zhou, G.; Niinemets, Ü. Isoprenoid and aromatic compound emissions in relation to leaf structure, plant growth form and species ecology in 45 East-Asian urban subtropical woody species. Urban For. Urban Green. 2020, 53, 126705. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Yu, X. Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ. Pollut. 2020, 259, 113955. [Google Scholar] [CrossRef]
- Lahr, E.; Schade, G.; Crossett, C.; Watson, M. Photosynthesis and isoprene emission from trees along an urban-rural gradient in Texas. Glob. Chang. Biol. 2015, 21, 4221–4236. [Google Scholar] [CrossRef] [PubMed]
- Douma, J.C.; Shipley, B. A multigroup extension to piecewise path analysis. Ecosphere 2021, 12, e03502. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, L.; Zuo, S.; Luo, Y.; Shao, G.; Hua, L.; Yang, Y. Geographical modeling of spatial interaction between human activity and forest connectivity in an urban landscape of southeast China. Landsc. Ecol. 2014, 29, 1741–1758. [Google Scholar] [CrossRef]
- Zhou, M.; He, D.; Qin, D.; You, W.; Wu, L.; Xiao, S. Impacts of land use change on vegetation coverage in Xiamen City from 1995 to 2015. J. For. Environ. 2017, 37, 440–445. [Google Scholar]
- Zhao, W.; Li, A.; Huang, Q.; Gao, Y.; Li, F.; Zhang, L. An improved method for assessing vegetation cooling service in regulating thermal environment: A case study in Xiamen, China. Ecol. Indic. 2019, 98, 531–542. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Zhang, H.; Song, W.; Wu, Z.; Wang, X. Design and characterization of a semi-open dynamic chamber for measuring biogenic volatile organic compound (BVOC) emissions from plants. Atmos. Meas. Tech. 2022, 15, 79–93. [Google Scholar] [CrossRef]
- Jing, X.; Lun, X.; Fan, C.; Ma, W. Emission patterns of biogenic volatile organic compounds from dominant forest species in Beijing, China. J. Environ. Sci. 2020, 95, 73–81. [Google Scholar] [CrossRef]
- Ortega, J.; Helmig, D.; Daly, R.W.; Tanner, D.M.; Guenther, A.B.; Herrick, J.D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques—Part B: Applications. Chemosphere 2008, 72, 365–380. [Google Scholar] [CrossRef]
- Ortega, J.; Helmig, D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques—Part A. Chemosphere 2008, 72, 343–364. [Google Scholar] [CrossRef]
- Karlsson, T.; Rinnan, R.; Holst, T. Variability of BVOC emissions from commercially used willow (Salix spp.) varieties. Atmosphere 2020, 11, 356. [Google Scholar] [CrossRef]
- Mermet, K.; Sauvage, S.; Dusanter, S.; Salameh, T.; Léonardis, T.; Flaud, P.-M.; Perraudin, É.; Villenave, É.; Locoge, N. Optimization of a gas chromatographic unit for measuring biogenic volatile organic compounds in ambient air. Atmos. Meas. Tech. 2019, 12, 6153–6171. [Google Scholar] [CrossRef]
- Guenther, A. Isoprene and Monoterpene Emission Rate Variability Model Evaluations and Sensitivity Analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Volf, M.; Volfova, T.; Seifert, C.L.; Ludwig, A.; Engelmann, R.; Jorge, L.R.; Richter, R.; Schedl, A.; Weinhold, A.; Wirth, C.; et al. A mosaic of induced and non-induced branches promotes variation in leaf traits, predation and insect herbivore assemblages in canopy trees. Ecol. Lett. 2021, 25, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Du, Y.; Du, J.; Liu, X.; Yuan, Z. Multiple-to-multiple path analysis model. PLoS ONE 2021, 16, e0247722. [Google Scholar] [CrossRef]
- Thomas, J.A.; Ditchman, N.; Beedle, R.B. The impact of knowledge, self-efficacy, and stigma on STI testing intention among college students. J. Am. Coll. Health 2020, 70, 1415–1425. [Google Scholar] [CrossRef]
- Alves, E.; Jardine, K.; Tota, J.; Jardine, A.; Yãnez-Serrano, A.; Karl, T.; Tavares, J.; Nelson, B.; Gu, D.; Stavrakou, T.; et al. Seasonality of isoprenoid emissions from a primary rainforest in central Amazonia. Atmos. Chem. Phys. 2016, 16, 3903–3925. [Google Scholar] [CrossRef]
- Ramirez-Gamboa, J.; Paton-Walsh, C.; Galbally, I.; Simmons, J.; Guerette, E.-A.; Griffith, A.; Chambers, S.; Williams, A. Seasonal variation of biogenic and anthropogenic VOCs in a semi-urban area near Sydney, Australia. Atmosphere 2020, 12, 47. [Google Scholar] [CrossRef]
- Liu, Y.; Schallhart, S.; Taipale, D.; Tykkä, T.; Räsänen, M.; Merbold, L.; Hellén, H.; Pellikka, P. Seasonal and diurnal variations in biogenic volatile organic compounds in highland and lowland ecosystems in southern Kenya. Atmos. Chem. Phys. 2021, 21, 14761–14787. [Google Scholar] [CrossRef]
- Helmig, D.; Daly, R.; Milford, J.; Guenther, A. Seasonal trends of biogenic terpene emissions. Chemosphere 2013, 93, 35–46. [Google Scholar] [CrossRef]
- Hong, Y.; Xu, X.; Liao, D.; Liu, T.; Ji, X.; Xu, K.; Liao, C.; Wang, T.; Lin, C.; Chen, J. Measurement report: Effects of anthropogenic emissions and environmental factors on the formation of biogenic secondary organic aerosol (BSOA) in a coastal city of southeastern China. Atmos. Chem. Phys. 2022, 22, 7827–7841. [Google Scholar] [CrossRef]
- Chen, G.; Liu, T.; Ji, X.; Xu, K.; Hong, Y.; Xu, L.; Li, M.; Fan, X.; Chen, Y.; Yang, C.; et al. Source Apportionment of VOCs and O3 Production Sensitivity at Coastal and Inland Sites of Southeast China. Aerosol Air Qual. Res. 2022, 22, 220289. [Google Scholar] [CrossRef]
- Tani, A.; Mochizuki, T. Review: Exchanges of volatile organic compounds between terrestrial ecosystems and the atmosphere. J. Agric. Meteorol. 2021, 77, 66–80. [Google Scholar] [CrossRef]
- Jason, Q.; Demi, G.; John, H.; Ian, E. Monoterpene synthases responsible for the terpene profile of anther glands in Eucalyptus polybractea R.T. Baker (Myrtaceae). Tree Physiol. 2020, 11, 849–864. [Google Scholar]
- Vanhatalo, A.; Ghirardo, A.; Juurola, E.; Schnitzler, J.-P.; Zimmer, I.; Hellén, H.; Hakola, H.; Bäck, J. Long-term dynamics of monoterpene synthase activities, monoterpene storage pools and emissions in boreal Scots pine. Biogeosciences 2018, 15, 5047–5060. [Google Scholar] [CrossRef]
- He, C.; Murray, F.; Tom, L. Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos. Environ. 2000, 34, 645–655. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Wang, Z.; Zhang, S. Studies on the emission rates of plants VOCs in China. Environ. Sci. 2004, 24, 654–657. [Google Scholar]
- Huang, A.; Li, N.; Guenther, A.; Greenberg, J.; Baker, B.; Graessli, M.; Bai, J. Investigation on emission properties of biogenic VOCs of landscape plants in Shenzhen. Environ. Sci. 2011, 32, 3555–3559. [Google Scholar]
- Yang, D.; Bai, Y.; Li, J.; Pan, N.; Yu, K.; Tang, L.; Peng, L.; Su, X. Study on hydrocarbon compounds from natural source in the Pearl River Delta area. Environ. Sci. 2001, 21, 422–426. [Google Scholar]
- Alves, E.; Harley, P.; Goncalves, J.; Moura, C.; Jardine, K. Effects of light and temperature on isoprene emission at different leaf developmental stages of Eschweilera coriacea in central Amazon. Acta Amaz. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- Bison, J.; Cardoso-Gustavson, P.; De Moraes, R.; Da Silva Pedrosa, G.; Cruz, L.; Freschi, L.; De Souza, S. Volatile organic compounds and nitric oxide as responses of a Brazilian tropical species to ozone: The emission profile of young and mature leaves. Environ. Sci. Pollut. Res. Int. 2018, 25, 3840–3848. [Google Scholar] [CrossRef] [PubMed]
- Mozaffar, A.; Schoon, N.; Bachy, A.; Digrado, A.; Heinesch, B.; Aubinet, M.; Fauconnier, M.; Delaplace, P.; Du Jardin, P.; Amelynck, C. Biogenic volatile organic compound emissions from senescent maize leaves and a comparison with other leaf developmental stages. Atmos. Environ. 2018, 176, 71–81. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhou, Y.; Zeng, L.; Dong, F.; Mei, X.; Liao, Y.; Watanabe, N.; Yang, Z. Analytical method for metabolites involved in biosynthesis of plant volatile compounds. RSC Adv. 2017, 7, 19363–19372. [Google Scholar] [CrossRef]
- Widhalm, J.; Jaini, R.; Morgan, J.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Xiong, F.; Sun, N.; Li, Y.; Liu, C.; Yin, S. Photosynthesis and related physiological parameters differences affected the isoprene emission rate among 10 typical tree species in subtropical metropolises. Int. J. Environ. Res. Public Health 2021, 18, 954. [Google Scholar] [CrossRef]
- Penuelas, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Guidolotti, G.; Rey, A.; Medori, M.; Calfapietra, C. Isoprenoids emission in Stipa tenacissima L.: Photosynthetic control and the effect of UV light. Environ. Pollut. 2016, 208, 336–344. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Veromann Jürgenson, L.L.; Niinemets, Ü. Gall- and erineum-forming Eriophyes mites alter photosynthesis and volatile emissions in an infection severity-dependent manner in broad-leaved trees Alnus glutinosa and Tilia cordata. Tree Physiol. 2021, 41, 1122–1142. [Google Scholar] [CrossRef]
- Oku, H.; Iwai, S.; Uehara, M.; Iqbal, A.; Mutanda, I.; Inafuku, M. Growth condition controls on G93 parameters of isoprene emission from tropical trees. J. Plant Res. 2021, 134, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Sellers, P.; Berry, J.; Collatz, G.; Field, C.; Hall, F. Canopy reflectance, photosynthesis, and transpiration. III. a reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens. Environ. 1992, 42, 187–216. [Google Scholar] [CrossRef]
- Zhang, N.; Su, X.; Zhang, X.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Monitoring daily variation of leaf layer photosynthesis in rice using UAV-based multi-spectral imagery and a light response curve model. Agric. For. Meterol. 2020, 291, 108098. [Google Scholar] [CrossRef]
- Kanazawa, A.; Chattopadhyay, A.; Kuhlgert, S.; Tuitupou, H.; Maiti, T.; Kramer, D. Light potentials of photosynthetic energy storage in the field: What limits the ability to use or dissipate rapidly increased light energy? R. Soc. Open Sci. 2021, 8, 211102. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Chen, J.; Bi, H.; Yu, X.; Fu, Y.; Liao, W. Influence of physiological and environmental factors on the diurnal variation in emissions of biogenic volatile compounds from Pinus tabuliformis. J. Environ. Sci. 2019, 81, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Terry, N. Limiting factors in photosynthesis. Plant Physiol. 1984, 75, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.; Cheesman, A. The benefits of recycling how photosynthetic bark can increase drought tolerance. New Phytol. 2015, 208, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Slot, M.; Winter, K. In situ temperature relationships of biochemical and stomatal controls of photosynthesis in four lowland tropical tree species. Plant Cell Environ. 2017, 40, 3055–3068. [Google Scholar] [CrossRef]
- Lerdau, M.; Guenther, A.; Monson, R. Plant production and emission of volatile organic compounds. BioScience 1997, 47, 373–383. [Google Scholar] [CrossRef]
- Sulaiman, H.; Liu, B.; Kaurilind, E.; Niinemets, Ü. Phloem-feeding insect infestation antagonizes volatile organic compound emissions and enhances heat stress recovery of photosynthesis in Origanum vulgare. Environ. Exp. Bot. 2021, 189, 104551. [Google Scholar] [CrossRef]
- Guidolotti, G.; Pallozzi, E.; Gavrichkova, O.; Scartazza, A.; Mattioni, M.; Loreto, F.; Calfapietra, C. Emission of constitutive isoprene, induced monoterpenes, and other volatiles under high temperatures in Eucalyptus camaldulensis: A (13) C labelling study. Plant Cell Environ. 2019, 42, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Fasbender, L.; Romek, K.; Yáñez-Serrano, A.; Kreuzwieser, J. Heat waves change plant carbon allocation among primary and secondary metabolism altering CO2 assimilation, respiration, and VOC emissions. Front. Plant Sci. 2020, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.; Schnitzler, J.; Vlot, A. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef]
- Tiiva, P.; Tang, J.; Michelsen, A.; Rinnan, R. Monoterpene emissions in response to long-term night-time warming, elevated CO2 and extended summer drought in a temperate heath ecosystem. Sci. Total Environ. 2017, 580, 1056–1067. [Google Scholar] [CrossRef]
- Barbieri, S.; Elustondo, M.; Urbicain, M. Retention of aroma compounds in basil dried with low pressure superheated steam. J. Food Eng. 2004, 65, 109–115. [Google Scholar] [CrossRef]
- Babu, K.; Singh, B.; Joshi, V.; Singh, V. Essential oil composition of Damask rose (Rosa damascena Mill.) distilled under different pressures and temperatures. Flavour Fragr. J. 2002, 17, 136–140. [Google Scholar] [CrossRef]
- Widegren, J.; Bruno, T. Vapor pressure measurements on low-volatility terpenoid compounds by the concatenated gas saturation method. Environ. Sci. Technol. 2010, 44, 388–393. [Google Scholar] [CrossRef]
- Edelmann, H.; Neinhuis, C.; Bargel, H. Influence of hydration and temperature on the rheological properties of plant cuticles and their impact on plant organ integrity. J. Plant Growth Regul. 2005, 24, 116–126. [Google Scholar] [CrossRef]
- Ma, F.; Barrett-Lennard, E.; Tian, C. Changes in cell size and tissue hydration (‘succulence’) cause curvilinear growth responses to salinity and watering treatments in euhalophytes. Environ. Exp. Bot. 2019, 159, 87–94. [Google Scholar] [CrossRef]
- Duan, C.; Zuo, S.; Wu, Z.; Qiu, Y.; Wang, J.; Lei, Y.; Liao, H.; Ren, Y. A review of research hotspots and trends in biogenic volatile organic compounds (BVOCs) emissions combining bibliometrics with evolution tree methods. Environ. Res. Lett. 2020, 16, 013003. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Meischner, M.; Grun, M.; Yanez-Serrano, A.; Fasbender, L.; Werner, C. Drought affects carbon partitioning into volatile organic compound biosynthesis in Scots pine needles. New Phytol. 2021, 232, 17736. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, M.; Llusià, J.; Filella, I.; Niinemets, Ü.; Arneth, A.; Wright, I.; Loreto, F.; Peñuelas, J. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 2018, 220, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Thompson, K.; Hodgson, J. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Ibrahim, M.; Maenpaa, M.; Hassinen, V.; Kontunen-Soppela, S.; Malec, L.; Rousi, M.; Pietikainen, L.; Tervahauta, A.; Karenlampi, S.; Holopainen, J.; et al. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J. Exp. Bot. 2010, 61, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Vescio, R.; Abenavoli, M.; Araniti, F.; Musarella, C.; Sofo, A.; Laface, V.; Spampinato, G.; Sorgonà, A. The assessment and the within-plant variation of the morpho-physiological traits and VOCs profile in endemic and rare salvia ceratophylloides Ard. (Lamiaceae). Plants 2021, 10, 474. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Hauff, K.; Bertin, N.; Tenhunen, J.; Steinbrecher, R.; Seufert, G. Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. New Phytol. 2001, 153, 243–356. [Google Scholar] [CrossRef]
- Liao, P.; Ray, S.; Boachon, B.; Lynch, J.; Deshpande, A.; McAdam, S.; Morgan, J.; Dudareva, N. Cuticle thickness affects dynamics of volatile emission from petunia flowers. Nat. Chem. Biol. 2021, 17, 138–145. [Google Scholar] [CrossRef]
- Geron, C.; Arnts, R. Seasonal monoterpene and sesquiterpene emissions from Pinus taeda and Pinus virginiana. Atmos. Environ. 2010, 44, 4240–4251. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Zuo, Z. Seasonal emission of monoterpenes from four chemotypes of Cinnamomum camphora. Ind. Crops Prod. 2021, 163, 113327. [Google Scholar] [CrossRef]
- Kuhn, U.; Rottenberger, S.; Biesenthal, T.; Wolf, A.; Schebeske, G.; Ciccioli, P.; Brancaleoni, E.; Frattoni, M.; Tavares, T.; Kesselmeier, J. Seasonal differences in isoprene and light-dependent monoterpene emission by Amazonian tree species. Glob. Change Biol. 2004, 10, 663–682. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Seufert, G.; Steinbrecher, R.; Tenhunen, J. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol. 2001, 153, 257–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).