Phase-Change-Material-Impregnated Wood for Potential Energy-Saving Building Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Impregnation of Wood Samples with PCM (PCMW)
2.3. Characterization of PCMs and PCMW
2.4. Leaching and Decay Test
2.5. Water Absorption Test
3. Results and Discussion
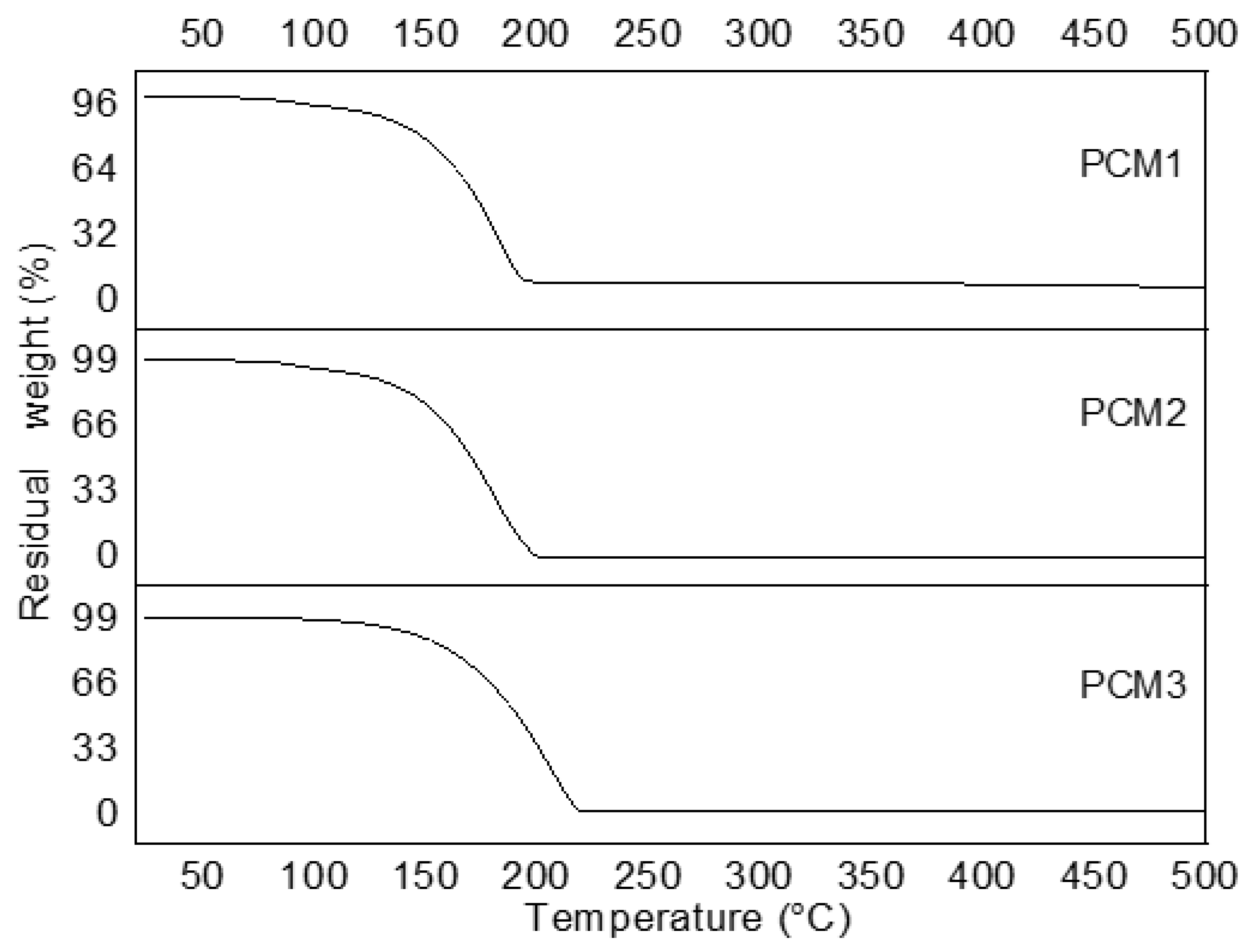
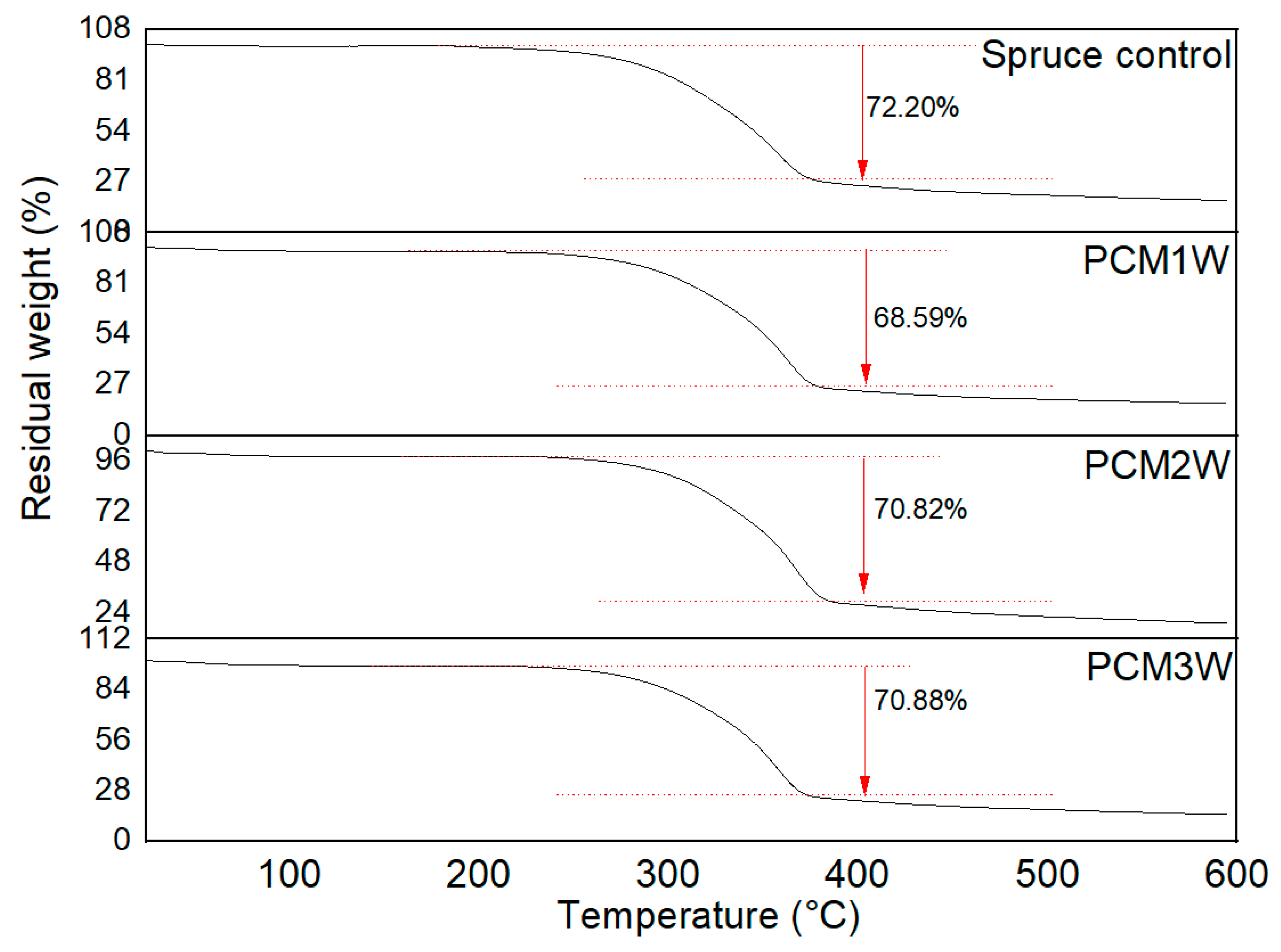
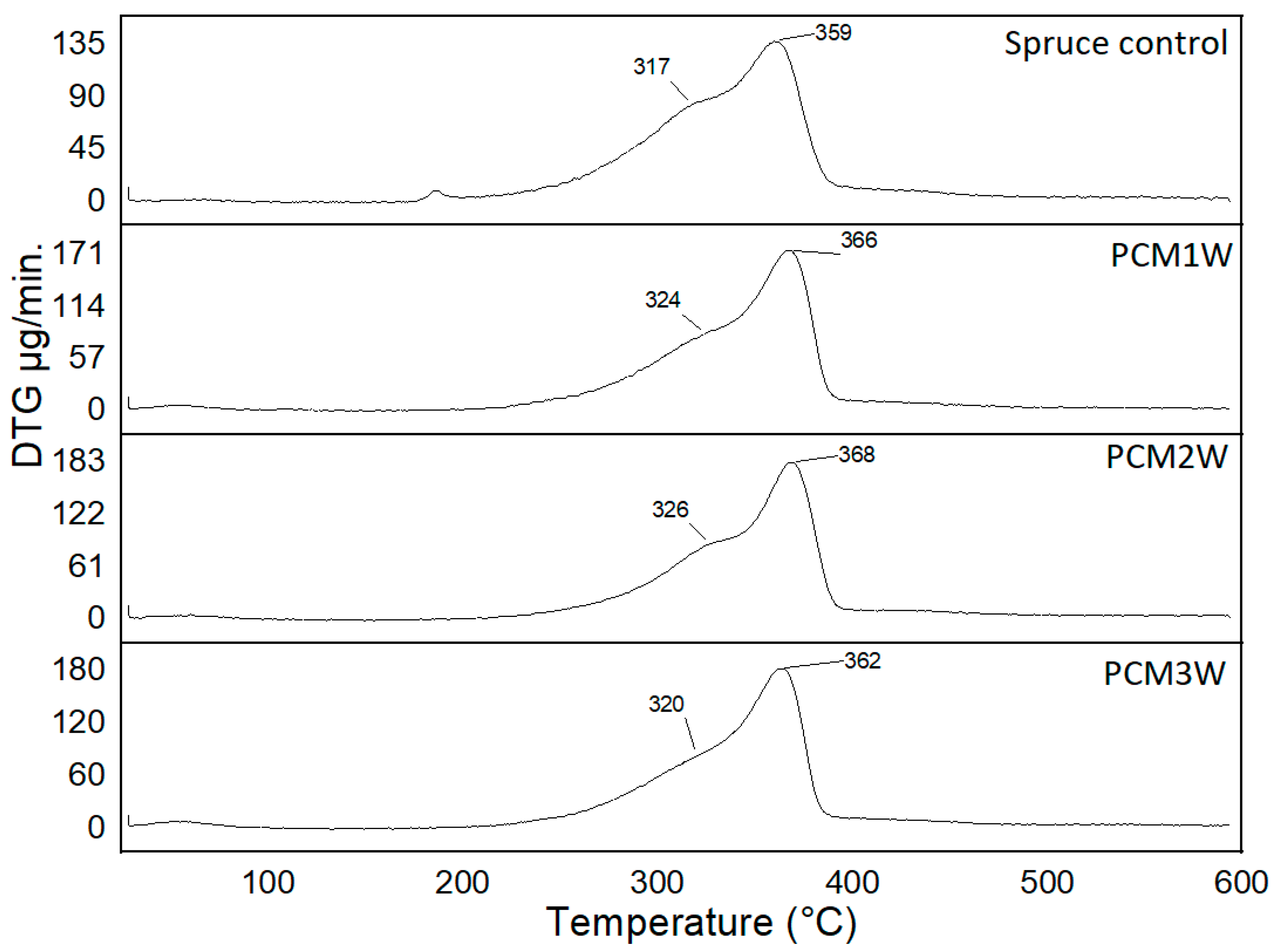
3.1. Thermal Stability Analysis of PCMs-Impregnated Woods
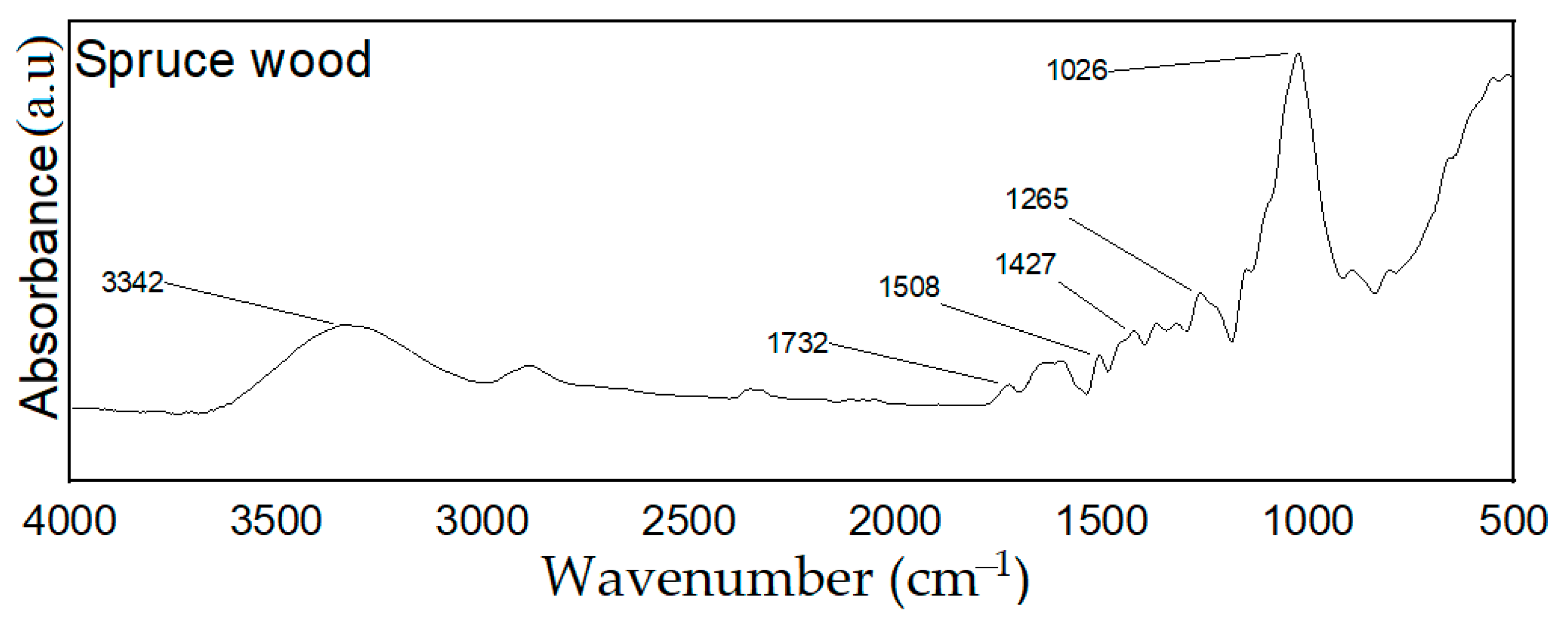
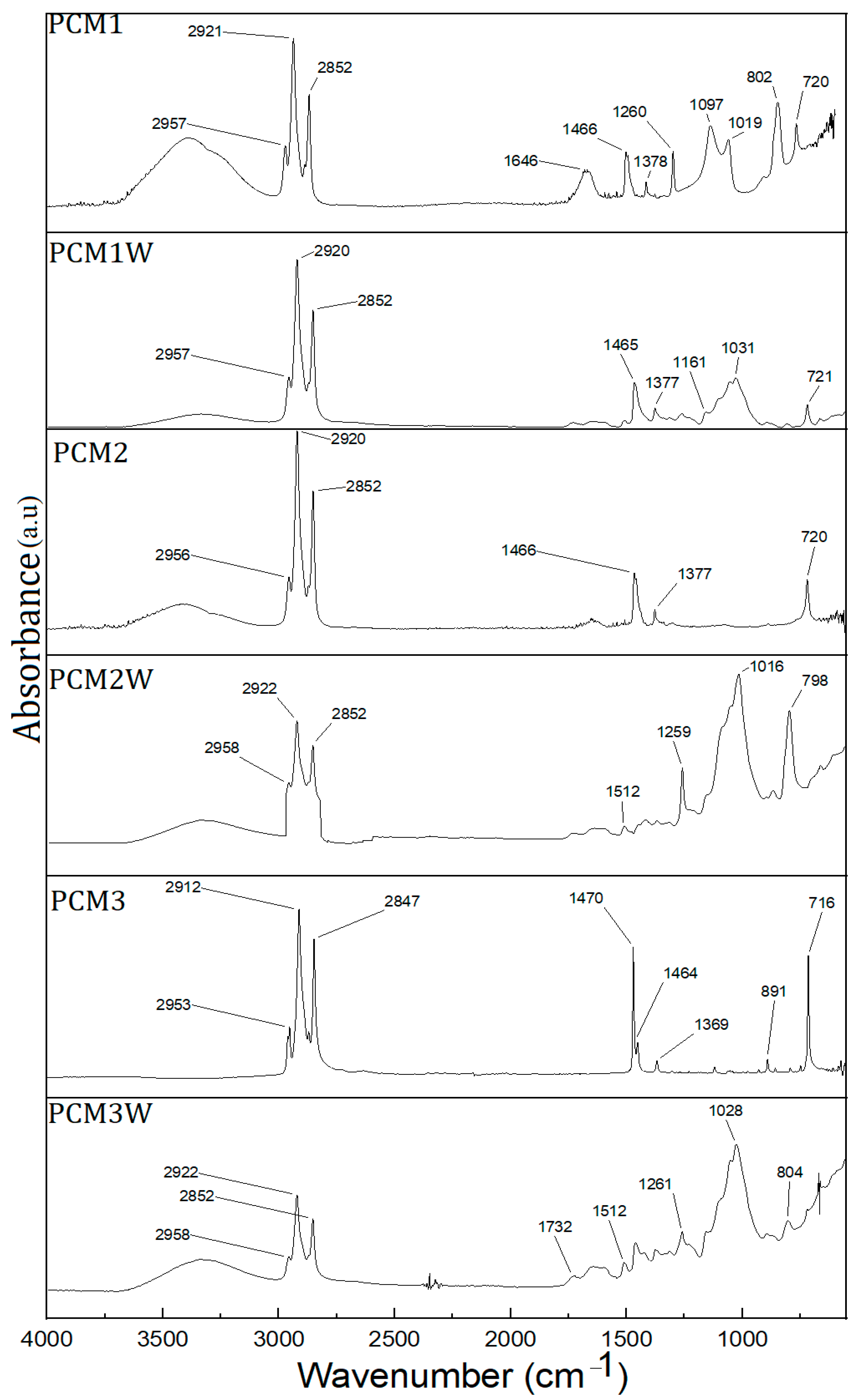
3.2. FT-IR Analysis of PCMs-Impregnated Wood Samples
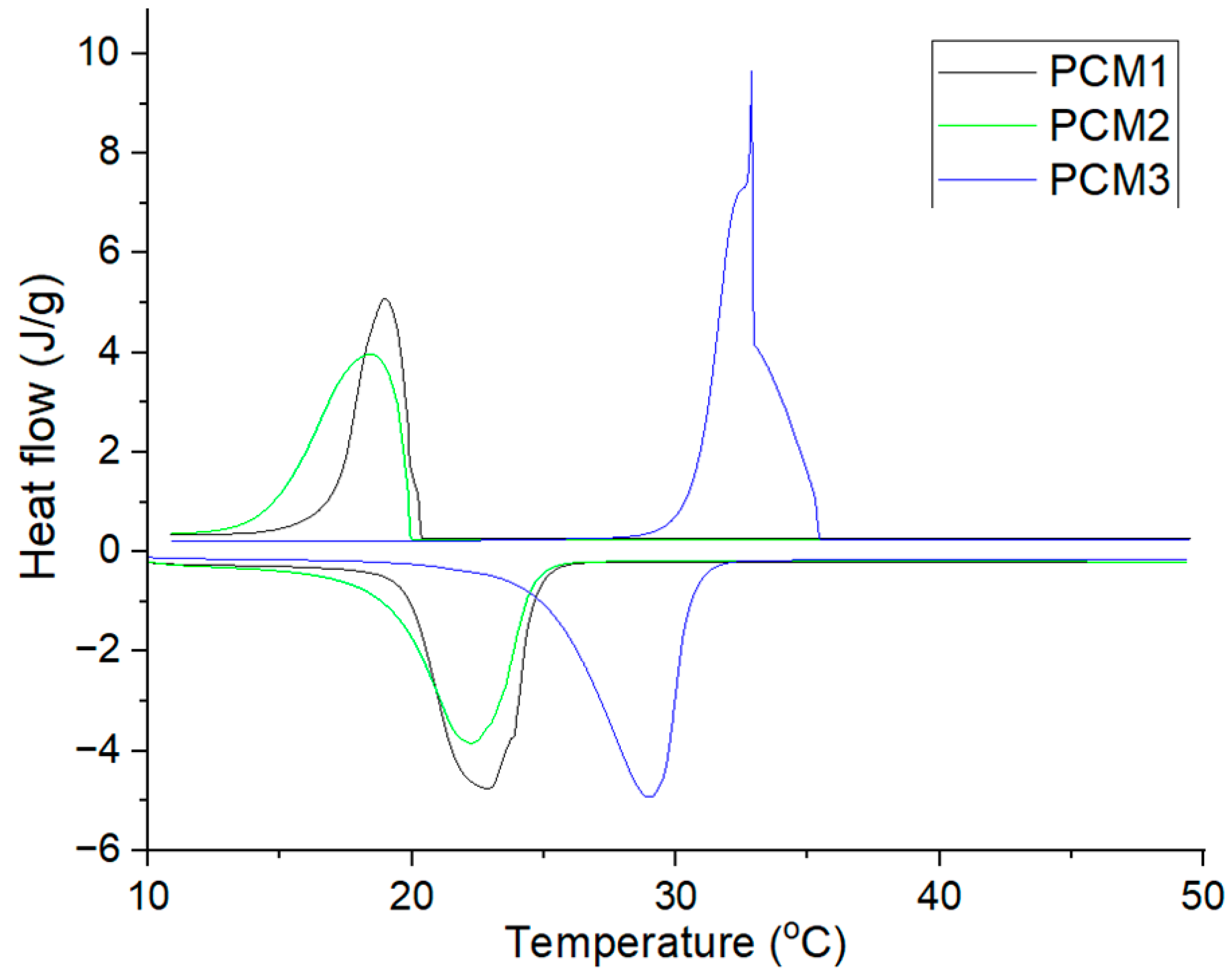
3.3. Thermal Properties Analysis of PCMs-Impregnated Woods
3.4. Mass Loss of PCMs-Impregnated Woods
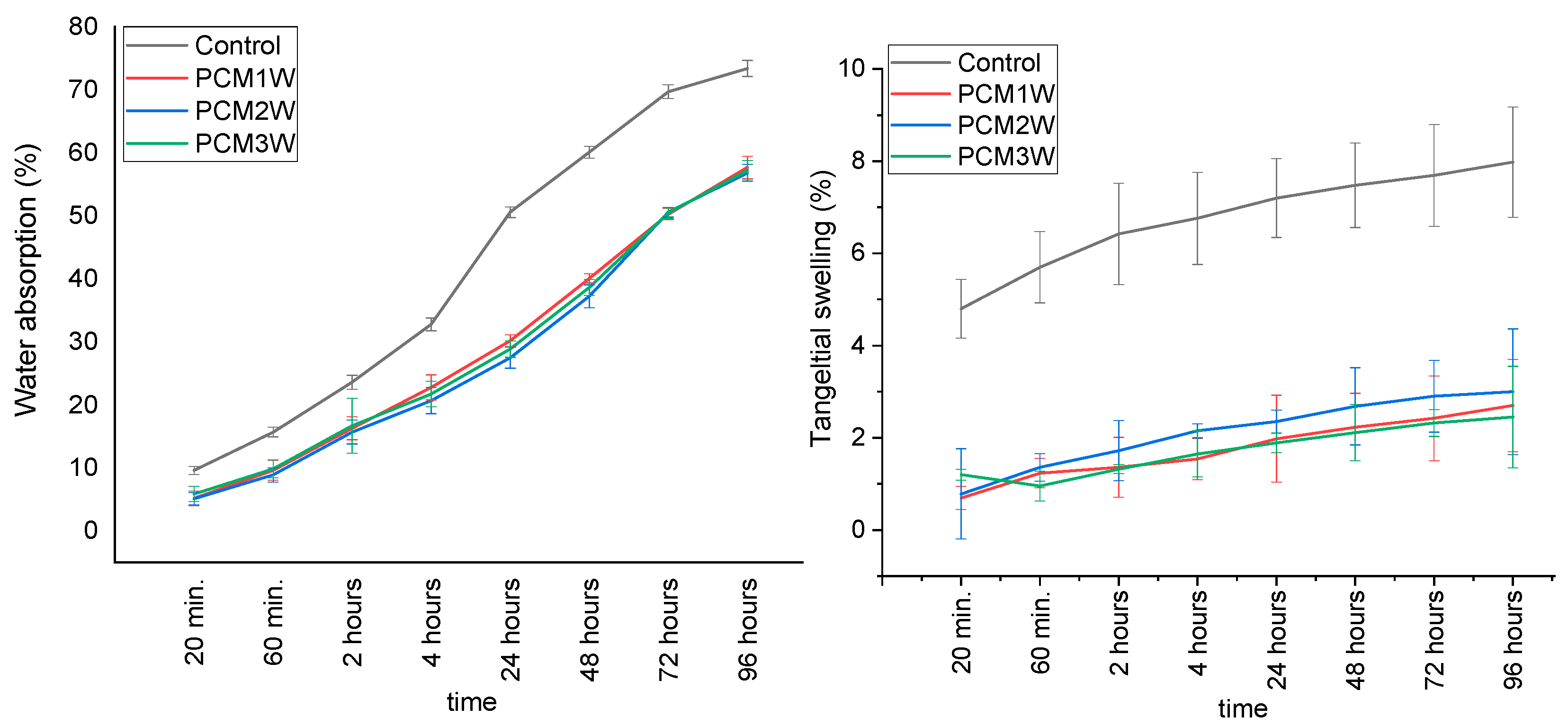
3.5. Hygroscopic Properties of PCMs-Impregnated Woods
4. Conclusions
- The DSC results revealed melting enthalpy values of up to 41.63 J/g to PCM1, which are beneficial for latent heat energy storage.
- The fungal test results showed that wood samples impregnated with PCMs showed resistance to fungi, specifying that it is safe to treat wood with PCMs.
- Spruce wood samples impregnated with PCMs have been shown to have potential as thermal regulation building materials. PCMs are capable of absorbing and releasing heat energy as they change phase (i.e., solid to liquid and vice versa), which can help to minimize temperature fluctuations in a building, thereby reducing energy consumption for heating and cooling. This is particularly useful in reducing temperature changes during the day and night.
- Potential future plans for PCMW include the following:
- The integration of PCMW into building materials, such as wall insulation and roof tiles, for passive thermal energy storage.
- Investigation of the potential for using PCMW in combination with thermal management systems, such as heat pumps and refrigeration systems, to improve overall energy efficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X.; Dai, X.; Liu, J. Building energy-consumption status worldwide and the state-of-the-art technologies for zero-energy buildings during the past decade. Energy Build. 2016, 128, 198–213. [Google Scholar] [CrossRef]
- Qiu, F.; Song, S.; Li, D.; Liu, Y.; Wang, Y.; Dong, L. Experimental investigation on improvement of latent heat and thermal conductivity of shape-stable phase-change materials using modified fly ash. J. Clean. Prod. 2020, 246, 118952. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and numerical analysis of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage. J. Energy Storage 2002, 56, 105975. [Google Scholar] [CrossRef]
- Pekdogan, T.; Tokuç, A.; Ezan, M.A.; Başaran, T. Experimental investigation on heat transfer and air flow behavior of latent heat storage unit in a facade integrated ventilation system. J. Energy Storage 2021, 44, 103367. [Google Scholar] [CrossRef]
- Ben Khedher, N. Numerical Study of the Thermal Behavior of a Composite Phase Change Material (PCM) Room. Eng. Technol. Appl. Sci. Res. 2018, 8, 2663–2667. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagière, P. Impregnation of wood with microencapsulated bio-based phase change materials for high thermal mass engineered wood flooring. Appl. Sci. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Montanari, C.; Li, Y.; Chen, H.; Yan, M.; Berglund, L.A. Transparent wood for thermal energy storage and reversible optical transmittance. ACS Appl. Mater. Interfaces 2019, 11, 20465–20472. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Liu, L.; Lu, Z.; Sanjayan, J.G.; Duan, W.H. Development of granular expanded perlite/paraffin phase change material composites and prevention of leakage. Sol. Energy 2016, 137, 179–188. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, W.; Yang, Y.; Zhao, J.; Liu, Y.; Guo, H. Transparent wood with phase change heat storage as novel green energy storage composites for building energy conservation. J. Clean. Prod. 2021, 296, 126598. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, L.-X.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.; Youngblood, J.P. Synthesis and Characterization of Microencapsulated Phase Change Materials with Poly(urea urethane) Shells Containing Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on para_n core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing di_erent soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar]
- Kaygusuz, K.; Alkan, C.; Sarı, A.; Uzun, O. Encapsulated Fatty Acids in an Acrylic Resin as Shape-stabilized Phase Change Materials for Latent Heat Thermal Energy Storage. Energy Sources Part. A Recover. Util. Environ. E 2008, 30, 1050–1059. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiu, J.; Tang, B.; Lu, R.; Zhang, S.-F. Novel semi-interpenetrating network structural phase change composites with high phase change enthalpy. AIChE J. 2017, 64, 688–696. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A.S. Phase change materials based on low-density polyethylene/paraffin wax blends. Eur. Polym. J. 2007, 43, 4695–4705. [Google Scholar] [CrossRef]
- Sánchez, L.; Lacasa, E.; Carmona, M.; Rodríguez, J.; Sánchez, P.; Silva, M.L.S.; Rodríguez, J. Applying an Experimental Design to Improve the Characteristics of Microcapsules Containing Phase Change Materials for Fabric Uses. Ind. Eng. Chem. Res. 2008, 47, 9783–9790. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on para_n/high density polyethylene composites. Renew. Energy 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Tian, C.; Wang, J. Preparation and characterization of form-stable para_n/polyurethane composites as phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 77, 13–21. [Google Scholar]
- Silakhori, M.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Baradaran, S.; Naghavi, M.S. Palmitic acid/polypyrrole composites as form-stable phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 80, 491–497. [Google Scholar] [CrossRef]
- Sari, A.; Akcay, M.; Soylak, M. Polymer–stearic acid blends aform formrm-stablese change material for thermal energy storage. Energy Sources 2005, 27, 1535–1546. [Google Scholar]
- Sari, A.; Kaygusuz, K. Poly (vinyl alcohol)/fatty acid blends for thermal energy storage. Energy Sources 2007, 29, 873–883. [Google Scholar] [CrossRef]
- Li, Z.; He, W.; Xu, J.; Jiang, M. Preparation and characterization of in situ grafted/crosslinked polyethylene glycol/polyvinyl alcohol composite thermal regulating fiber. Sol. Energy Mater. Sol. Cells 2015, 140, 193–201. [Google Scholar] [CrossRef]
- Sentürk, S.B.; Kahraman, D.; Alkan, C.; Gokce, I. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Cheng, L.; Feng, J. Form-stable phase change materials based on delignified wood flour for thermal management of buildings. Compos. Part A 2020, 129, 105690. [Google Scholar] [CrossRef]
- Liang, J.; Zhimeng, L.; Ye, Y.; Yanjun, W.; Jingxin, L.; Changlin, Z. Fabrication and characterization of fatty acid/wood-flour composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2018, 171, 88–99. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Ou, R.; Sun, L.; Wang, Q.; Li, L. Preparation and characterization of modified porous wood flour/lauric-myristic acid eutectic mixture as a form-stable phase change material. Energy Fuel. 2018, 32, 5453–5461. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Barreneche, C.; Vecstaudza, J.; Bajare, D.; Fernandez, A. PCM/wood composite to store thermal energy in passive building envelopes. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2017, 251, 012111. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Cao, J.; Peng, Y. Paraffin/wood flour/high-density polyethylene composites for thermal energy storage material in buildings: A morphology, thermal performance, and mechanical property study. Polym Compos. 2018, 39, E1643–E1652. [Google Scholar] [CrossRef]
- Temiz, A.; Hekimoğlu, G.; Köse Demirel, G.; Sarı, A.; Mohamad Amini, M.H. Phase change material impregnated wood for passive thermal management of timber buildings. Int. J. Energy Res. 2020, 44, 10495–10505. [Google Scholar] [CrossRef]
- EN 84; Wood Preservatives—Accelerated Ageing of Treated Wood Prior to Biological Testing—Leaching Procedure. European Committee for Standardization: Brussels, Belgium, 1997.
- EN 113-2; Durability of Wood and Wood-Based Products—Test Method against Wood Destroying Basidiomycetes—Part 2: Assessment of Inherent or Enhanced Durability. European Committee for Standardization: Brussels, Belgium, 2020.
- Barzegar, R.; Yozgatligil, A.; Olgun, H.; Atimtay, A.T. TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J. Energy Inst. 2020, 93, 889–898. [Google Scholar] [CrossRef]
- Amini, M.H.M.; Temiz, A.; Hekimoğlu, G.; Demirel, G.K.; Sarı, A. Properties of Scots pine wood impregnated with capric acid for potential energy saving building material. Holzforschung 2022, 76, 744–753. [Google Scholar] [CrossRef]
- Archer, K.; Leebow, S. Primary Wood Processing: Principles and Practice; Chapter 9—J.C.F. Walker, Wood Preservation; Springer: Dordrecht, The Netherlands, 2006; pp. 297–338. ISBN 978-1-4020-4393-2. [Google Scholar]
- Lesar, B.; Humar, M. Use of wax emulsion for improvement of wood durability and sorption properties. Eur. J. Wood Wood Prod. 2011, 69, 231–238. [Google Scholar] [CrossRef]
- Reinprecht, L.; Repák, M. The impact of paraffin-thermal modification of beech wood on its biological, physical and mechanical properties. Forests 2019, 10, 1102. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, H.; Ma, E.; Liu, R. Resistance to fungal decay of paraffin wax emulsion/copper azole compound system treated wood. Int. Biodeterior. Biodegrad. 2018, 129, 61–66. [Google Scholar] [CrossRef]
- Humar, M.; Krzisnik, D.; Lesar, B.; Thaler, N.; Ugovsek, A.; Zupancic, K.; Zlahtic, M. Thermal modification of wax-impregnated wood to enhance its physical, mechanical, and biological properties. Holzforschung 2016, 70, 411–419. [Google Scholar] [CrossRef]
- Chau, T.; Ma, E.; Yang, T.; Cao, J. Moisture Sorption and Hygroexpansion of Paraffin Wax Emulsion–Treated Southern Pine (Pinus spp.) under Dynamic Conditions. For. Prod. J. 2017, 67, 463–470. [Google Scholar] [CrossRef]


| Product Name | Normal Paraffin n-C14 |
|---|---|
| Product properties | Liquid |
| Appearance | Transparent |
| Enthalpy | 180–200 J/kg |
| Specific heat | 3.22 kj/kgK |
| Coefficient of thermal conductivity | 0.21 Wm/K−1 |
| Color | Colorless |
| Melting point (PCM1, PCM2, PCM3) | 20 °C, 25 °C, 30 °C |
| Density | 0.77 g/cm3 |
| Solubility | Water Insoluble (0.09 ug/L, 25 °C) Soluble in: ether, alcohol, acetone |
| Chemical stability | Generally stable |
| Degradation Interval (°C) | % Mass Loss | |
|---|---|---|
| PCM1 | 95–224 | 100 |
| PCM2 | 95–203 | 100 |
| PCM3 | 128–201 | 100 |
| Spruce wood | 180–400 | 72.20 |
| PCM1W | 200–390 | 68.59 |
| PCM2W | 200–390 | 70.82 |
| PCM3W | 200–390 | 70.88 |
| Samples | Melting | Solidifying | ||||
|---|---|---|---|---|---|---|
| Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | |
| PCM1 | 20.63 | 22.07 | 168.71 | 20.27 | 19.27 | 179.09 |
| PCM1W | 19.19 | 21.49 | 40.34 | 19.65 | 18.41 | 41.63 |
| PCM2 | 19.72 | 21.77 | 158.97 | 19.92 | 18.43 | 170.16 |
| PCM2W | - | - | - | - | - | - |
| PCM3 | 25.75 | 28.30 | 211.26 | 25.09 | 24.07 | 210.15 |
| PCM3W | 24.77 | 26.63 | 30.33 | 25.04 | 24.18 | 19.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can, A.; Lee, S.H.; Antov, P.; Abd Ghani, M.A. Phase-Change-Material-Impregnated Wood for Potential Energy-Saving Building Materials. Forests 2023, 14, 514. https://doi.org/10.3390/f14030514
Can A, Lee SH, Antov P, Abd Ghani MA. Phase-Change-Material-Impregnated Wood for Potential Energy-Saving Building Materials. Forests. 2023; 14(3):514. https://doi.org/10.3390/f14030514
Chicago/Turabian StyleCan, Ahmet, Seng Hua Lee, Petar Antov, and Muhammad Aizat Abd Ghani. 2023. "Phase-Change-Material-Impregnated Wood for Potential Energy-Saving Building Materials" Forests 14, no. 3: 514. https://doi.org/10.3390/f14030514
APA StyleCan, A., Lee, S. H., Antov, P., & Abd Ghani, M. A. (2023). Phase-Change-Material-Impregnated Wood for Potential Energy-Saving Building Materials. Forests, 14(3), 514. https://doi.org/10.3390/f14030514










