Somatic Embryogenesis Induction and Genetic Stability Assessment of Plants Regenerated from Immature Seeds of Akebia trifoliate (Thunb.) Koidz
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Explant Disinfection
2.2. Explant Inoculation and SE Initiation
2.3. Proliferation and Maturation of SEs
2.4. Whole Plant Regeneration
2.5. Acclimatization and Transplantation
2.6. Evaluation of Leaf Morphological Characteristics
2.7. Nuclei Isolation and Relative Nuclear DNA Content Analysis
2.8. Measurements of Stomatal and Gas Exchange Parameters
2.9. Genomic DNA Isolation and SSR Analysis
2.10. Statistical Analysis
3. Results
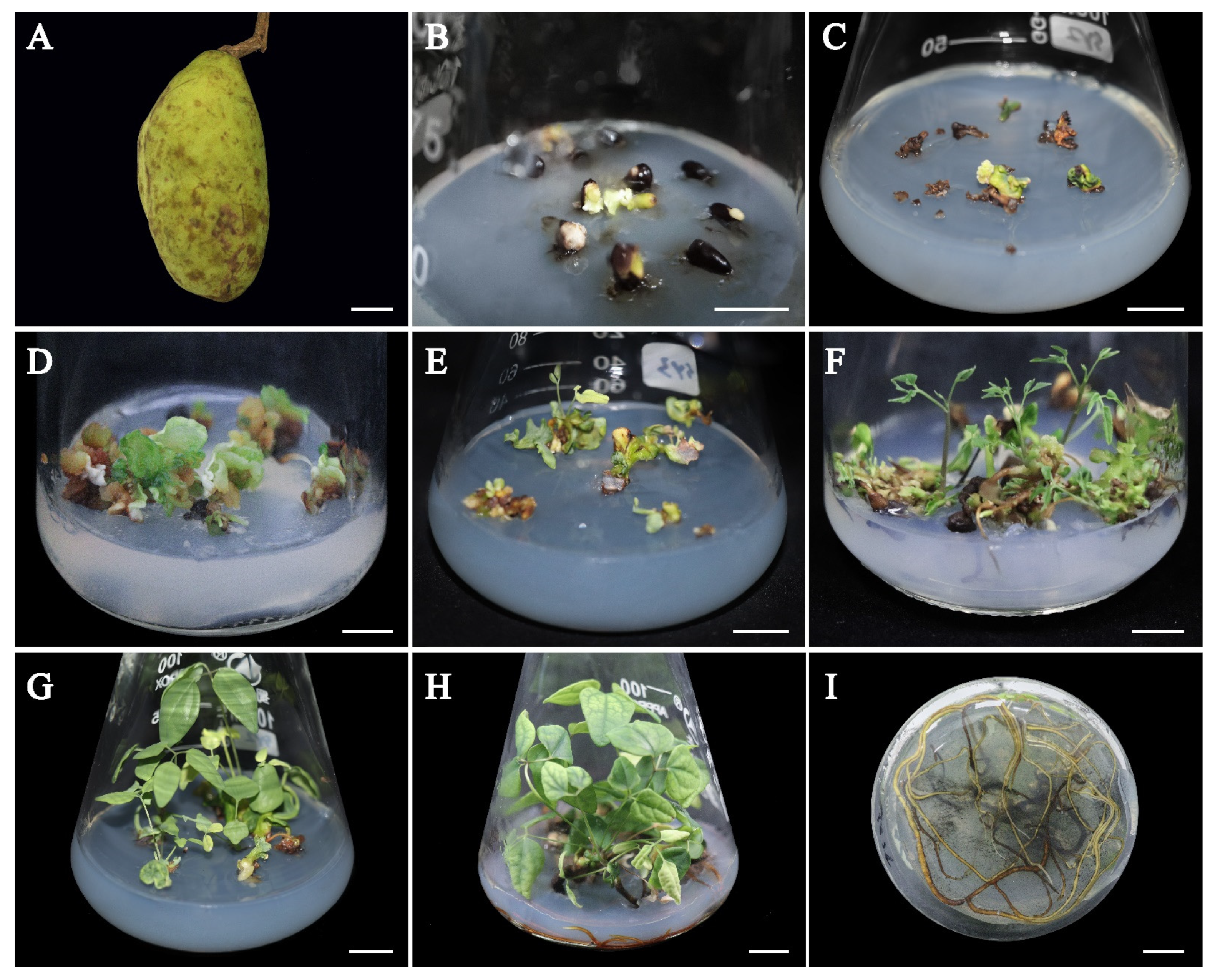
3.1. Initiation of SEs from Immature Seeds
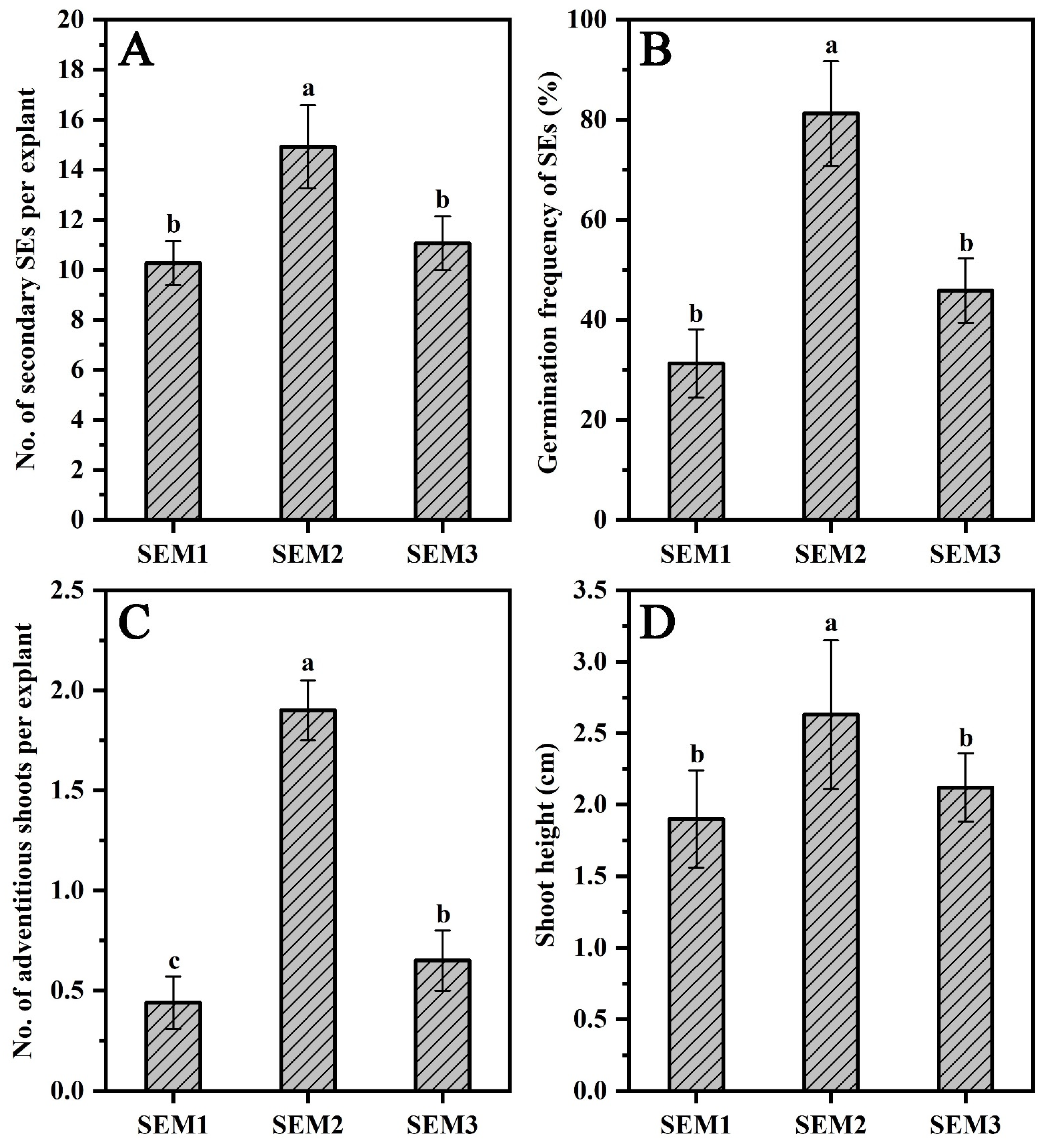
3.2. Proliferation and Maturation of SEs
3.3. In Vitro Rooting
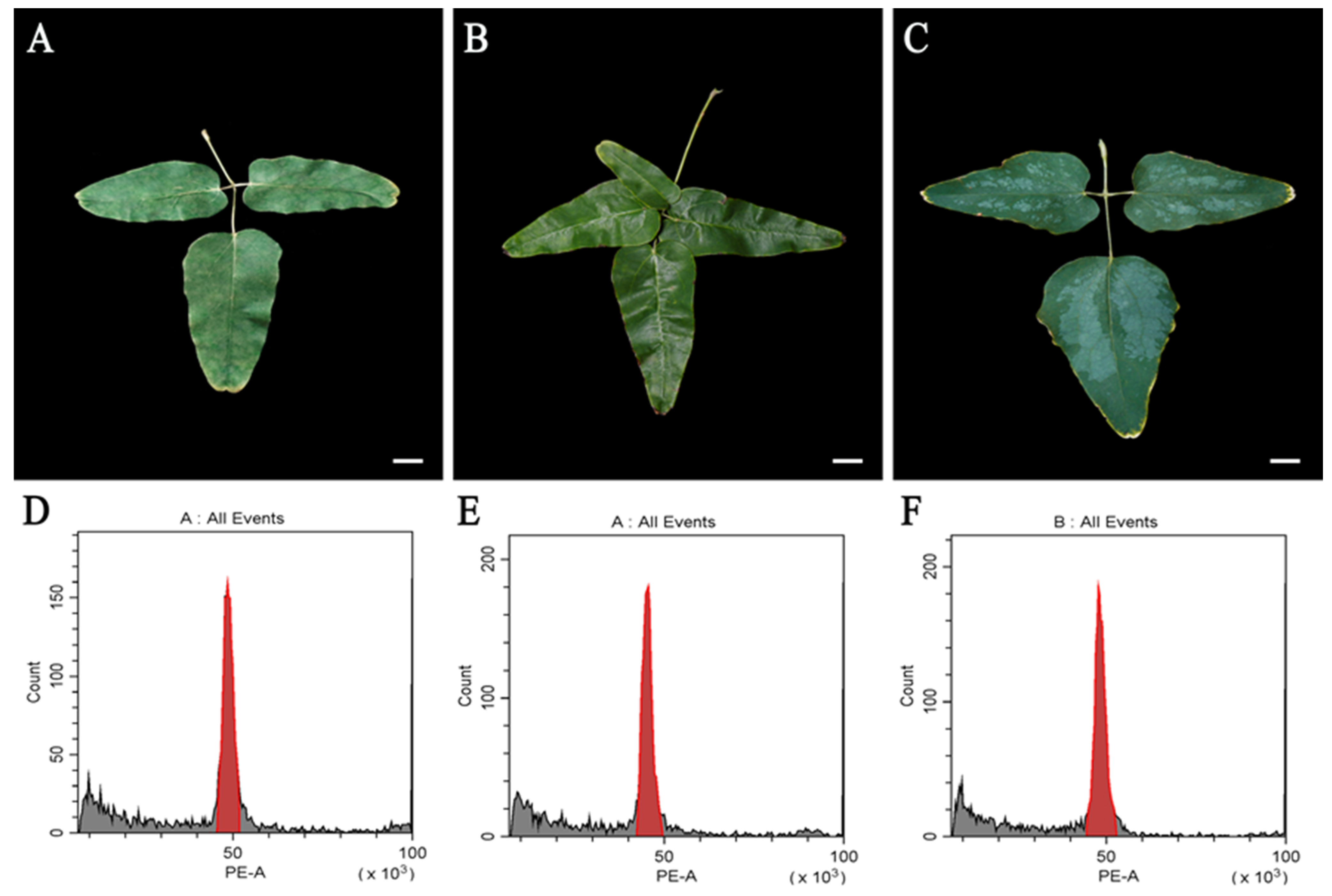
3.4. Plant Establishment and Genetic Stability Analysis Based on Leaf Morphological Characteristics
3.5. Main Morphological Variation Types and Flow Cytometry, Stomatal, and Gas Exchange Analysis
3.6. SSR Analysis of the Wild Parent and the in Vitro Regenerants
4. Discussion
4.1. Factors Affecting SE Induction
4.2. Pathways of Plant Regeneration via Somatic Embryogenesis
4.3. SE Maturation and Plant Regeneration
4.4. Genetic Stability Analysis of the Regenerated Plants from the Immature Seeds via Somatic Embryogenesis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, S.; Yao, X.; Zhong, C.; Zhao, T.; Huang, H. Effectiveness of recurrent selection in Akebia trifoliata (Lardizabalaceae) breeding. Sci. Hortic. 2019, 246, 79–85. [Google Scholar] [CrossRef]
- Huang, P.; Zang, F.; Li, C.; Lin, F.; Zang, D.; Li, B.; Zheng, Y. The Akebia genus as a novel forest crop: A review of its genetic resources, nutritional components, biosynthesis, and biological studies. Front. Plant Sci. 2022, 13, 936571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, H.; Zhou, X.; Wang, D.; Zhong, Y.; Xia, Q.; Deng, Y.; Zhao, Y. Antimicrobial, antioxidant and physical properties of chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite and its application. Food Chem. 2021, 361, 130111. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, D.; Dobrowolska, E.; Sharafan, M.; Ekiert, H.; Tomczyk, M.; Szopa, A. Akebia quinata and Akebia trifoliata—A review of phytochemical composition, ethnopharmacological approaches and biological studies. J. Ethnopharmacol. 2021, 280, 114486. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Zhong, C.; Chen, X.; Huang, H. Akebia: A potential new fruit crop in China. HortScience 2010, 45, 4–10. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Z.; Chen, J.; Niu, J.; Shi, Y.; Wang, Y.; Chen, T.; Sun, Z.; Chen, J.; Luan, M. Physicochemical properties, content, composition and partial least squares models of A. trifoliata seeds oil. Food Chem. X 2021, 12, 100131. [Google Scholar] [CrossRef]
- Xiong, D.S.; Guo, C.Q.; Xie, B. Seed quality characteristics of Akebia trifoliata. Chin. Tradit. Herb. Drugs 2005, 36, 1710–1713, (In Chinese with English Abstract). [Google Scholar]
- Sherif, N.A.; Benjamin, J.F.; Kumar, T.S.; Rao, M.V. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018, 132, 303–316. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, F.S.; Song, S.L.; Wang, C.X.; Sun, H.M. Highly effective organogenesis and somatic embryogenesis of Clivia. Sci. Hortic. 2022, 306, 111443. [Google Scholar] [CrossRef]
- Su, H.; Han, X.; Li, T.; Xu, L.; Xu, X.; Hu, L.; Liao, L. Callus induction and differentiation of hypocotyl of Akebia trifoliata. Jiangsu Agric. Sci. 2015, 43, 50–52. (In Chinese) [Google Scholar]
- Wu, L.L.; Ke, B.F.; Gong, C.; Ma, Y.; Lei, X.L.; Li, J.A. Tissue culture and rapid propagation of Akebia trifoliate var. australis. Plant Physiol. J. 2015, 51, 903–908, (In Chinese with English Abstract). [Google Scholar]
- Guan, Y.; Li, S.G.; Fan, X.F.; Su, Z.H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, I.A.; Castander-Olarieta, A.; Hargreaves, C.L.; Gough, K.; Reeves, C.B.; van Ballekom, S.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Hybrid pine (Pinus attenuate × Pinus radiata) somatic embryogenesis: What do you prefer, mother or nurse? Forests 2021, 12, 45. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Wawrzyniak, M.K.; Kijowska-Oberc, J.; Staszak, A.M.; Ratajczak, E. Somatic embryogenesis of Norway spruce and Scots pine: Possibility of application in modern forestry. Forests 2022, 13, 155. [Google Scholar] [CrossRef]
- Varis, S.; Tikkinen, M.; Välimäki, S.; Aronen, T. Light spectra during somatic embryogenesis of Norway spruce—Impact on growth, embryo productivity, and embling survival. Forests 2021, 12, 301. [Google Scholar] [CrossRef]
- Gaj, M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Wu, G.Y.; Wei, X.L.; Wang, X.; Wei, Y. Induction of somatic embryogenesis in different explants from Ormosia henryi Prain. Plant Cell Tissue Organ Cult. 2020, 142, 229–240. [Google Scholar] [CrossRef]
- Faisal, M.; Abdel-Salam, E.M.; Alatar, A.A.; Qahtan, A.A. Induction of somatic embryogenesis in Brassica juncea L. and analysis of regenerants using ISSR-PCR and flow cytometer. Saudi J. Biol. Sci. 2021, 28, 1147–1153. [Google Scholar] [CrossRef]
- Zou, S.; Yao, X.; Zhong, C.; Li, D.; Wang, Z.; Huang, H. Recurrent somatic embryogenesis and development of somatic embryos in Akebia trifoliata (Thunb.) Koidz (Lardizabalaceae). Plant Cell Tissue Organ Cult. 2020, 139, 493–504. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, X.; Hu, S.; Li, Y.; Yang, J.; Chen, F. Somatic embryogenesis and plantlet regeneration of Akebia trifoliate. J. Southwest Forestry Univ. 2022, 42, 34–41, (In Chinese with English abstract). [Google Scholar]
- Priyanka, V.; Kumar, R.; Dhaliwal, I.; Kaushik, P. Germplasm conservation: Instrumental in agricultural biodiversity–A review. Sustainability 2021, 13, 6743. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Shi, Y.; Wang, X.; Sun, Z.M.; Huang, K.; Gong, C.; Luan, M.; Chen, J. Development of SSR markers via de novo transcriptome assembly in Akebia trifoliata (Thunb.) Koidz. Genome 2019, 62, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.B.; McCown, B.H. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Zhang, Y.S.; Chen, J.J.; Cao, Y.M.; Duan, J.X.; Cai, X.D. Induction of tetraploids in ‘Red Flash’ caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci. Hortic. 2020, 272, 109524. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, S.; Wang, Y.; Jiang, D.; Zhang, Y.; Hu, L.; Zhu, Y.; Jia, Q.; Yin, J.; Liu, Y.; et al. Field Performance of Disease-Free Plants of Ginger Produced by Tissue Culture and Agronomic, Cytological, and Molecular Characterization of the Morphological Variants. Agronomy 2023, 13, 74. [Google Scholar] [CrossRef]
- Fulton, T.M.; Chunwongse, J.; Tanksley, S.D. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 1995, 13, 207–209. [Google Scholar] [CrossRef]
- Li, T.; Dong, J.; Liao, L.; Jin, H.; Han, X.; Wen, F.; Xu, L. Isolation and characterization of microsatellite markers for Akebia trifoliata. Guihaia 2018, 38, 1117–1124, (In Chinese with English Abstract). [Google Scholar]
- Yu, S.; Zhao, X.; Wang, Y.; Jiang, D.; Zhang, Y.; Hu, L.; Liu, Y.; Cai, X. Morphological, cytological, and molecular-based genetic stability analysis of in vitro-propagated plants from newly induced aneuploids in caladium. Agriculture 2022, 12, 1708. [Google Scholar] [CrossRef]
- De Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and indirect somatic embryogenesis induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.A.; Alavi, S.V.; Majd, A.; Fallahian, F. Plant regeneration through direct and indirect somatic embryogenesis from immature seeds of citrus. Eur. J. Exp. Biol. 2013, 3, 307–310. [Google Scholar]
- Luo, Y.; Han, Y.; Wei, W.; Han, Y.; Yuan, J.; He, N. Transcriptome and metabolome analyses reveal the efficiency of in vitro regeneration by TDZ pretreatment in mulberry. Sci. Hortic. 2023, 310, 111678. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant. 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Singh, N.D.; Sahoo, N.; Sarin, N.B.; Jaiwal, P.K. The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Millsp). Plant Sci. 2003, 164, 341–347. [Google Scholar] [CrossRef]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Montalbán, I.A.; Moncaleán, P. Rooting of Pinus radiata somatic embryos: Factors involved in the success of the process. J. For. Res. 2019, 30, 65–71. [Google Scholar] [CrossRef]
- Cao, X.; Gao, F.; Qin, C.; Chen, S.; Cai, J.; Sun, C.; Weng, Y.; Tao, J. Optimizing somatic embryogenesis initiation, maturation and preculturing for cryopreservation in Picea pungens. Forests 2022, 13, 2097. [Google Scholar] [CrossRef]
- Garcia, C.; Furtado de Almeida, A.; Costa, M.; Britto, D.; Valle, R.; Royaert, S.; Marelli, J.P. Abnormalities in somatic embryogenesis caused by 2,4-D: An overview. Plant Cell Tissue Organ Cult. 2019, 137, 193–212. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Xu, S.; Deng, Z. Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ caladium. Plant Cell Tissue Organ Cult. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech. 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhang, Y.S.; Duan, J.X.; Cao, Y.M.; Cai, X.D. Morphological, cytological, and pigment analysis of leaf color variants regenerated from long-term subcultured caladium callus. In Vitro Cell. Dev. Biol. Plant. 2021, 57, 60–71. [Google Scholar] [CrossRef]
- Cao, Z.; Sui, S.; Cai, X.; Yang, Q.; Deng, Z. Somaclonal variation in ‘Red Flash’ caladium: Morphological, cytological and molecular characterization. Plant Cell Tissue Organ Cult. 2016, 126, 269–279. [Google Scholar] [CrossRef]


| Basic Medium | PGRs (g L−1) | Somatic Embryogenesis Frequency (%) | Browning Rate (%) | |||
|---|---|---|---|---|---|---|
| TDZ | 6-BA | 2,4-D | ||||
| SEI1 | MS | 1.0 | – | 1.0 | 0.0 ± 0.0 c | 92.6 ± 5.7 b |
| SEI2 | 1.0 | 1.0 | – | 20.4 ± 8.4 b | 77.8 ± 9.9 c | |
| SEI3 | – | 1.0 | 1.0 | 0.0 ± 0.0 c | 100.0 ± 0.0 a | |
| SEI4 | WPM | 1.0 | – | 1.0 | 0.0 ± 0.0 c | 96.3 ± 9.1 ab |
| SEI5 | 1.0 | 1.0 | – | 35.2 ± 14.8 a | 61.1 ± 9.3 d | |
| SEI6 | – | 1.0 | 1.0 | 0.0 ± 0.0 c | 100.0 ± 0.0 a | |
| Medium | PGRs (mg L−1) | Root Induction Rate (%) | Adventitious Root Number Per Plant | Total Root Length Per Plant (cm) | Plant Height (cm) | Total Leaf Number Per Plant | |
|---|---|---|---|---|---|---|---|
| IBA | TDZ | ||||||
| RI1 | 1.0 | 0.5 | 33.3 ± 12.9 b | 6.5 ± 1.0 b | 21.6 ± 3.7 a | 4.1 ± 0.5 ab | 12.7 ± 2.1 b |
| RI2 | 1.0 | 1.0 | 100.0 ± 0.0 a | 4.8 ± 1.3 b | 9.2 ± 1.3 b | 3.5 ± 0.3 b | 12.3 ± 1.5 b |
| RI3 | 2.0 | 0.5 | 95.8 ± 11.2 a | 13.7 ± 2.8 a | 19.2 ± 1.9 a | 5.2 ± 0.9 a | 20.3 ± 4.2 a |
| RI4 | 2.0 | 1.0 | 41.7 ± 12.9 b | 6.2 ± 1.9 b | 17.5 ±1.8 a | 4.0 ± 0.5 ab | 19.7 ± 2.3 a |
| Characters | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| Terminal leaflet length (cm) | 3.63 | 6.93 | 5.53 | 0.69 | 12.51 |
| Terminal leaflet width (cm) | 2.73 | 4.90 | 3.55 | 0.60 | 16.94 |
| Lateral leaflet length (cm) | 3.42 | 6.12 | 4.67 | 0.62 | 13.24 |
| Lateral leaflet width (cm) | 2.35 | 3.88 | 2.82 | 0.42 | 14.84 |
| Leaf rachis length (cm) | 1.67 | 8.00 | 3.88 | 1.89 | 48.83 |
| Leaf rachis diameter (mm) | 0.69 | 1.09 | 0.93 | 0.11 | 11.83 |
| Leaf SPAD value | 37.03 | 58.82 | 51.47 | 5.19 | 10.08 |
| Characteristics | Normal Type | Type Ι | Type ΙΙ |
|---|---|---|---|
| Terminal leaflet length (cm) | 5.36 ± 0.51 b | 5.92 ± 0.41 b | 6.86 ± 0.71 a |
| Terminal leaflet width (cm) | 3.28 ± 0.44 b | 3.10 ± 0.33 b | 5.04 ± 0.65 a |
| Leaf length/width ratio of terminal leaflets | 1.64 ± 0.09 b | 1.93 ± 0.25 a | 1.37 ± 0.06 c |
| Lateral leaflet length (cm) | 4.23 ± 0.87 b | 4.43 ± 0.48 b | 5.81 ± 0.83 a |
| Lateral leaflet width (cm) | 2.46 ± 0.25 b | 2.71 ± 0.29 b | 3.36 ± 0.75 a |
| Leaf length/width ratio of lateral leaflets | 1.73 ± 0.24 a | 1.76 ± 0.31 a | 1.69 ± 0.09 a |
| Leaf rachis length (cm) | 2.96 ± 0.43 b | 5.46 ± 0.25 a | 2.44 ± 0.54 b |
| Leaf rachis diameter (mm) | 0.77 ± 0.16 b | 0.91 ± 0.07 ab | 1.07 ± 0.06 a |
| Leaf SPAD value | 51.05 ± 7.55 ab | 48.13 ± 3.97 b | 55.40 ± 4.68 a |
| Stomatal guard cell length (μm) | 23.80 ± 2.24 a | 21.36 ± 2.08 a | 21.67 ± 2.80 a |
| Stomatal guard cell width (μm) | 17.83 ± 2.59 a | 14.55 ± 1.46 b | 13.28 ± 1.74 b |
| Stomatal density (no./mm2) | 210.94 ± 20.23 a | 218.24 ± 2.54 a | 203.04 ± 20.44 a |
| Net photosynthesis rate (μmol CO2 m−2 s−1) | 7.32 ± 0.86 ab | 6.74 ± 0.20 b | 7.93 ± 0.54 a |
| Transpiration rate (mmol H2O m−2 s−1) | 1.86 ± 0.01 a | 1.59 ± 0.09 b | 1.95 ± 0.32 a |
| Stomatal conductance (mol H2O m−2 s−1) | 0.18 ± 0.01 a | 0.13 ± 0.01 b | 0.19 ± 0.02 a |
| Primers | No. of Amplified Bands | No. of Polymorphic Bands | Percentage of Polymorphic Bands (%) |
|---|---|---|---|
| MT15 | 47 | 28 | 59.57 |
| MT28 | 47 | 13 | 14.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cao, Y.; Wang, Y.; Cai, X. Somatic Embryogenesis Induction and Genetic Stability Assessment of Plants Regenerated from Immature Seeds of Akebia trifoliate (Thunb.) Koidz. Forests 2023, 14, 473. https://doi.org/10.3390/f14030473
Zhang Y, Cao Y, Wang Y, Cai X. Somatic Embryogenesis Induction and Genetic Stability Assessment of Plants Regenerated from Immature Seeds of Akebia trifoliate (Thunb.) Koidz. Forests. 2023; 14(3):473. https://doi.org/10.3390/f14030473
Chicago/Turabian StyleZhang, Yiming, Yunmei Cao, Yida Wang, and Xiaodong Cai. 2023. "Somatic Embryogenesis Induction and Genetic Stability Assessment of Plants Regenerated from Immature Seeds of Akebia trifoliate (Thunb.) Koidz" Forests 14, no. 3: 473. https://doi.org/10.3390/f14030473
APA StyleZhang, Y., Cao, Y., Wang, Y., & Cai, X. (2023). Somatic Embryogenesis Induction and Genetic Stability Assessment of Plants Regenerated from Immature Seeds of Akebia trifoliate (Thunb.) Koidz. Forests, 14(3), 473. https://doi.org/10.3390/f14030473






