Leaf Functional Traits of Zanthoxylum planispinum ‘Dintanensis’ Plantations with Different Planting Combinations and Their Responses to Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Treatment Setting
2.3. Soil Sampling and Analysis
2.4. Leaf Sampling and Leaf Functional Trait Analysis
2.5. Data Analysis
3. Results
3.1. Characteristics of Leaf Functional Traits of Z. planispinum in Different Planting Combinations
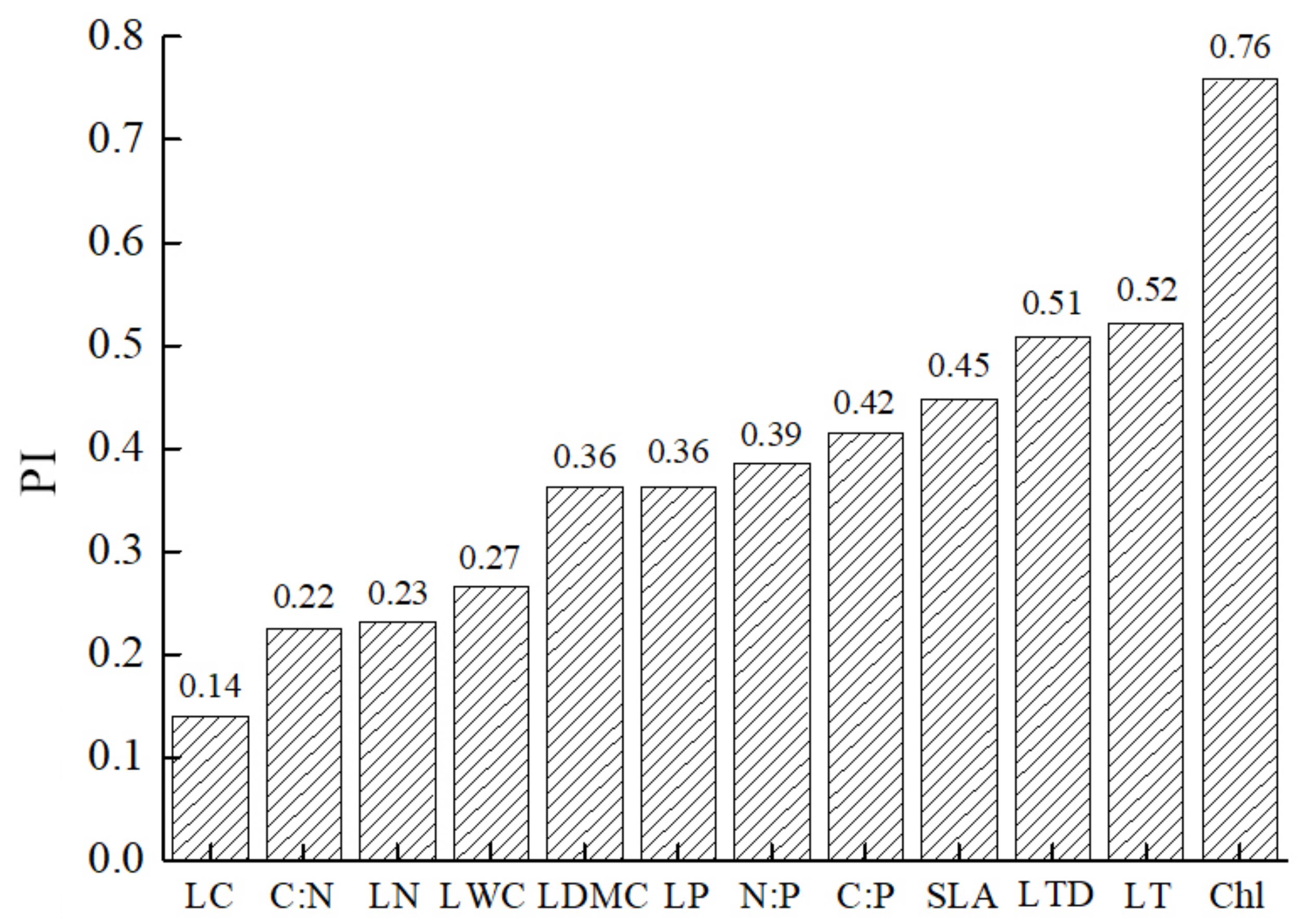
3.2. Plasticity of Leaf Functional Traits of Z. planispinum in Different Planting Combinations
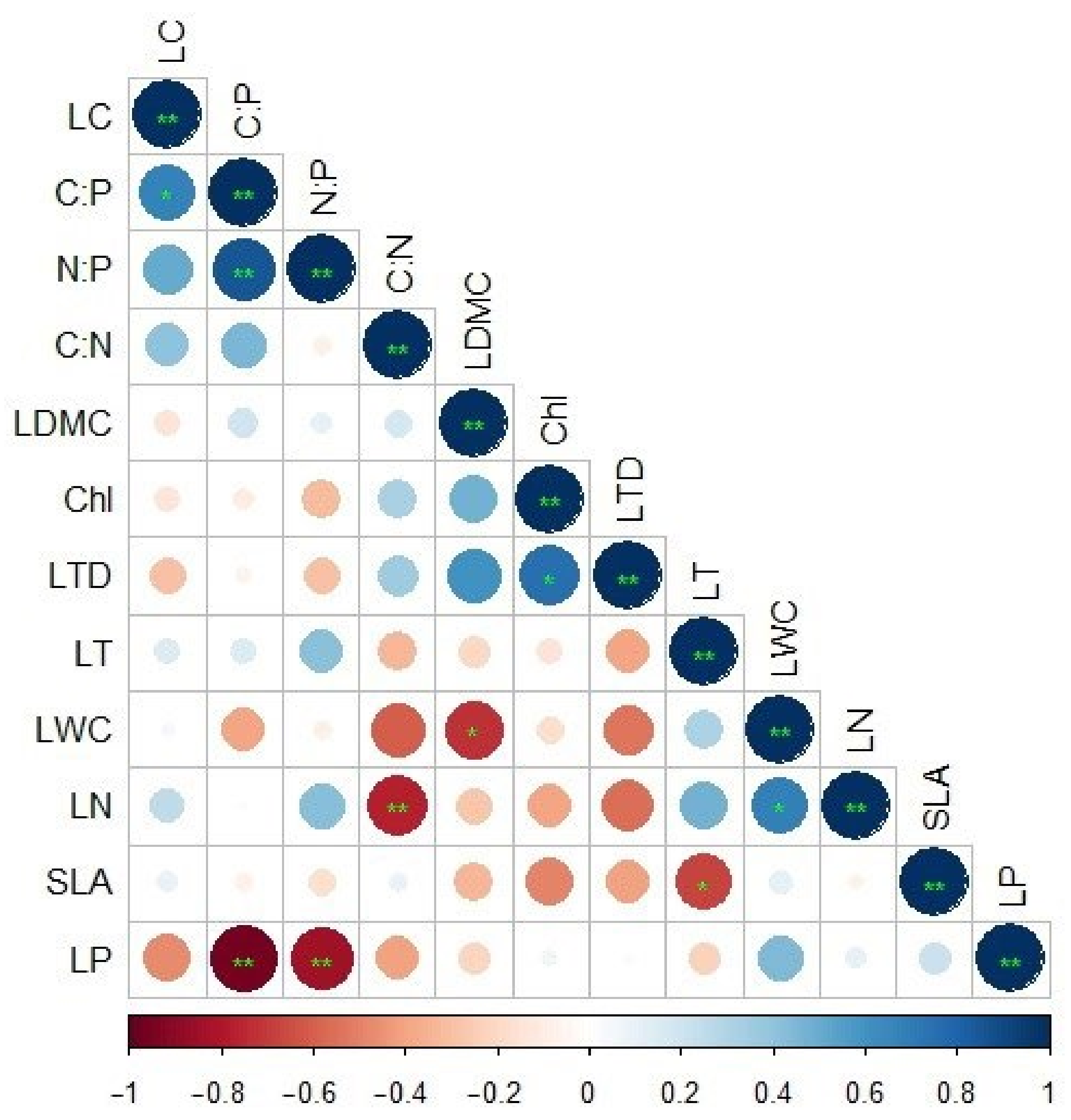
3.3. Correlation Analysis of the Leaf Functional Traits of Z. planispinum in Different Planting Combinations
3.4. Analysis of the Adaptability of Z. planispinum in Different Planting Combinations
3.5. Effects of Soil Factors on Leaf Functional Traits
4. Discussion
4.1. Effect of Planting Combinations on the Leaf Functional Traits of Z. planispinum
4.2. Coupling Relationship between the Leaf Functional Traits of Z. planispinum
4.3. Response of Leaf Functional Traits to Soil Factors
5. Conclusions
- (1)
- Z. planispinum tended to have a slow investment strategy after planting with P. salicina. The combination with the S. tonkinensi showed a rapid growth strategy. Following combination with L. japonica, Z. planispinum tended to form a combination of traits that resisted drought and infertile environmental stress. The combination with L. japonica made the investment strategy of Z. planispinum adopt a transition from slow to fast. The results showed that species combination could affect the adaptive mechanism of Z. planispinum.
- (2)
- Z. planispinum was relatively more adaptive when combined with P. salicina or L. japonica. However, the lowest adaptive capacity occurred when the Z. planispinum was planted in combination with S. tonkinensis. The results indicated that planting combinations can promote or inhibit the growth of Z. planispinum.
- (3)
- The leaf functional traits of Z. planispinum were affected by SWC, MBC, MBN, MBP, AN, TP, ACa, C:N, C:P, and N:P, involving the effects of soil physical properties, soil elements, and their stoichiometry and microbial properties. In the future, it will be necessary to further study the comprehensive effect of soil action on leaf functional traits across a wider range of sites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database–Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.J.; Wang, H.; Gou, W.; White, J.F.; Kingsley, K.L.; Wu, G.Q.; Su, P.X. Leaf functional traits of dominant desert plants in the Hexi Corridor, Northwestern China: Trade-off relationships and adversity strategies. Glob. Ecol. Conserv. 2021, 28, e01666. [Google Scholar] [CrossRef]
- Wang, M.; Wan, P.C.; Guo, J.C.; Xu, J.S.; Chai, Y.F.; Yue, M. Relationships among leaf, stem and root traits of the dominant shrubs from four vegetation zones in Shaanxi Province, China. Isr. J. Ecol. Evol. 2017, 63, 25–32. [Google Scholar] [CrossRef]
- Hou, X.L.; Han, H.; Tigabu, M.; Cai, L.P.; Meng, F.R.; Liu, A.Q.; Ma, X.Q. Changes in soil physico-chemical properties following vegetation restoration mediate bacterial community composition and diversity in Changting, China. Ecol. Eng. 2019, 138, 171–179. [Google Scholar] [CrossRef]
- Wang, L.X.; Pang, X.Y.; Li, N.; Qi, K.B.; Huang, J.S.; Yin, C.Y. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Lü, X.T.; Reed, S.; Yu, Q.; He, N.P.; Wang, Z.W.; Han, X.G. Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob. Chang. Biol. 2013, 19, 2775–2784. [Google Scholar] [CrossRef]
- Thoms, C.; Gattinger, A.; Jacob, M.; Thomas, F.M.; Gleixner, G. Direct and indirect effects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol. Biochem. 2010, 42, 1558–1565. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.J.; Saeed, S.; Zhang, B.; Luo, M. The difference of soil properties between pure and mixed Chinese fir (Cunninghamia lanceolata) plantations depends on tree species. Glob. Ecol. Conserv. 2020, 22, e01009. [Google Scholar] [CrossRef]
- Bongers, F.J.; Schmid, B.; Bruelheide, H.; Bongers, F.; Li, S.; von Oheimb, G.; Li, Y.; Cheng, A.P.; Ma, K.P.; Liu, X.J. Functional diversity effects on productivity increase with age in a forest biodiversity experiment. Nat. Ecol. Evol. 2021, 5, 1594–1603. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Wang, L.X.; Lu, N.; Li, J.Y. Water use characteristics of the common tree species in different plantation types in the Loess Plateau of China. Agr. For. Meteorol. 2020, 288, 108020. [Google Scholar] [CrossRef]
- Peng, C.J.; Song, M.H.; Zhou, C.L.; Li, Y.K.; Li, X.J.; Cao, G.M. Relationship between leaf functional traits of herbaceous plants and soil factors in different coverage gradients of Potentilla fruticosa shrub under grazing. Acta Bot. Boreal. Occident. Sin. 2020, 40, 870–881. [Google Scholar] [CrossRef]
- Li, X.E.; Song, X.Y.; Zhao, J.; Lu, H.F.; Qian, C.; Zhao, X. Shifts and plasticity of plant leaf mass per area and leaf size among slope aspects in a subalpine meadow. Ecol. Evol. 2021, 11, 14042–14055. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, M.X.; Xu, L.; Zhu, J.X.; Dai, G.H.; He, N.P. C:N:P stoichiometry in terrestrial ecosystems in China. Sci. Total Environ. 2021, 795, 148849. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.D.; Cui, Q.J.; Gao, J. Latitudinal, soil and climate effects on key leaf traits in northeastern China. Glob. Ecol. Conserv. 2020, 22, e00904. [Google Scholar] [CrossRef]
- Li, Y.Q.; He, W.; Wu, J.; Zhao, P.; Chen, T.; Zhu, L.W.; Ouyang, L.; Ni, G.Y.; Hölscher, D. Leaf stoichiometry is synergistically-driven by climate, site, soil characteristics and phylogeny in karst areas, Southwest China. Biogeochemistry 2021, 155, 283–301. [Google Scholar] [CrossRef]
- Bauters, M.; Verbeeck, H.; Doetter, S.; Ampoorter, E.; Baert, G.; Vermeir, P.; Verheyen, K.; Boeckx, P. Functional Composition of Tree Communities Changed Topsoil Properties in an Old Experimental Tropical Plantation. Ecosystems 2017, 20, 861–871. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, C.H.; Chen, H.S.; Yue, Y.M.; Zhang, W.; Zhang, M.Y.; Qi, X.K.; Fu, Z.Y. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Kuang, Y.W.; Kang, M. Untangling the influence of phylogeny, soil and climate on leaf element concentrations in a biodiversity hotspot. Funct. Ecol. 2015, 29, 165–176. [Google Scholar] [CrossRef]
- Tu, Y.L.; Wei, C.S.; Zou, Z.L.; Lu, Y.M. A new Zanthoxylum Genus—Z. planipinum var. dingtanensis and the research of its species classification. Guizhou Sci. 2001, 19, 77–80. (In Chinese) [Google Scholar]
- Yu, Y.H.; Song, Y.P.; Li, Y.T. Management practices effects on Zanthoxylum planispinum ‘dintanensis’ fruit quality. Agron. J. 2022, 114, 2095–2104. [Google Scholar] [CrossRef]
- Zou, J.; Yu, L.F.; Huang, Z.S. Variation of Leaf Carbon Isotope in Plants in Different Lithological Habitats in a Karst Area. Forests 2019, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.T.; Yu, Y.H.; Song, Y.P. Stoichiometry of Soil, Microorganisms, and Extracellular Enzymes of Zanthoxylum planispinum var. dintanensis Plantations for Different Allocations. Agronomy 2022, 12, 1709. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 22–173. (In Chinese) [Google Scholar]
- Lu, R.K. Methods for Soil and Agriculture Chemistry Analysis, 3rd ed.; Chinese Agricultural Science and Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, H.; Wang, Z.H.; Wen, Z.; Yang, T.Z. Effects of plant functional traits on ecosystem services: A review. Chin. J. Plant Ecol. 2021, 45, 1140–1153. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Kong, H.Y.; Liu, H. Stoichiometric characteristics of soil C, N, P and K in different Pinus massoniana forests. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2016, 44, 68–82. (In Chinese) [Google Scholar]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Wright, I.J.; Ackerly, D.D.; Bongers, F.; Harms, K.E.; Ibarra-Manriquez, G.; Martine-Ramos, M.; Mazer, S.J.; Muller-Landau, H.C.; Paz, H.; Pitman, N.C.A.; et al. Relationships among ecologically important dimensions of plant trait variation in seven Neotropical forests. Ann. Bot.-Lond. 2007, 99, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant functional traits: Soil and ecosystem services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Thomas, F.M.; Yu, R.D.; Schafer, P.; Zhang, X.M.; Lang, P. How diverse are Populus “diversifolia” leaves? Linking leaf morphology to ecophysiological and stand variables along water supply and salinity gradients. Flora 2017, 233, 68–78. [Google Scholar] [CrossRef]
- Parkhurst, D.F. Diffusion of CO2 and other gases inside leaves. New Phytol. 1994, 126, 449–479. [Google Scholar] [CrossRef]
- Wu, T.H.; Long, L.C.; Xiong, L.; Liu, Q. Variation and adaptation of leaf functional traits of different growth type in Karst forests. Chin. J. Appl. Environ. Biol. 2023, 29, 1–10. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Persson, J.; Fink, P.; Goto, A. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Zhang, T.T.; Liu, W.Y.; Huang, J.B.; Hu, T.; Tang, D.D.; Chen, Q. Characteristics of plant ecological stoichiometry homeostasis. Guihaia 2019, 39, 701–712. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Ma, F.; Xu, T.T.; Liu, J.L.; Xiao, G.J.; Li, M.; Bi, J.T.; Na, X.F. Variations in Carbon, Nitrogen and Phosphorus Stoichiometry of Caragana liouana Originated from Nine Provenances in a Common Garden. Acta Bot. Boreal. Occident. Sin. 2017, 37, 1381–1389. [Google Scholar] [CrossRef]
- Bertolet, B.L.; Corman, J.R.; Casson, N.J.; Sebestyen, S.D.; Kolka, R.K.; Stanley, E.H. Influence of soil temperature and moisture on the dissolved carbon, nitrogen, and phosphorus in organic matter entering lake ecosystems. Biogeochemistry 2018, 139, 293–305. [Google Scholar] [CrossRef]
- Cortes, S.S.; Whitworth-Hulse, J.I.; Piovano, E.L.; Gurvich, D.E.; Magligano, P.N. Changes in rainfall partitioning caused by the replacement of native dry forests of Lithraea molleoides by exotic plantations of Pinus elliottii in the dry Chaco mountain forests, central Argentina. J. Arid Land 2020, 12, 717–729. [Google Scholar] [CrossRef]
- Singh, S.P.; Mahapatra, B.S.; Pramanick, B.; Yadav, V.R. Effects of irrigation levels, planting methods and mulching on nutrient uptake, yield, quality, water and fertilizer productivity of field mustrad (Brassica rapa L.) under sandy loam soil. Agric. Water Manag. 2021, 244, 106539. [Google Scholar] [CrossRef]
- Whyte, G.; Howard, K.; Hardy, G.E.S.; Burgess, T.I. The Tree Decline Recovery Seesaw; a conceptual model of the decline and recovery of drought stressed plantation trees. For. Ecol. Manag. 2016, 370, 102–113. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Das, B.; Manivannan, S.; Manjunath, B.L.; Verma, R.R.; Desai, S.; Kulkarni, R.M.; Latare, A.M.; Sale, R.; Murgaonkar, D.; et al. Soil and water conservation measures improve soil carbon sequestration and soil quality under cashews. Int. J. Sediment Res. 2021, 36, 190–206. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, A.M.; Yan, S.W.; Rafay, L.; Du, K.; Wang, D.J.; Ge, Y.G.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot vally. J. Soil Sediment 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Hu, H.Q.; Wang, L.H.; Li, Y.L.; Sun, J.W.; Zhou, Q.; Huang, X.H. Insight into mechanism of lanthanum (III) induced damage to plant photosynthesis. Ecotoxicol. Environ. Saf. 2016, 127, 43–50. [Google Scholar] [CrossRef]
- Amoozager, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Xu, C.H.; Xiang, W.H.; Gou, M.M.; Chen, M.M.; Chen, L.; Lei, P.F.; Fang, X.; Deng, X.W.; Ouyang, S. Effects of forest restoration on soil carbon, phosphorus, and their stoichiometry in Hunan, Southern China. Sustainability 2018, 10, 1874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- He, L.H. Quality Formation and Regulation of Wheat in Shanxi; China Agricultural Science and Technology Press: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Sun, Y.; Wang, C.T.; Chen, X.L.; Liu, S.R.; Lu, X.J.; Chen, H.Y.H.; Ruan, H.H. Phosphorus additions imbalance terrestrial ecosystem C:N:P stoichiometry. Glob. Chang. Biol. 2022, 28, 7353–7365. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Visser, J.; Lonhienne, T.G.A.; Schmidt, L. Past, present and future of organic nutrients. Plant Soil 2012, 359, 1–18. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.H.; Han, W.X.; Fang, J.Y. Global patterns of soil microbial nitrogen and phosphorus stoichiometry inforest ecosystems. Glob. Ecol. Biogeogr. 2014, 23, 979–987. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Zaehle, S.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hanson, P.J.; Hickler, T.; Jain, A.K.; Luo, Y.; Parton, W.; et al. Using ecosystem experiments to improve vegetation models. Nat. Clim. Chang. 2015, 5, 528–534. [Google Scholar] [CrossRef]
- Chen, L.L.; Deng, Q.; Yuan, Z.Y.; Mu, X.M.; Kallenbach, R.L. Age-related C:N:P stoichiometry in two plantation forests in the Loess Plateau of China. Ecol. Eng. 2018, 120, 14–22. [Google Scholar] [CrossRef]
- Murray, D.S.; Shattuck, M.D.; McDowell, W.H.; Wymore, A.S. Nitrogen wet deposition stoichiometry: The role of organic nitrogen, seasonality, and snow. Biogeochemistry 2022, 160, 301–314. [Google Scholar] [CrossRef]


| Plantation Types | Species Combinations | Longitude | Latitude | Growing Area (ha) | Altitude (m asl) | Density (m) | Height (m) | Crown Width (m) | Coverage (%) |
|---|---|---|---|---|---|---|---|---|---|
| Trt 1 | Z. planispinum + P. salicina | 105°40′28.33″ E | 25°37′57.41″ N | 1.34 | 764 | 3 × 3 | 3.5 | 2 × 2.3 | 70 |
| Trt 2 | Z. planispinum + S. tonkinensis | 105°40′19.79″ E | 25°39′25.75″ N | 0.67 | 728 | 2 × 2 | 2.0 | 1.2 × 1.8 | 60 |
| Trt 3 | Z. planispinum + A. hypogaea | 105°38′36.32″ E | 25°39′23.64″ N | 0.67 | 791 | 2 × 2 | 2.5 | 2.5 × 2.8 | 85 |
| Trt 4 | Z. planispinum + L. japonica | 105°38′36.35″ E | 25°39′22.29″ N | 6.67 | 814 | 3.5 × 3 | 2.5 | 1.5 × 2.5 | 70 |
| Trt 5 | Z. planispinum | 105°38′35.64″ E | 25°39′23.35″ N | 33.35 | 788 | 3 × 4 | 2.2 | 2.5 × 2.3 | 65 |
| Soil Parameters | Trt 1 | Trt 2 | Trt 3 | Trt 4 | Trt 5 |
|---|---|---|---|---|---|
| SWC | 31.60 ± 6.29 ab | 36.73 ± 2.65 a | 25.05 ± 1.38 bc | 28.69 ± 0.30 bc | 21.15 ± 0.14 c |
| pH | 6.70 ± 0.42 d | 7.35 ± 0.33 bc | 7.92 ± 0.05 ab | 7.28 ± 0.05 cd | 8.08 ± 0.05 a |
| SOC | 37.73 ± 7.32 ab | 29.40 ± 0.57 ab | 28.68 ± 12.62 ab | 50.83 ± 13.33 a | 26.50 ± 2.19 b |
| TN | 3.53 ± 0.46 ab | 2.64 ± 0.07 b | 2.78 ± 0.74 b | 4.60 ± 0.44 a | 2.76 ± 0.23 b |
| TP | 1.37 ± 0.02 a | 0.82 ± 0.03 b | 1.10 ± 0.43 ab | 1.52 ± 0.17 a | 1.26 ± 0.04 ab |
| TK | 6.95 ± 0.34 b | 6.11 ± 1.51 b | 12.33 ± 0.25 a | 11.88 ± 0.53 a | 10.88 ± 0.03 a |
| TCa | 0.95 ± 0.28 b | 1.48 ± 0.39 b | 1.85 ± 0.71 b | 1.88 ± 0.18 b | 6.05 ± 0.21 a |
| AN | 275.00 ± 74.25 ab | 160.00 ± 5.66 b | 161.75 ± 61.87 b | 350.00 ± 55.15 a | 153.75 ± 15.91 b |
| AP | 45.80 ± 13.29 a | 23.38 ± 11.63 a | 26.55 ± 10.54 a | 36.68 ± 10.01 a | 20.08 ± 2.44 a |
| AK | 393.00 ± 107.48 a | 195.85 ± 32.03 b | 172.75 ± 57.63 b | 223.75 ± 98.64 ab | 141.25 ± 2.47 b |
| ACa | 317.50 ± 14.85 b | 334.75 ± 0.35 b | 347.75 ± 24.40 ab | 371.00 ± 8.49 a | 350.50 ± 7.07 ab |
| Soil C:N ratio | 10.65 ± 0.70 a | 11.13 ± 0.53 a | 10.07 ± 1.85 a | 10.97 ± 1.85 a | 9.59 ± 0.00 a |
| Soil C:P ratio | 27.68 ± 5.79 abc | 35.83 ± 0.79 a | 25.82 ± 1.33 bc | 33.10 ± 4.99 ab | 21.03 ± 2.38 c |
| Soil N:P ratio | 2.59 ± 0.37 ab | 3.22 ± 0.22 a | 2.60 ± 0.34 ab | 3.02 ± 0.05 a | 2.19 ± 0.25 b |
| MBC | 243.00 ± 4.95 a | 254.75 ± 2.47 a | 252.00 ± 2.83 a | 262.75 ± 21.57 a | 262.25 ± 26.52 a |
| MBN | 12.40 ± 1.70 a | 13.58 ± 1.31 a | 14.38 ± 0.60 a | 13.90 ± 1.06 a | 14.08 ± 0.18 a |
| MBP | 128.00 ± 23.33 a | 144.50 ± 4.95 a | 148.00 ± 8.49 a | 154.50 ± 13.44 a | 139.00 ± 3.54 a |
| Trait | Unit | Ecological Connotation |
|---|---|---|
| LT | mm | LT is closely related to the rate of light energy utilization and photosynthetic efficiency, affecting the water supply and storage of leaves and the process of material and energy exchange in photosynthesis; the larger the value, the more suitable the plant for resource-deficient habitats. |
| SLA | cm2 | SLA reflects the carbon acquisition strategies, growth strategies, and adaptation characteristics of plants to different habitats and affects their relative growth rates; the higher the photosynthetic rate, the higher the transpiration. |
| LDMC | mg·g−1 | LDMC reflects the ability of plants to acquire and maintain environmental resources and the tissue construction of leaves. Higher values indicate that the leaves are better able to lock up nutrients in the body and reduce losses. |
| LWC | % | Leaf water content is important in breeding for drought tolerance and water retention traits of plants; higher values indicate higher drought resistance. |
| Chl | - | The higher the Chl content, the more photosynthetically active and shade-tolerant the plant. |
| LTD | g·cm−3 | LTD is related to resource acquisition, indicating the ability of plants to store nutrients and water and resist external interference; the higher the value, the stronger the ability to resist interference. |
| LC | g·kg−1 | The higher the LC value, the stronger the water supply capacity of the plant in a xerophytic environment. |
| LN | g·kg−1 | The higher the LN value, the better the chlorophyll synthesis and photosynthetic efficiency. |
| LP | g·kg−1 | LP promotes protein synthesis and physiological repair, and improves plant cold tolerance. |
| Leaf C:N ratio | - | C:N is proportional to the growth rate; the higher the value, the higher the carbon fixation advantage and nutrient utilization strategy, and the stronger the carbon assimilation ability. |
| Leaf C:P ratio | - | C:P represents the ability of plants to assimilate carbon when absorbing nutrients and the efficiency of carbon fixation in plants; the higher the value, the higher the carbon fixation advantage and nutrient utilization strategy, and the stronger the carbon assimilation ability. |
| Leaf N:P ratio | - | N:P indicates that plants are limited by nitrogen and phosphorus. If the value is >16, the plants are limited by phosphorus, if it is <14, the plants are limited by nitrogen, and between 14 and 16, both elements are limiting plant growth. |
| Plantation Type | LC (g·kg−1) | LN (g·kg−1) | LP (g·kg−1) | Leaf C:N Ratio | Leaf C:P Ratio | Leaf N:P Ratio |
|---|---|---|---|---|---|---|
| Trt 1 | 46.38 ± 1.43 a | 2.79 ± 0.08 a | 2.74 ± 0.34 a | 16.60 ± 0.01 a | 17.02 ± 1.58 a | 1.03 ± 0.10 a |
| Trt 2 | 44.64 ± 4.21 a | 2.93 ± 0.11 a | 3.42 ± 0.72 a | 15.23 ± 0.86 a | 13.49 ± 4.07 a | 0.88 ± 0.22 a |
| Trt 3 | 45.33 ± 4.39 a | 3.11 ± 0.23 a | 3.00 ± 0.16 a | 14.58 ± 0.33 a | 15.16 ± 2.27 a | 1.04 ± 0.13 a |
| Trt 4 | 44.40 ± 2.83 a | 3.20 ± 0.14 a | 2.92 ± 0.42 a | 13.88 ± 0.28 a | 15.31 ± 1.21 a | 1.10 ± 0.11 a |
| Trt 5 | 42.98 ± 0.90 a | 2.85 ± 0.45 a | 3.46 ± 0.51 a | 15.28 ± 2.74 a | 12.57 ± 2.12 a | 0.82 ± 0.01 a |
| Coefficient variation/% | 5.8 | 8.1 | 14.8 | 9.0 | 16.7 | 15.2 |
| Factors | Load Matrix of Principal Component | |||
|---|---|---|---|---|
| PCA1 | PCA2 | PCA3 | PCA4 | |
| LT | 0.291 | −0.508 | 0.309 | 0.625 |
| SPAD | −0.235 | 0.534 | −0.249 | 0.647 |
| SLA | −0.097 | 0.114 | 0.210 | −0.943 |
| LDMC | 0.104 | 0.115 | −0.873 | 0.212 |
| LWC | −0.305 | −0.440 | 0.769 | 0.090 |
| LTD | −0.217 | 0.520 | −0.614 | 0.388 |
| LC | 0.727 | 0.196 | 0.452 | −0.018 |
| LN | 0.107 | −0.823 | 0.364 | 0.091 |
| LP | −0.928 | −0.076 | 0.220 | −0.088 |
| Leaf C:N ratio | 0.366 | 0.912 | −0.047 | −0.055 |
| Leaf C:P ratio | 0.979 | 0.121 | −0.107 | −0.009 |
| Leaf N:P ratio | 0.887 | −0.406 | −0.076 | 0.052 |
| Eigenvalue | 3.905 | 3.586 | 2.068 | 1.050 |
| Variance contribution rate/% | 29.838 | 23.051 | 19.480 | 16.035 |
| Cumulative variance contribution rate/% | 29.838 | 52.889 | 72.370 | 88.405 |
| Sample Site | Factor Score | Soil Quality Index | Rank | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |||
| Trt 1 | 1.409 | 1.934 | −2.516 | 0.959 | 0.530 | 1 |
| Trt 2 | −0.93 | −0.187 | 1.526 | −3.292 | −0.552 | 5 |
| Trt 3 | 0.466 | −0.746 | 0.631 | −0.178 | 0.061 | 3 |
| Trt 4 | 0.904 | −1.821 | 0.731 | 1.303 | 0.201 | 2 |
| Trt 5 | −1.844 | 0.820 | −0.373 | 1.208 | −0.240 | 4 |
| Leaf Functional Traits | Stepwise Regression Equation | Standardized Regression Coefficients | R-Square | P |
|---|---|---|---|---|
| LT | LT = 0.071 + 0.002 × MBP | BMBP = 0.715 | 0.449 | 0.020 |
| Chl | Chl = 29.749 – 3.814 × N:P−0.65 × MBN | BN:P = −0.937, BMBN = −0.373 | 0.796 | 0.002 |
| SLA | SLA = 127.27 – 17.515 × TP + 1.067 × SWC − 0.055 × AN | BTP = −0.412, BSWC = 0.515, BAN = −0.396 | 0.960 | 0.000 |
| LTD | LTD = 0.868–0.226 × N:P + 0.017 × C:P − 0.001 × MBC + 0.001 × SWC − 0.033 × C:N | BN:P = −4.464, BC:P = 4.493, BMBC = −0.321, BSWC = 0.21, BC:N = −1.474 | 0.983 | 0.000 |
| Leaf C:N ratio | C:N = 20.979 – 0.058 × ACa + 0.055 × MBC | BACa = −0.908, BMBC = 0.564 | 0.628 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, Y.; Song, Y. Leaf Functional Traits of Zanthoxylum planispinum ‘Dintanensis’ Plantations with Different Planting Combinations and Their Responses to Soil. Forests 2023, 14, 468. https://doi.org/10.3390/f14030468
Li Y, Yu Y, Song Y. Leaf Functional Traits of Zanthoxylum planispinum ‘Dintanensis’ Plantations with Different Planting Combinations and Their Responses to Soil. Forests. 2023; 14(3):468. https://doi.org/10.3390/f14030468
Chicago/Turabian StyleLi, Yitong, Yanghua Yu, and Yanping Song. 2023. "Leaf Functional Traits of Zanthoxylum planispinum ‘Dintanensis’ Plantations with Different Planting Combinations and Their Responses to Soil" Forests 14, no. 3: 468. https://doi.org/10.3390/f14030468
APA StyleLi, Y., Yu, Y., & Song, Y. (2023). Leaf Functional Traits of Zanthoxylum planispinum ‘Dintanensis’ Plantations with Different Planting Combinations and Their Responses to Soil. Forests, 14(3), 468. https://doi.org/10.3390/f14030468






