Valorization of Wood-Based Waste from Grapevine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Type of grape: white graft—Pesecká léánka (Feteasca regala), red graft—Frankovka modrá (Vitis vinifera ‘Blaufränkisch’), native, graft;
- -
- Pruning time: the second half of February 2022;
- -
- Location: Nitra—Dolné Krškany, Nitra wine region, Slovakia;
- -
- WGS84 (φ, λ): 48.256958°, 18.082436°
- -
- Soil: brown earth soils with sandy-clay soils;
- -
- Direction of the vineyard: southwest, sloping terrain (south, southwest).
2.2. Methods
2.2.1. Chemical Analyses
2.2.2. Fourier-Transform Infrared (ATR-FTIR) Analysis
2.2.3. Mechanical Strength
2.2.4. Fiber Tester Analysis
2.2.5. Calorimetry
3. Results and Discussion
3.1. Chemical Composition of the Grapevine Cuttings
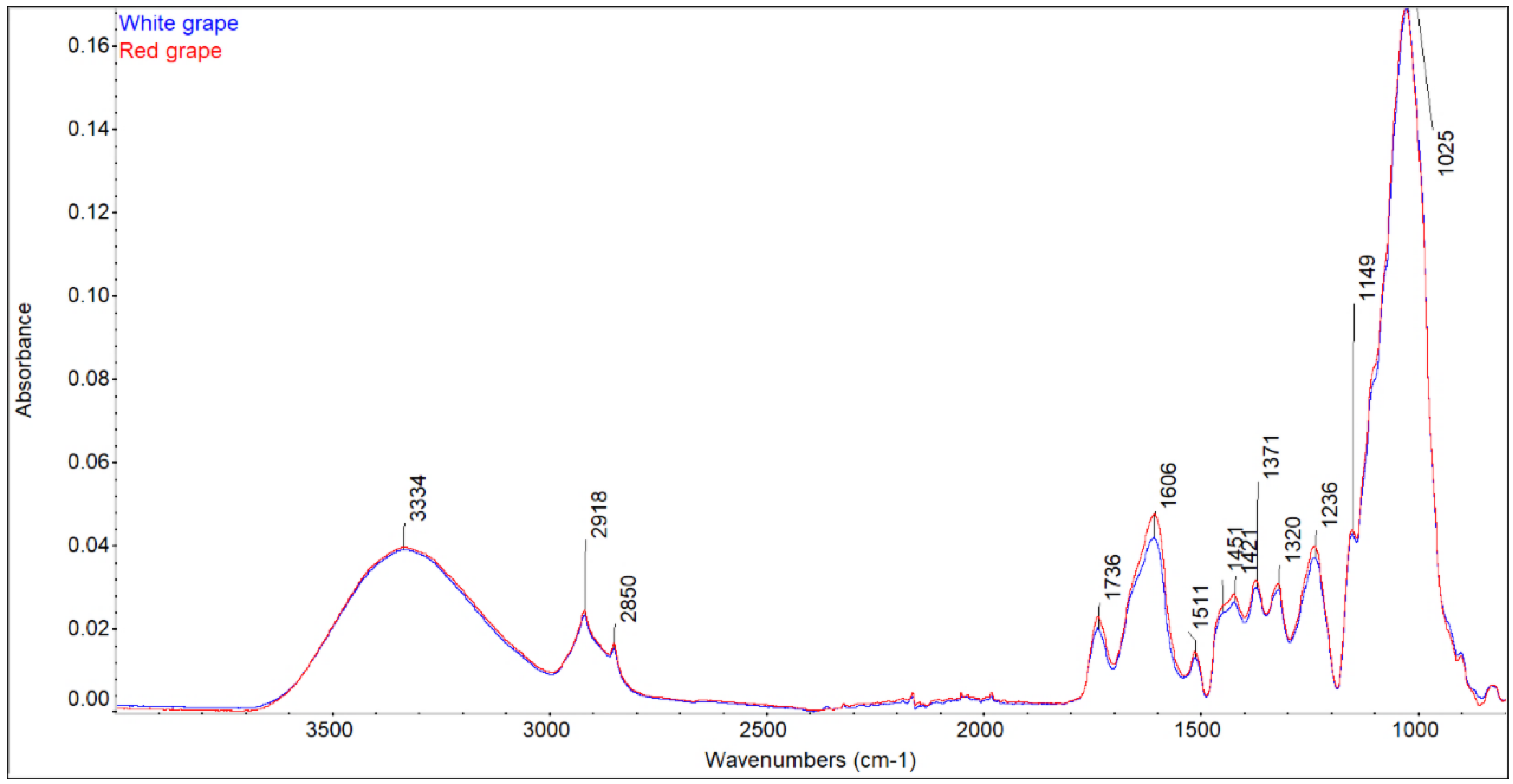
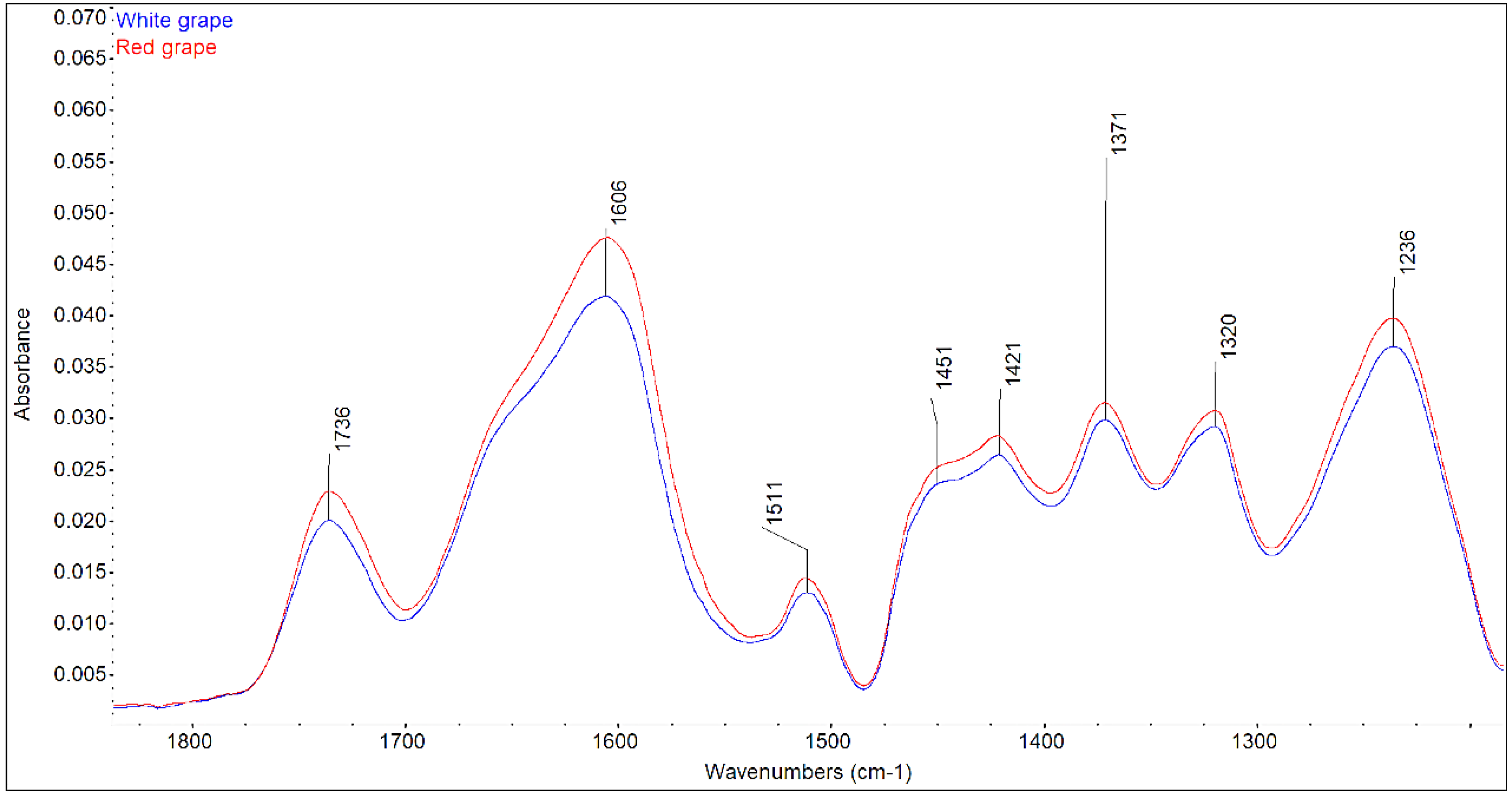
3.2. ATR-FTIR Analysis of Grapevine Cuttings
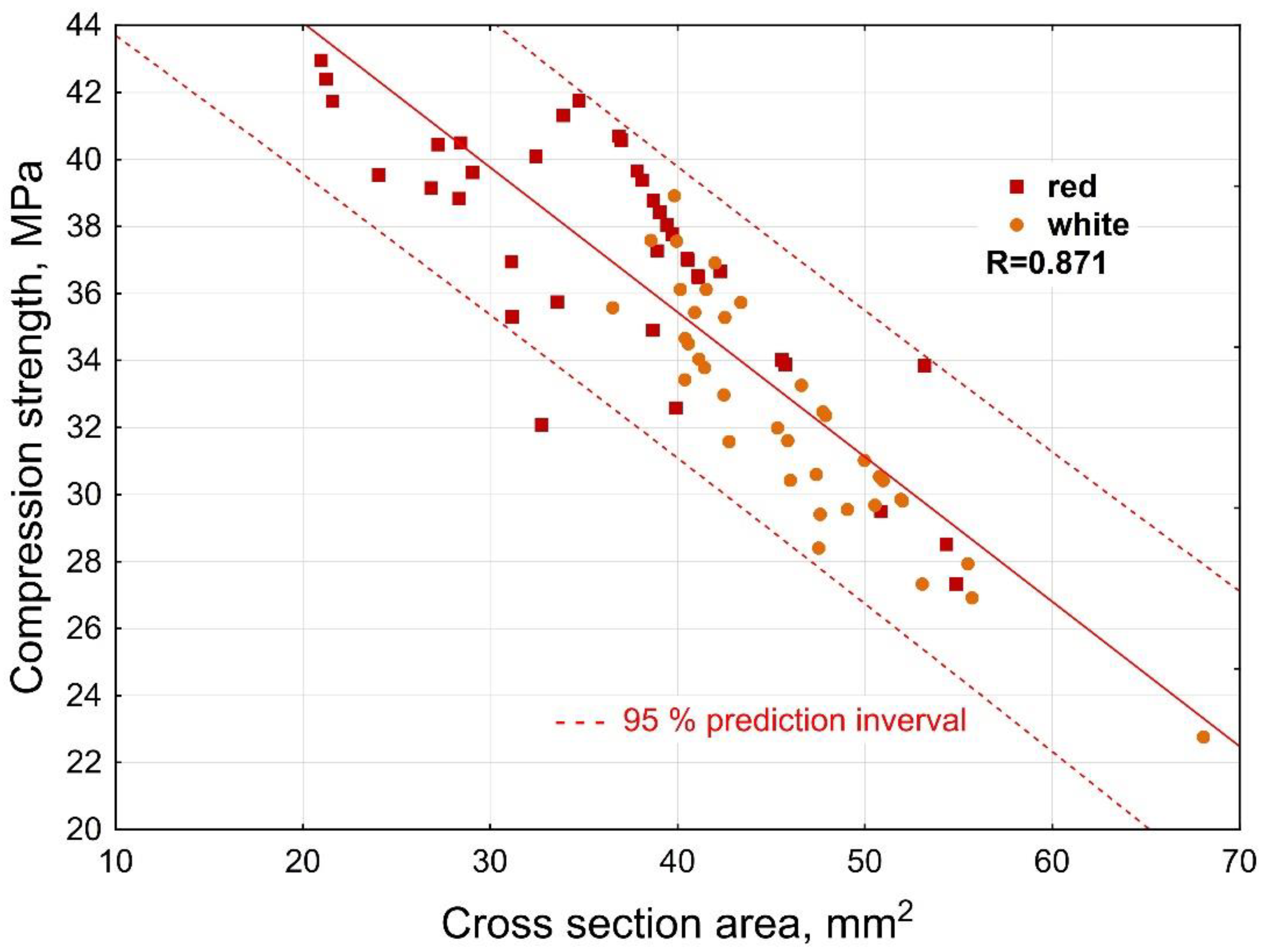
3.3. Mechanical Properties
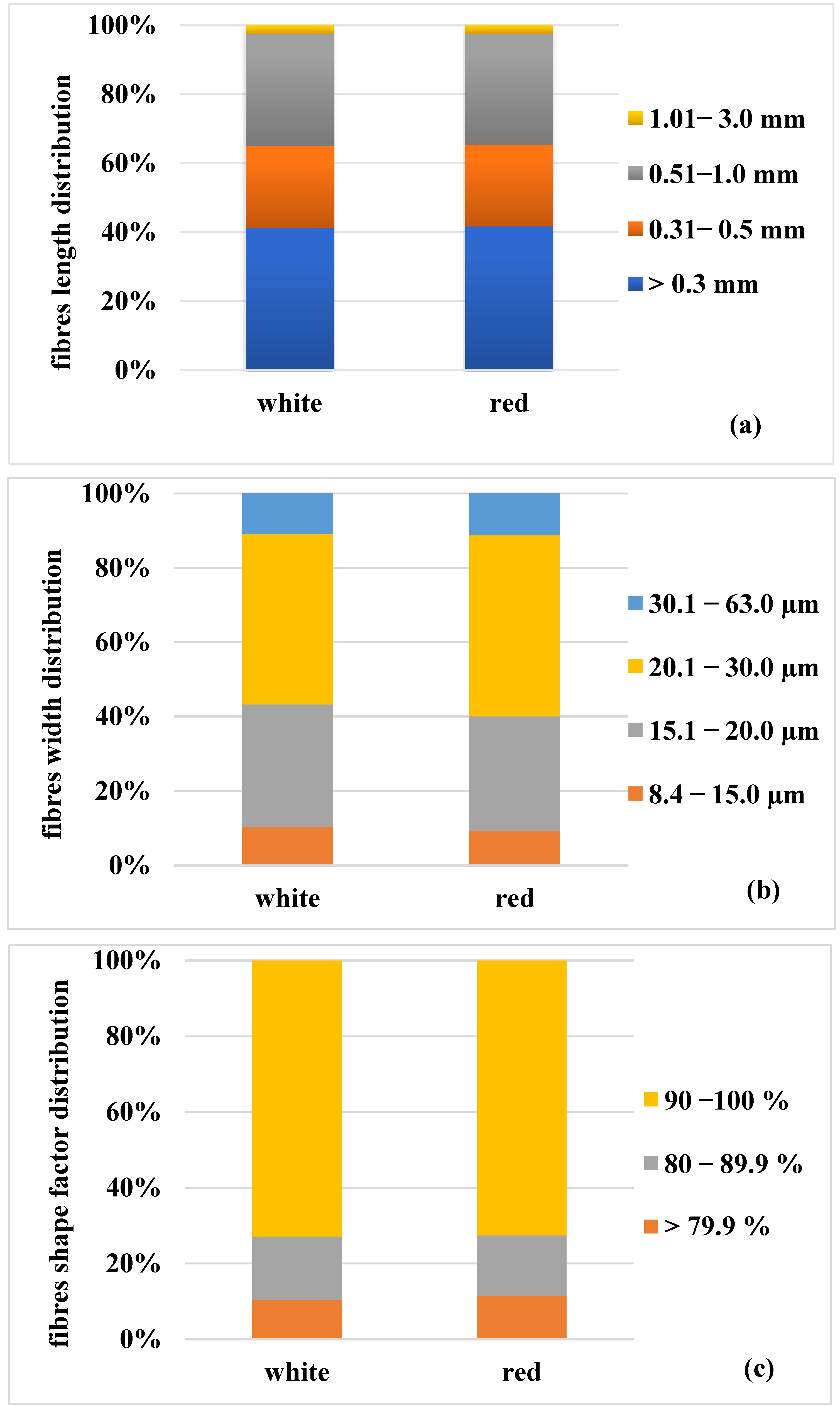
3.4. Fiber Analysis
3.5. Calorific Value Determination
4. Conclusions
- -
- The content of carbohydrates of grape cuttings provides a prerequisite for their acid or enzymatic hydrolysis to produce monosaccharides or second-generation bioethanol.
- -
- The higher glucose content in red grape samples compared to the white ones was confirmed by FTIR, as well as by chemical analysis.
- -
- The strength properties of both red and white cuttings showed the potential of the material to produce particleboards, fiberboards, or pulp and paper.
- -
- The fibers’ morphological properties of grape canes are comparable to those of non-wood species, which is a predisposition to the possibility of its further use for pulp and paper production.
- -
- Differences between cuttings from varieties Pesecká leánka (white graft) and Frankovka modrá (red graft) were obvious in terms of elements, extractive and saccharide (glucose and xylose) content, and compression strength.
- -
- The energetic potential of grape canes is comparable to wood; the slightly higher calorific value of red grapes is due to a higher content of carbon and lignin, and a lower content of carbohydrates compared to white grapes.
- -
- Grape pellets had a lower calorific value and very high amount of ash compared to the grape canes; thus, they are less suitable for energy use.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Dam, J.; Faaij, A.P.C.; Lewandowski, I.; Fischer, G. Biomass production potentials in Central and Eastern Europe under different scenarios. Biomass Bioenergy 2007, 31, 345–366. [Google Scholar] [CrossRef]
- Jobbágy, J.; Krištof, K.; Schmidt, A.; Križan, M.; Urbanovičová, O. Evaluation of the mechanized harvest of grapes with regards to harvest losses and economical aspects. Agron. Res. 2018, 16, 426–442. [Google Scholar]
- Maj, G.; Klimek, K.; Kapłan, M.; Wrzesińska-Jędrusiak, E. Using wood-based waste from grapevine cultivation for energy purposes. Energies 2022, 15, 890. [Google Scholar] [CrossRef]
- Spinelli, R.; Nati, C.; Pari, L.; Mescalchin, E.; Magagnotti, N. Production and quality of biomass fuels from mechanized collection and processing of vineyard pruning residues. Appl. Energy 2012, 89, 374–379. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Baydar, N.G. 2011. Chemical composition of grape canes. Ind. Crop. Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 25 August 2022).
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery wastes as precursors of sustainable porous carbons for environmental applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Slovakia Wine Guide: Traditional Regions & Grape Varieties. Available online: https://littlebigslovakia.com/does-slovakia-make-wine/ (accessed on 23 September 2022).
- Jobbágy, J.; Dočkalík, M.; Krištof, K.; Burg, P. Mechanized grape harvest efficiency. Appl. Sci. 2021, 11, 4621. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Escobar-Avello, D.; Ferreira, I.; Vieira, C.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. Grape canes (Vitis vinifera L.) applications on packaging and particleboard industry: New bioadhesive based on grape extracts and citric acid. Polymers 2022, 14, 1137. [Google Scholar] [CrossRef]
- Ferreyra, S.G.; Antoniolli, A.; Bottini, R.; Fontana, A. Bioactive compounds and total antioxidant capacity of cane residues from different grape varieties. J. Sci. Food Agric. 2020, 100, 376–383. [Google Scholar] [CrossRef]
- Gharwalová, L.; Hutár, D.; Masák, J.; Kolouchová, I. Antioxidant activity and phenolic content of organic and conventional vine cane extracts. Czech J. Food Sci. 2018, 36, 289–295. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Dávila, I.; Labidi, J.; Gonzalez-Garcia, S. Comparative environmental Life Cycle Assessment of integral revalorization of vine shoots from a biorefinery perspective. Sci. Total Environ. 2018, 624, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.; Yadollahi, A.; Ford, C.M.; Shojaeiyan, A.; Ayyari, M.; Hokmabadi, H. Chemodiversity evaluation of grape (Vitis vinifera) vegetative parts during summer and early fall. Ind. Crop. Prod. 2017, 108, 267–277. [Google Scholar] [CrossRef]
- Loupit, G.; Prigent, S.; Franc, C.; De Revel, G.; Richard, T.; Cookson, S.J.; Fonayet, J.V. Polyphenol profiles of just pruned grapevine canes from wild Vitis accessions and Vitis Vinifera cultivars. J. Agric. Food Chem. 2020, 68, 13397–13407. [Google Scholar] [CrossRef] [PubMed]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.D.; Fierascu, R.C. Grapevine wastes: A rich source of antioxidants and other biologically active compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Rodrigues, D.; Bochi, V.C.; Hermosín-Gutiérrez, I.; Godoy, H.T. Effect of drying methods on the phenolic content and antioxidant capacity of Brazilian winemaking by products and their stability over storage. Int. J. Food Sci. Nutr. 2015, 66, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Moreno-Rojas, J.M.; Mulero, J.; Puertas, B.; Giron, F.; Guerrero, R.F.; et al. Valorization of grape stems. Ind. Crop. Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.; Prozil, S.; Prozil, S.; Evtuguin, D.V.; Arshanitsa, A.; Solodovnik, V.; Telysheva, G.M. Evaluation of grape stalks as a feedstock for pellets production. In Proceedings of the 13th European Workshop on Lignocellulosics and Pulp, Seville, Spain, 24–27 June 2014; p. 4. [Google Scholar]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape stalk valorization for fermentation purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Basanta, R.; Delgado, M.A.G.; Martínez, J.E.C.; Vázquez, H.M.; Vázquez, G.B. Sustainability of waste recycling from the sugar agribusiness: A review. Cienc. Tecnol. Aliment. 2007, 5, 293–305. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; de Souza Oliveira, R.P.; Domínguez, J.M. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Biores. Bioproc. 2018, 5, 46. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood waste from fruit trees: Biomolecules and their applications in agri-food industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Hendrikse, S.I.S.; Sherrell, P.C.; Ellis, A.V. Grapevine waste in sustainable hybrid particleboard production. Waste Manag. 2020, 118, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, M.E.; Ibarra, D.; Martín-Sampedro, R.; Espinosa, E.; Bascón, I.; Rodríguez, A. Alternative raw materials for pulp and paper production in the concept of a lignocellulosic biorefinery. Cellulose 2019, 12, 78. [Google Scholar] [CrossRef]
- Egüés, I.; Serrano, L.; Amendola, D.; de Faveri, D.M.; Spigno, G.; Labidi, J. Fermentable sugars recovery from grape stalks for bioethanol production. Renew. Energy 2013, 60, 553–558. [Google Scholar] [CrossRef]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J. Changes in the grape cane stilbene content under various conditions of storage. ACS Sustain. Chem. Eng. 2019, 7, 19584–19590. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.M.; Richard, T. Antioxidant and cytoprotective activities of grapevine stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crop. Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Richard, T.; Cantos-Villar, E. Grapevine cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit juice industry wastes as a source of bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Tomás-Barberán, F.A. Introduction to novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6785–6786. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Shi, X.; He, F.; Wu, T.; Jiang, L.; Normakhamatov, N.; Sharipov, A.; Wang, T.; Wen, M.; Aisa, H.A. Valorization of food processing waste to produce valuable polyphenolics. J. Agric. Food Chem. 2022, 70, 8855–8870. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory: Golden, CO, USA, 2021. Available online: http://www.nrel.gov/biomass/analytical_procedures.html (accessed on 20 February 2021).
- Feng, X.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar]
- ISO 1928; Solid Mineral Fuels. Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Net Calorific Value. Bristish Standards Institution: London, UK, 2020.
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crop. Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Spigno, G.; Maggi, L.; Amendola, D.; Dragoni, M.; De Faveri, D. Influence of cultivar on the lignocellulosic fractionation of grape stalks. Ind. Crop. Prod. 2013, 46, 283–289. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT–Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Briones, R.; Torres, L.; Saravia, Y.; Serrano, L.; Labidi, J. Liquefied agricultural residues for film elaboration. Ind. Crop. Prod. 2015, 78, 19–28. [Google Scholar] [CrossRef]
- Sun, X.-F.; Jing, Z.; Fowler, P.; Wu, Y.; Rajaratnam, M. Structural characterization and isolation of lignin and hemicelluloses from barley straw. Ind. Crop. Prod. 2011, 33, 588–598. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crop. Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Bhagia, S.; Ďurkovič, J.; Lagaňa, R.; Kardošová, M.; Kačík, F.; Cernescu, A.; Schäfer, P.; Yoo, C.G.; Ragauskas, A.J. Nanoscale FTIR and Mechanical Mapping of Plant Cell Walls for Understanding Biomass Deconstruction. ACS Sustain. Chem. Eng. 2022, 10, 3016–3026. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. 2021, X, 100168. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, A.; Casquero, P.A.; Carro-Huerga, G.; García-González, J.; Álvarez-García, S.; Juan-Valdés, A. Failure under stress of grapevine wood: The effects of the cerambycid Xylotrechus arvicola on the biomechanics properties of Vitis vinifera. Maderas Cienc. Tecnol. 2020, 22, 167–178. [Google Scholar] [CrossRef]
- Aicher, S.; Stapf, G. Compressive strength parallel to the fiber of spruce with high moisture content. Eur. J. Wood Prod. 2016, 74, 527–542. [Google Scholar] [CrossRef]
- Ntalos, G.A.; Grigoriou, A.H. Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Ind. Crop. Prod. 2002, 16, 59–68. [Google Scholar] [CrossRef]
- de Assis, T.; Reisinger, L.; Dasmohapatra, S.; Pawlak, J.; Jameel, H.; Pal, L.; Kavalew, D.; Gonzalez, R. Performance and sustainability vs. the shelf price of tissue paper kitchen towels. Bioresources 2018, 13, 6868–6892. [Google Scholar] [CrossRef]
- Niskanen, K. Paper Physics, Book 16, Papermaking Science and Technology, a Series of 19 Books; Fapet Oy: Helsinki, Finland, 1998; 324 p. [Google Scholar]
- de Assis, T.; Pawlak, J.; Pal, L.; Jameel, H.; Venditti, R.; Reisinger, L.W.; Kavalew, D.; Gonzalez, R.W. Comparison of wood and non-wood market pulps for tissue paper application. Bioresources 2019, 14, 6781–6810. [Google Scholar] [CrossRef]
- Salminen, L.I.; Liukkonen, S.; Alava, M.J. Ground wood fiber length distributions. Bioresources 2014, 9, 1168–1178. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood, Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2003; 613 p. [Google Scholar]
- Ferreira, P.J.; Matos, S.; Figueiredo, M.M. Size characterization of fibres and fines in hardwood kraft pulps. Part. Part. Syst. Charact. 1999, 16, 20–24. [Google Scholar] [CrossRef]
- Čabalová, I.; Bélik, M.; Kučerová, V.; Jurczyková, T. Chemical and morphological composition of Norway spruce wood (Picea abies L.) in the dependence of its storage. Polymers 2021, 13, 1619. [Google Scholar] [CrossRef]
- Stankovská, M.; Gigac, J.; Fišerová, M.; Opálená, E. Relationship between structural parameters and water absorption of bleached softwood and hardwood kraft pulps. Wood Res. 2019, 64, 261–272. [Google Scholar]
- Ferdous, T.; Ni, Y.; Quaiyyum, M.A.; Uddin, M.N.; Jahan, M.S. Non-Wood Fibers: Relationships of Fiber Properties with Pulp Properties. ACS Omega 2021, 6, 21613–21622. [Google Scholar] [CrossRef] [PubMed]
- Fišerová, M.; Gigac, J.; Balberčák, J. Relationship between fibre characteristics and tensile strength of hardwood and softwood kraft pulps. Cell. Chem. Technol. 2009, 44, 249–253. [Google Scholar]
- Oluwadare, A.O.; Ashimiyu, O.S. The relationship between fibre characteristics and pulp-sheet properties of Leucaenaleucocephala (Lam.) De Wit. Middle-East. J. Sci. Res. 2007, 2, 63–68. [Google Scholar]
- Molteberg, D.; Høibø, O. Development and variation of wood density, kraft pulp yield and fibre dimension in young Norway spruce (Picea abies). Wood Sci. Technol. 2006, 40, 173–189. [Google Scholar] [CrossRef]
- Mohlin, U.B.; Dahlbom, J.; Hornatowska, J. Fibre deformation and sheet strength. Tappi J. 1996, 79, 105–111. [Google Scholar]
- Olisa, Y.P.; Ajoko, T.J. Gross calorific value of combustible solid waste in a mass burn incineration plant, Benin City, Nigeria. J. Air Waste Manag. Assoc. 2018, 22, 1377–1380. [Google Scholar] [CrossRef]
- Lunguleasa, A.; Spirchez, C.; Zeleniuc, O. Evaluation of the calorific values of wastes from some tropical wood species. Maderas Cienc. Tecnol. 2020, 22, 269–280. [Google Scholar] [CrossRef]
- Lieskovský, M.; Jankovský, M.; Trenčiansky, M.; Merganič, J.; Dvořák, J. Ash content vs. the economics of using wood chips for energy: Model based on data from central Europe. Bioresources 2017, 12, 1579–1592. [Google Scholar] [CrossRef]
- Günther, B.; Gebauer, K.; Barkowski, R.; Rosenthal, M.; Bues, C.T. Calorific value of selected wood species and wood products. Eur. J. Wood Wood Prod. 2012, 70, 755–757. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Burg, P.; Ludín, D.; Rutkowski, K.; Krakowiak-Bal, A.; Trávníček, P.; Zemánek, P.; Turan, J.; Višacki, V. Calorific evaluation and energy potential of grape pomace. Int. Agrophys. 2016, 30, 261–265. [Google Scholar] [CrossRef]
- EN ISO 17225-1; Solid Biofuels-Fuel Specifications and Classes-Part 1: General Requirements. ISO: Geneva, Switzerland, 2021.
- Chandrasekaran, S.R.; Hopke, P.K.; Rector, L.; Allen, G.; Lin, L. Chemical Composition of Wood Chips and Wood Pellets. Energy Fuels 2012, 26, 4932–4937. [Google Scholar] [CrossRef]
- STN ISO 10694; Soil Quality. Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). ISO: Geneva, Switzerland, 2001.
- ISO 13878; Soil Quality. Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis). ISO: Geneva, Switzerland, 1998.
- ISO 11885; Water Quality. Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
| Sample | White | Red |
|---|---|---|
| EX-W | 16.65 ± 0.11 | 16.18 ± 0.25 |
| EX-E | 1.63 ± 0.01 | 1.39 ± 0.01 |
| EX-SUM | 18.28 ± 0.13 | 17.57 ± 0.26 |
| LIG | 20.15 ± 0.77 | 20.57 ± 0.02 |
| GLC | 32.81 ± 0.86 | 34.26 ± 0.15 |
| XYL | 13.24 ± 0.40 | 11.52 ±0.97 |
| GAL | 3.04 ± 0.20 | 2.89 ± 0.06 |
| ARA | 3.06 ± 0.13 | 2.73 ± 0.08 |
| MAN | 3.13 ± 0.15 | 2.80 ± 0.35 |
| SACCHARIDES | 55.27 ± 1.44 | 54.19 ± 0.74 |
| ASH | 0.94 ± 0.13 | 1.54 ± 0.16 |
| N | Mean (MPa) | Min (MPa) | Max (MPa) | Var. (%) | |
|---|---|---|---|---|---|
| Red | 39 | 37.34 | 27.34 | 42.97 | 10.01 |
| White | 37 | 32.34 | 22.77 | 38.93 | 10.80 |
| All | 76 | 34.90 | 12.57 |
| No. | Sample of Grape Cuttings/Element | White | Red | Pellets | Ash from Pellets | Used Method | Principle |
|---|---|---|---|---|---|---|---|
| 1. | Carbon (g/kg) | 455 | 462 | 454 | 75 | STN ISO 10684 [72] | EA-TCD |
| 2. | Nitrogen (g/kg) | 6.61 | 5.98 | 6.52 | 2.75 | ISO 13878 [73] | |
| 3. | Phosphorus (g/kg) | 1.15 | 1.07 | 1.01 | 5.85 | ISO 11885 [74] | AES-ICP |
| 4. | Calcium (mg/kg) | 5698 | 9651 | 6892 | 48,943 | ||
| 5. | Magnesium (mg/kg) | 1592 | 2003 | 2234 | 12,649 | ||
| 6. | Potassium (mg/kg) | 3277 | 4023 | 3588 | 7396 | ||
| 7. | Sodium (mg/kg) | 103 | 94.50 | 87.30 | 2142 | ||
| 8. | Iron (mg/kg) | 183 | 804 | 1857 | 5802 | ||
| 9. | Manganese (mg/kg) | 27.60 | 40.80 | 44.10 | 215 | ||
| 10. | Zinc (mg/kg) | 26.70 | 56.20 | 23.60 | 39.60 | ||
| 11. | Aluminum (mg/kg) | 37 | 123 | 379 | 3125 | ||
| 12. | Boron (mg/kg) | 12.60 | 15.60 | 15.40 | 106 | ||
| 13. | Copper (mg/kg) | 9.39 | 22.30 | 10.80 | 19.70 | ||
| 14. | Silicon (mg/kg) | 90 | 291 | 576 | 88.80 | ||
| 15. | Chrome (mg/kg) | 2.15 | 2.76 | 12.80 | 73.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čabalová, I.; Krilek, J.; Kačík, F.; Lagaňa, R.; Jurczyková, T. Valorization of Wood-Based Waste from Grapevine. Forests 2023, 14, 442. https://doi.org/10.3390/f14030442
Čabalová I, Krilek J, Kačík F, Lagaňa R, Jurczyková T. Valorization of Wood-Based Waste from Grapevine. Forests. 2023; 14(3):442. https://doi.org/10.3390/f14030442
Chicago/Turabian StyleČabalová, Iveta, Jozef Krilek, František Kačík, Rastislav Lagaňa, and Tereza Jurczyková. 2023. "Valorization of Wood-Based Waste from Grapevine" Forests 14, no. 3: 442. https://doi.org/10.3390/f14030442
APA StyleČabalová, I., Krilek, J., Kačík, F., Lagaňa, R., & Jurczyková, T. (2023). Valorization of Wood-Based Waste from Grapevine. Forests, 14(3), 442. https://doi.org/10.3390/f14030442









