Impacts of Different Reforestation Methods on Fungal Community and Nutrient Content in an Ex-Tea Plantation
Abstract
1. Introduction
2. Materials and Methods
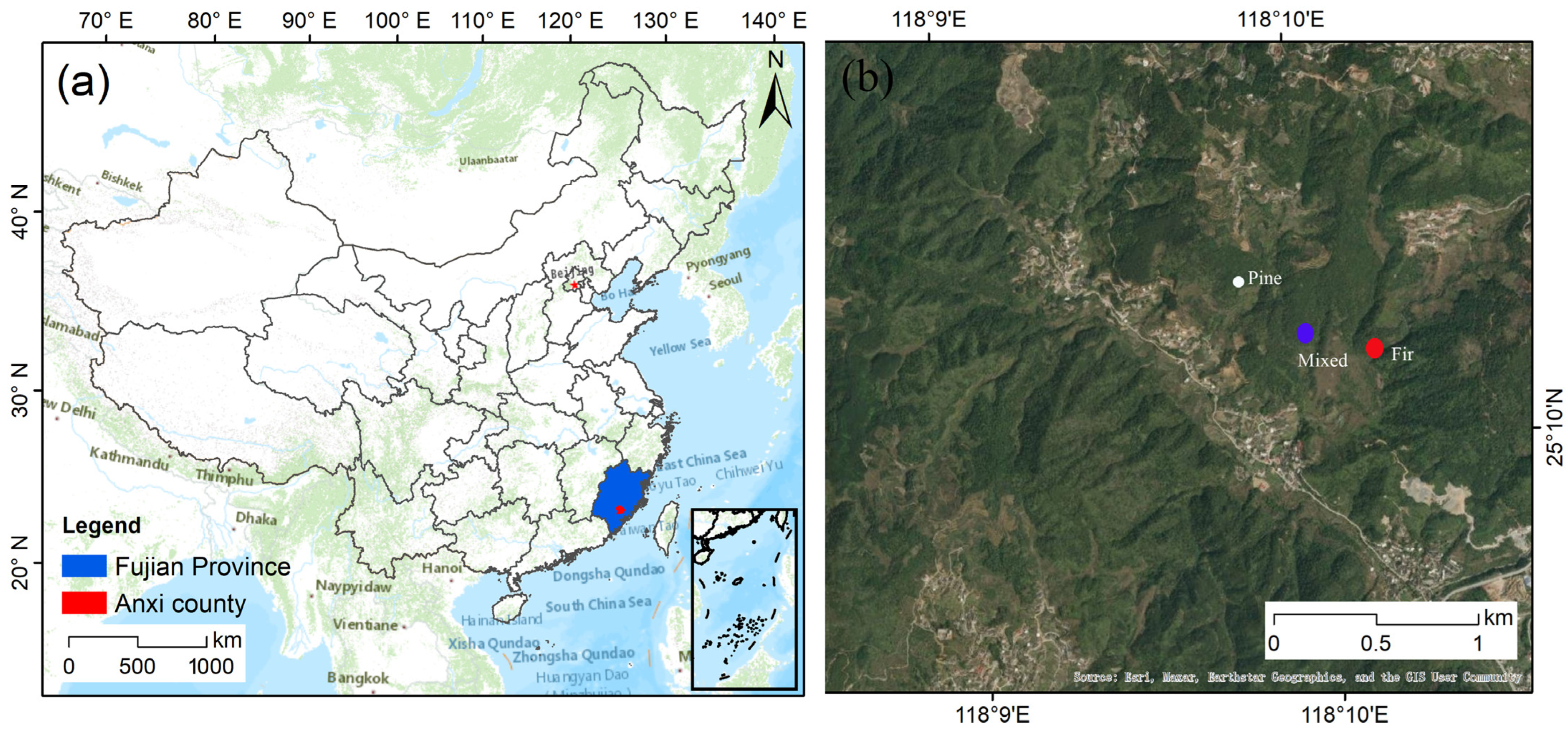
2.1. Study Area
2.2. Experimental Design and Soil Sampling
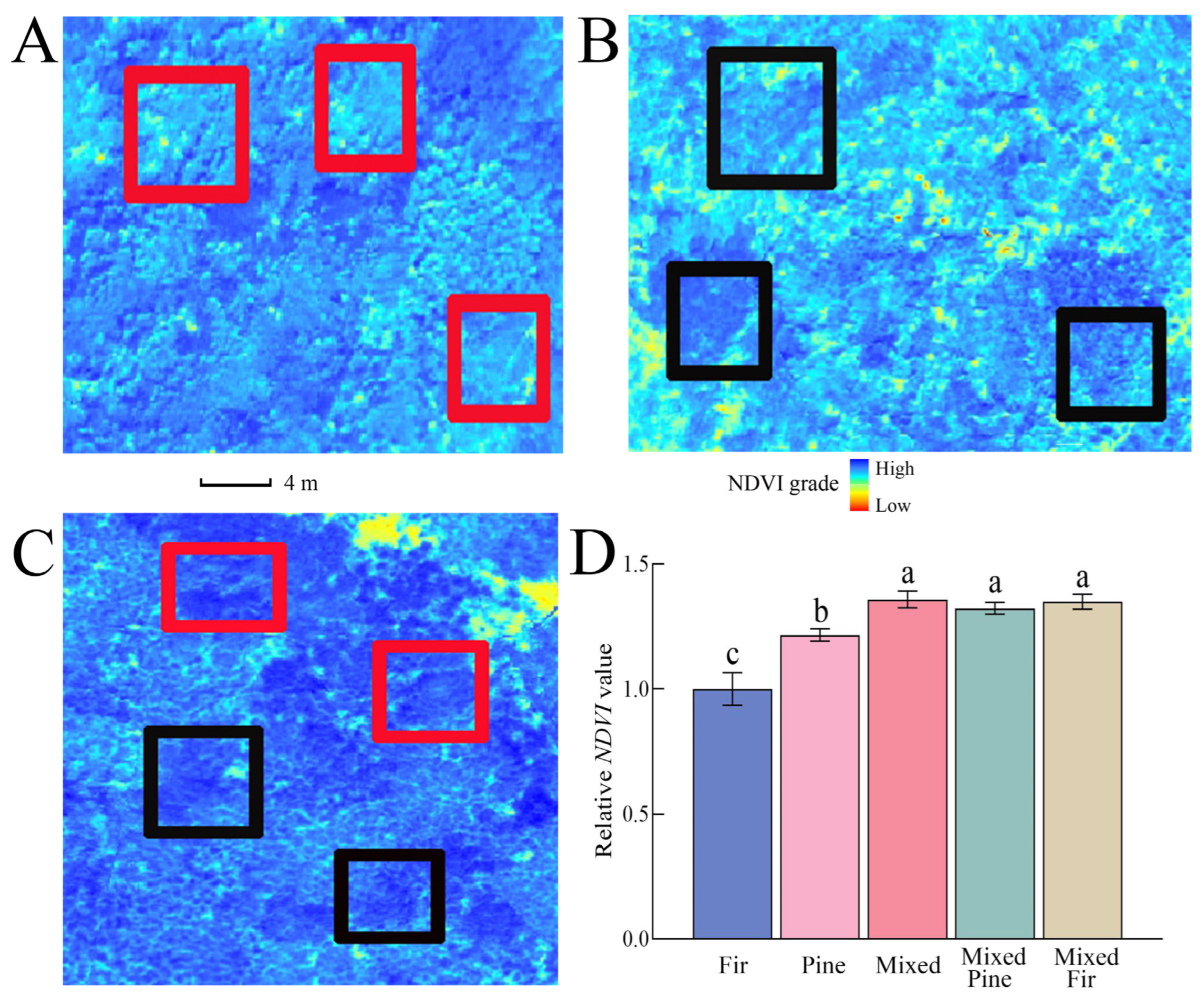
2.3. Unmanned Aerial Vehicle Image Acquisition and Processing
2.4. Analysis of Soil Chemical Properties
2.5. Soil Fungal DNA Extraction and Sequencing
2.6. Statistical Analysis
3. Results
3.1. NDVI under Different Planting Modes
3.2. Soil Chemical Properties
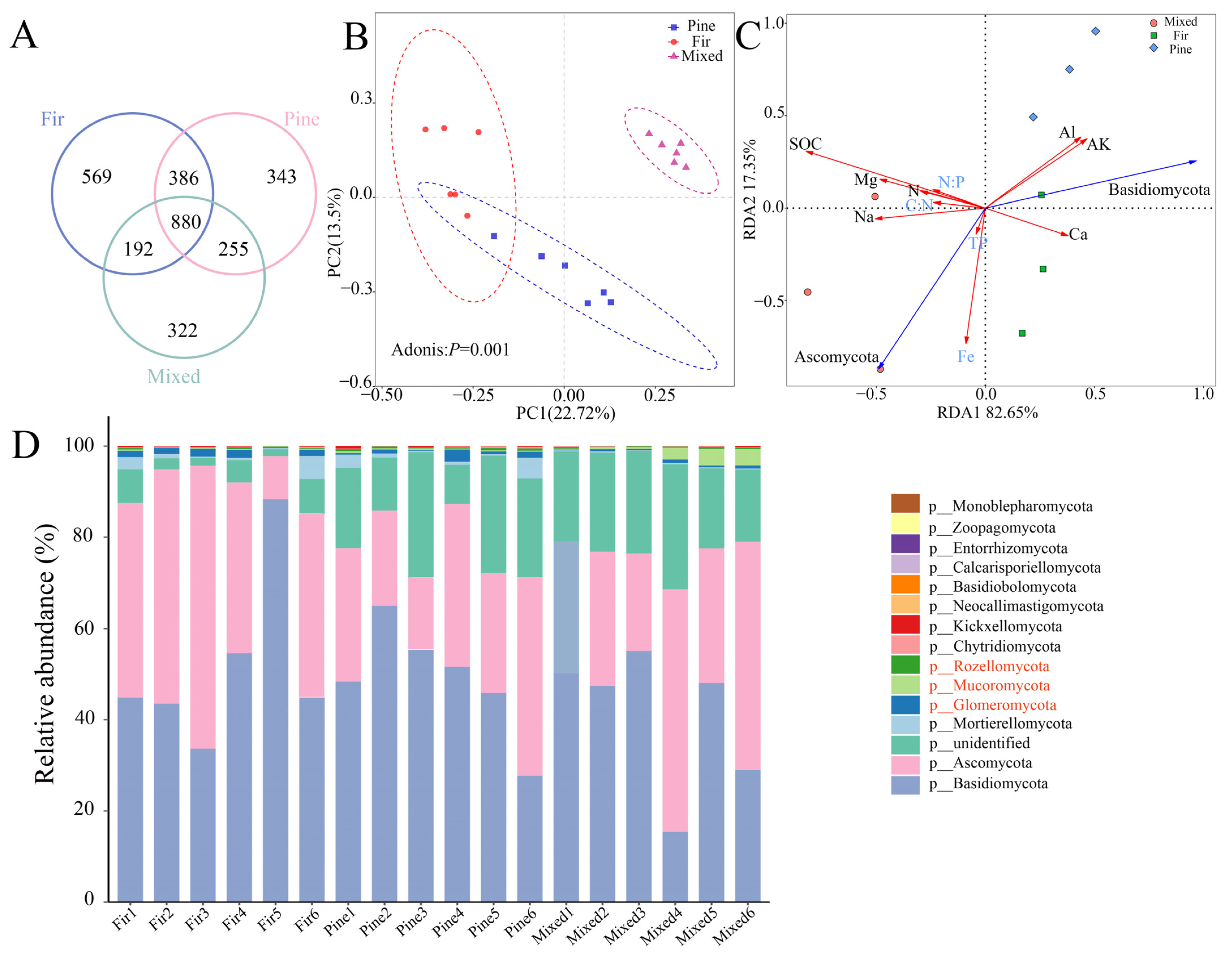
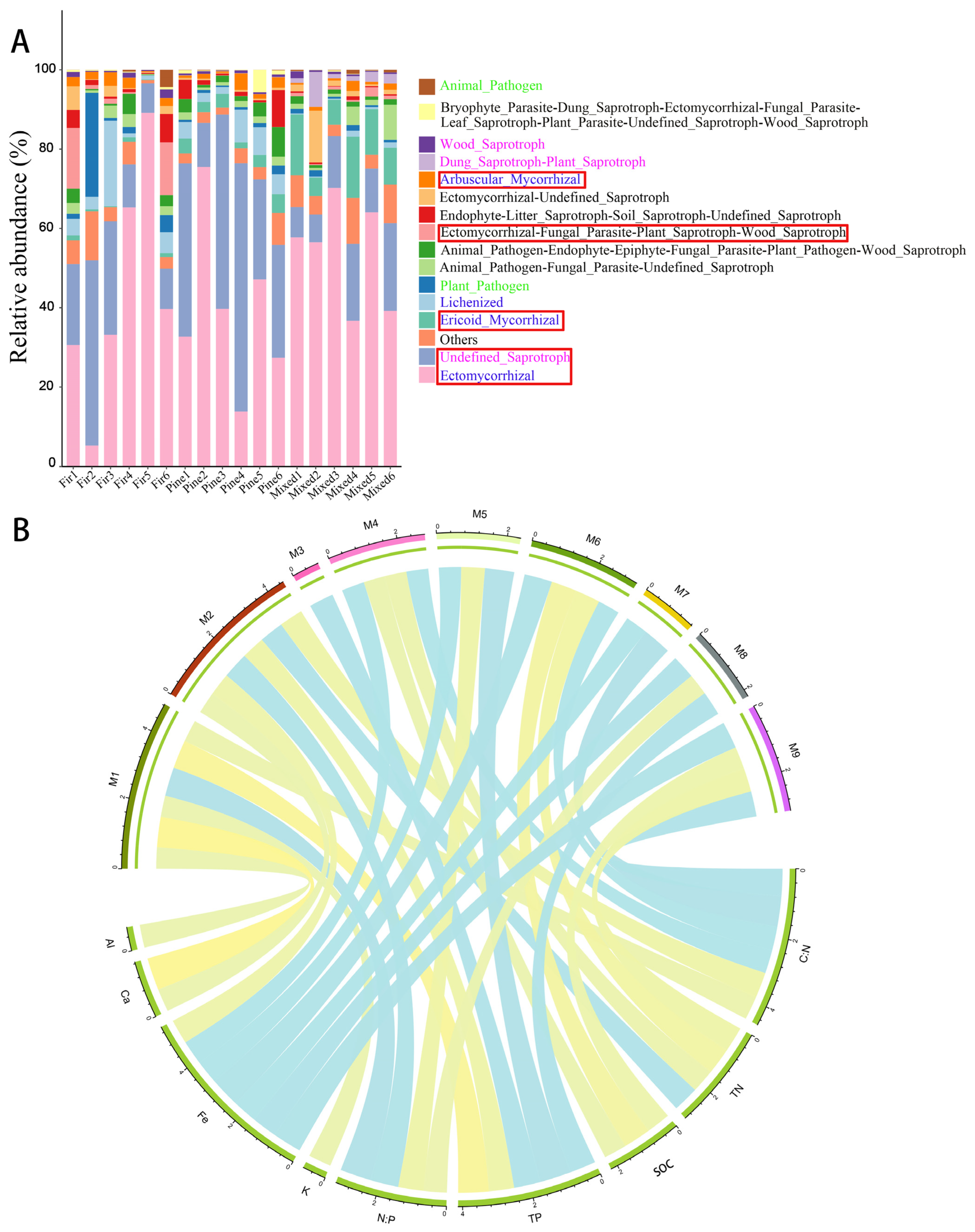
3.3. Diversity and Structure of Fungal Communities in Different Forests
3.4. Correlations between Fungal Community Composition and Soil Chemical Properties
4. Discussion
4.1. Variations in Soil Chemical Properties
4.2. Variations in Fungal Diversity, Community Composition, and Functional Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manawasinghe, I.S.; Jayawardena, R.S.; Li, H.L.; Zhou, Y.Y.; Zhang, W.; Phillips, A.J.L.; Wanasinghe, D.N.; Dissanayake, A.J.; Li, X.H.; Li, Y.H.; et al. Microfungi associated with Camellia sinensis: A case study of leaf and shoot necrosis on tea in Fujian, China. Mycosphere 2021, 12, 430–518. [Google Scholar] [CrossRef]
- UNFAO. The Food and Agriculture Organization Corporate Statistical Database (FAOSTAT); Food and Agriculture Organization of the United Nations (UNFAO): Rome, Italy, 2019. [Google Scholar]
- Bureau of Statistics. Fujian Statistical Yearbook 2019; Bureau of Statistics of Fujian Province: Fuzhou, China, 2019. [Google Scholar]
- Guo, L.; Du, Z.H.; Wang, Z.; Lin, Z.; Guo, Y.L.; Chen, M.J. Location affects fatty acid composition in Camellia sinensis cv Tieguanyin fresh leaves. J. Food Sci. Technol. 2020, 57, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Zhang, G.L.; Chu, X.B.; Zhu, H.Y.; Zou, D.S.; Li, L.C.; Du, L.S. The response of soil nutrients and microbial community structures in long-term tea plantations and diverse agroforestry intercropping systems. Sustainability 2021, 13, 7799. [Google Scholar] [CrossRef]
- Gao, S.L.; Hu, S.S.; He, P.; Feng, K.; Pan, R.Y.; Zhang, S.A.; Guo, B.; Lee, T.C.; Lin, J.K. Effects of reducing chemical fertilizer on the quality components of Tieguanyin tea leaves. IOP Conf. Ser. Earth Environ. Sci. 2020, 559, 012020. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.W.; Arafat, Y.; Lin, W.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Characterizing rhizosphere microbial communities in long-term monoculture tea orchards by fatty acid profiles and substrate utilization. Eur. J. Soil Biol. 2017, 81, 48–54. [Google Scholar] [CrossRef]
- Li, N.; Zhang, D.; Li, L.W.; Zhang, Y.L. Mapping the spatial distribution of tea plantations using high-spatiotemporal-resolution imagery in northern Zhejiang, China. Forests 2019, 10, 856. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Fitter, A.H. Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: Growth responses of the host plant and its AM fungal partner. J. Exp. Bot. 2004, 55, 525–534. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Sophie, M.; Richard, F.; Annie, D.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Dighton, J. Fungi in Ecosystem Processes; CRC Press: New York, NY, USA, 2018; pp. 21–77. [Google Scholar]
- He, J.Y.; Jiang, X.H.; Lei, Y.X.; Cai, W.J.; Zhang, J.J. Temporal and spatial variation and driving forces of soil erosion on the Loess Plateau before and after the implementation of the Grain-for-Green project: A case study in the Yanhe river basin, China. Int. J. Environ. Res. Publish Health 2022, 19, 8446. [Google Scholar] [CrossRef]
- Xie, S.W.; Feng, H.X.; Yang, F.; Zhao, Z.D.; Hu, X.D.; Wei, C.Y.; Liang, T.; Li, H.T.; Geng, Y.B. Does dual reduction in chemical fertilizer and pesticides improve nutrient loss and tea yield and quality? A pilot study in a green tea garden in Shaoxing, Zhejiang Province, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 2464–2476. [Google Scholar] [CrossRef]
- Dong, M.F.; Kai, F.; Peng, L.; Xiao, C.W. Ecological no-tillage technology of sloping tea garden in China. J. Korean Tea Soc. 2015, 21, 67–71. [Google Scholar]
- Duan, Y.; Shang, X.W.; Liu, G.D.; Zou, Z.W.; Zhu, X.J.; Ma, Y.C.; Li, F.; Fang, W.P. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant Biol. 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.S.; Xia, D.; Cai, C.F.; Ding, S.W. Effects of land uses on soil physic-chemical properties and erodibility in collapsing-gully alluvial fan of Anxi County, China. J. Integr. Agric. 2016, 15, 1863–1873. [Google Scholar] [CrossRef]
- Chang, Y.X.; Zou, T.H.; Yoshino, K.H.; Luo, S.Z.; Zhou, S.G. Ecological policy benefit valuation based on public feedback: Forest ecosystem services in Wuyishan nature reserve, China. Sci. Total Environ. 2019, 673, 622–630. [Google Scholar] [CrossRef]
- Nunes, S.; Gastauer, M.; Cavalcante, R.B.L.; Ramos, S.J.; Caldeira, C.F.; Silva, D.; Rodrigues, R.R.; Salomão, R.; Oliveira, M.; Souza-Filho, P.W.M.; et al. Challenges and opportunities for large-scale reforestation in the Eastern Amazon using native species. For. Ecol. Manag. 2020, 466, 118120. [Google Scholar] [CrossRef]
- Yang, G.J.; Liu, J.G.; Zhao, C.J.; Li, Z.H.; Huang, Y.B.; Yu, H.Y.; Xu, B.; Yang, X.D.; Zhu, D.M.; Zhang, X.Y.; et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Huete, A.R. Vegetation indices, remote sensing and forest monitoring. Geogr. Compass 2012, 6, 513–532. [Google Scholar] [CrossRef]
- Tucker, C.J.; Fung, I.Y.; Keeling, C.D.; Gammon, R.H. Relationship between atmospheric CO2 variations and a satellite-derived vegetation index. Nature 1986, 319, 195–199. [Google Scholar] [CrossRef]
- Xu, H.D.; Yu, M.K.; Cheng, X.R. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Zou, X.D.; Liang, A.J.; Wu, B.Z.; Su, J.; Zheng, R.H.; Li, J. UAV-based high-throughput approach for fast growing Cunninghamia lanceolata (Lamb.) cultivar screening by machine learning. Forests 2019, 10, 815. [Google Scholar] [CrossRef]
- Ashfaq, A.; Majid, H.; Saqib, A.; Kashif, A.; Muhammad, M.W.; Zamir, A.; Arshad, A.; Moazzam, N.S.; Bilal, A.; Harrison, M.T.; et al. Ecological stoichiometry in Pinus massoniana L. plantation: Increasing nutrient limitation in a 48-year chronosequence. Forests 2022, 13, 469. [Google Scholar]
- Zhang, B.; Wang, H.L.; Yao, S.H.; Bi, L.D. Litter quantity confers soil functional resilience through mediating soil biophysical habitat and microbial community structure on an eroded bare land restored with mono Pinus massoniana. Soil Biol. Biochem. 2013, 57, 556–567. [Google Scholar] [CrossRef]
- Hou, X.L.; Han, H.; Mulualem, T.; Cai, L.P.; Meng, F.R.; Liu, A.Q.; Ma, X.Q. Changes in soil physico-chemical properties following vegetation restoration mediate bacterial community composition and diversity in Changting, China. Ecol. Eng. 2019, 138, 171–179. [Google Scholar] [CrossRef]
- Cao, S.; Hu, R.Y.; Wu, X.L.; Sun, Y.H.; Wu, B.; Duan, H.J.; Lin, H.Z.; Wu, M.J.; Fang, L.M.; Yu, X.L.; et al. Two chemical mutagens modulate the seed germination, growth, and phenotypic characteristics of Chinese fir (Cunninghamia lanceolata). J. For. Res. 2021, 32, 2077–2085. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Jiao, P.Y.; Guo, W.; Du, D.J.; Hu, Y.L.; Tan, X.; Liu, X. Changes in bulk and rhizosphere soil microbial diversity and composition along an age gradient of Chinese fir (Cunninghamia lanceolata) plantations in subtropical China. Front. Microbiol. 2022, 12, 777862. [Google Scholar] [CrossRef]
- Bao, T.; Deng, S.L.; Yu, K.Y.; Li, W.Y.; Dong, A.R. Metagenomic insights into seasonal variations in the soil microbial community and function in a Larix gmelinii forest of Mohe, China. J. For. Res. 2021, 32, 371–383. [Google Scholar] [CrossRef]
- Tao, X.Y.; Li, Y.J.; Yan, W.Q.; Wang, M.J.; Tan, Z.F.; Jiang, J.M.; Luan, Q.F. Heritable variation in tree growth and needle vegetation indices of slash pine (Pinus elliottii) using unmanned aerial vehicles (UAVs). Ind. Crops Prod. 2021, 173, 114073. [Google Scholar] [CrossRef]
- Wu, B.Z.; Liang, A.J.; Zhang, H.F.; Zhu, T.F.; Zou, Z.Y.; Yang, D.M.; Tang, W.Y.; Li, J.; Su, J. Application of conventional UAV-based high-throughput object detection to the early diagnosis of pine wilt disease by deep learning. For. Ecol. Manag. 2021, 486, 118986. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhu, W.Q.; Xie, Z.Y.; Zhan, P.; Huang, X.; Sun, L.X.; Duan, Z. Assessing the effects of time interpolation of NDVI composites on phenology trend estimation. Remote Sens. 2021, 13, 5018. [Google Scholar] [CrossRef]
- Cai, S.P.; Jia, J.Y.; He, C.Y.; Zeng, L.Q.; Fang, Y.; Qiu, G.W.; Lan, X.; Su, J.; He, X.Y. Multi-omics of pine wood nematode pathogenicity associated with culturable associated microbiota through an artificial assembly approach. Front. Plant Sci. 2022, 12, 798539. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package (R Package Version 2.6-4). 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 February 2023).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research (R package version 2.2.9); Northwestern University: Evanston, IL, USA, 2022; Available online: https://CRAN.R-project.org/package=psych (accessed on 3 February 2023).
- Schratz, P.; Joseph, M.; Gelfand, S. Circle: R Client Package for Circle CI (R package version 0.7.2). 2022. Available online: https://CRAN.R-project.org/package=circle (accessed on 3 February 2023).
- Xu, P.; Yang, L.Y.; Liu, M.C.; Peng, F. Soil Characteristics and Nutrients in Different Tea Garden Types in Fujian Province, China. J. Resour Ecol. 2014, 5, 356–363. [Google Scholar]
- González, I.; Sixto, H.; Rodríguez-Soalleiro, R.; Oliveira, N. Nutrient contribution of litterfall in a short rotation plantation of pure or mixed plots of Populus alba L. and Robinia pseudoacacia L. Forests 2020, 11, 1133. [Google Scholar] [CrossRef]
- Zhang, W.W.; Liu, W.; He, S.W.; Chen, Q.C.; Han, J.G.; Zhang, Q.F. Mixed plantations of Metasequoia glyptostroboides and Bischofia polycarpa change soil fungal and archaeal communities and enhance soil phosphorus availability in Shanghai, China. Ecol. Evol. 2021, 11, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Niu, Y.B.; Xun, M.Y.; Jin, J.Y.; Tang, Y.K.; Chen, Y.M. Soil carbon, nitrogen, and phosphorus storages and their stoichiometry due to mixed afforestation with Hippophae rhamnoides in the Loess Hilly Region, China. Forests 2021, 12, 1718. [Google Scholar] [CrossRef]
- Feudis, M.D.; Cardelli, V.; Massaccesi, L.; Lagomarsino, A.; Fornasier, F.; Westphalen, D.J.; Cocco, S.; Corti, G.; Alberto Agnelli, A. Influence of altitude on biochemical properties of European beech (Fagus sylvatica L.) forest soils. Forests 2017, 8, 213. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Vernal nitrogen and phosphorus retention by forest understory vegetation and soil microbes. Plant Soil 2003, 256, 443–453. [Google Scholar] [CrossRef]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Jerry, M.M.; Charles, A.S.H. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Hu, Y.L.; Wang, S.L.; Zeng, D.H. Effects of single Chinese fir and mixed leaf litters on soil chemical, microbial properties and soil enzyme activities. Plant Soil 2006, 282, 379–386. [Google Scholar] [CrossRef]
- Versini, A.; Mareschal, L.; Matsoumbou, T.; Zeller, B.; Ranger, J.; Laclau, J.P. Effects of litter manipulation in a tropical Eucalyptus plantation on leaching of mineral nutrients, dissolved organic nitrogen and dissolved organic carbon. Geoderma 2014, 233, 426–436. [Google Scholar] [CrossRef]
- Ali, J.; Jewel, Z.A.; Mahender, A.; Anandan, A.; Hernandez, J.; Li, Z. Molecular Genetics and Breeding for Nutrient Use Efficiency in Rice. Int. J. Mol. Sci. 2018, 19, 1762. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Xiang, W.H.; Ouyang, S.A.; Xiao, W.F.; Li, S.G.; Chen, L.; Lei, P.F.; Deng, X.W.; Zeng, Y.L.; Zeng, L.X.; et al. Tree growth rate and soil nutrient status determine the shift in nutrient-use strategy of Chinese fir plantations along a chronosequence. For. Ecol. Manag. 2020, 460, 117896. [Google Scholar] [CrossRef]
- Liu, B.T.; Han, F.; Ning, P.; Li, H.B.; Rengel, Z. Root traits and soil nutrient and carbon availability drive soil microbial diversity and composition in a northern temperate forest. Plant Soil 2022, 479, 281–299. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.T.; Kim, Y.; Tong, Z.K.; Zhang, J.H. The introduction of Phoebe bournei into Cunninghamia lanceolata monoculture plantations increased microbial network complexity and shifted keystone taxa. For. Ecol. Manag. 2022, 509, 120072. [Google Scholar] [CrossRef]
- Chen, K.Y.; Hu, L.; Wang, C.T.; Yang, W.G.; Zi, H.B.; Lerdau, M. Herbaceous plants influence bacterial communities, while shrubs influence fungal communities in subalpine coniferous forests. For. Ecol. Manag. 2021, 500, 119656. [Google Scholar] [CrossRef]
- Hartmann, M.; Widmer, F. Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl. Environ. Microb. 2006, 72, 7804–7812. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.Z.; Shi, Z.; Wang, S.N.; Wang, Z.T.; Liu, Y.; Wen, X.X.; Mo, F.; Han, J.; Liao, Y.C. Crop development has more influence on shaping rhizobacteria of wheat than tillage practice and crop rotation pattern in an arid agroecosystem. Appl. Soil Ecol. 2021, 165, 104016. [Google Scholar] [CrossRef]
- Bastida, F.; Hernández, T.; Albaladejo, J.; García, C. Phylogenetic and functional changes in the microbial community of long-term restored soils under semiarid climate. Soil Biol. Biochem. 2013, 65, 12–21. [Google Scholar] [CrossRef]
- Bahadori, M.; Wang, J.T.; Shen, J.P.; Lewis, S.; Rezaei Rashti, M.; Chen, C.R. Soil organic matter and geochemical characteristics shape microbial community composition and structure across different land uses in an Australian wet tropical catchment. Land Degrad Dev. 2022, 33, 817–831. [Google Scholar] [CrossRef]
- Shang, R.G.; Li, S.F.; Huang, X.B.; Liu, W.D.; Lang, X.D.; Su, J.R. Effects of soil properties and plant diversity on soil microbial community composition and diversity during secondary succession. Forests 2021, 12, 805. [Google Scholar] [CrossRef]
- Cheng, H.T.; Zhou, X.H.; Dong, R.S.; Wang, X.M.; Liu, G.D.; Li, Q.F. Priming of soil organic carbon mineralization and its temperature sensitivity in response to vegetation restoration in a karst area of Southwest China. Sci. Total Environ. 2022, 851, 158400. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.N.; Li, Q.; Yan, J.H.; Liu, C.; Zhong, J.X. Vegetation restoration facilitates belowground microbial network complexity and recalcitrant soil organic carbon storage in southwest China karst region. Sci. Total Environ. 2022, 820, 153137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Pan, Y.P.; Liu, Y.; Li, M. High-level diversity of basal fungal lineages and the control of fungal community assembly by stochastic processes in mangrove sediments. Appl. Environ. Microb. 2021, 87, e00928-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wang, N.; Zhang, J.Y.; Hu, Y.B.; Cai, D.J.; Guo, J.H.; Wu, D.; Sun, G.Y. Soil physicochemical properties and the rhizosphere soil fungal community in a mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. Forests 2019, 10, 167. [Google Scholar] [CrossRef]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The influence of phosphorus source and the nature of nitrogen substrate on the biomass production and lipid accumulation in oleaginous Mucoromycota fungi. Appl. Microbiol. Biot. 2020, 104, 8065–8076. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008; pp. 145–187. [Google Scholar]
- Mensah, J.A.; Koch, A.M.; Antunes, P.M.; Kiers, E.T.; Hart, M.; Heike, B. High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 2015, 25, 533–546. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential use of beneficial microorganisms for soil amelioration, phytopathogen biocontrol, and sustainable crop production in smallholder agroecosystems. Front. Sustain. Food Syst. 2021, 5, 606306. [Google Scholar] [CrossRef]
| Forest Type | Tree Species | Stand Age (years) | Elevation/m | Average DBH (cm) | Average Tree Height (m) | Density (ha−1) |
|---|---|---|---|---|---|---|
| Fir | Cunninghamia lanceolata | 27 | 500 | 19.6 | 13.6 | 1604 |
| Pine | Pinus massoniana | 23 | 555 | 12.9 | 14.2 | 1689 |
| Mixed | Pinus massoniana Cunninghamia lanceolata | 24 | 650 | 25.3 | 17.0 | 1868 |
| SOC (g kg−1) | TN (g kg−1) | C:N Ratio | N:P Ratio | TP (g kg−1) | AK (mg kg−1) | |
|---|---|---|---|---|---|---|
| Fir | 11.8 ± 3.11 b | 0.87 ± 0.32 b | 13.9 ± 1.48 a | 1.87 ± 0.46 b | 0.46 ± 0.05 a | 0.10 ± 0.02 ab |
| Pine | 19.2 ± 1.90 a | 1.54 ± 0.06 a | 12.4 ± 1.13 ab | 4.28 ± 0.34 b | 0.36 ± 0.04 b | 0.15 ± 0.07 a |
| Mixed | 18.1 ± 3.83 a | 1.64 ± 0.44 a | 11.2 ± 1.02 b | 7.46 ± 2.07 a | 0.22 ± 0.03 c | 0.04 ± 0.03 b |
| TG1 [17] | 13.33 | 1.0 | / | / | 0.6 | 0.18 |
| TG2 [41] | 6.3–11.4 | 0.18–0.57 | / | / | 0.15–0.27 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, A.; Wen, X.; Yu, W.; Su, S.; Lin, Y.; Fan, H.; Su, J.; Wu, C. Impacts of Different Reforestation Methods on Fungal Community and Nutrient Content in an Ex-Tea Plantation. Forests 2023, 14, 432. https://doi.org/10.3390/f14020432
Liang A, Wen X, Yu W, Su S, Lin Y, Fan H, Su J, Wu C. Impacts of Different Reforestation Methods on Fungal Community and Nutrient Content in an Ex-Tea Plantation. Forests. 2023; 14(2):432. https://doi.org/10.3390/f14020432
Chicago/Turabian StyleLiang, Anjie, Xinyi Wen, Wenjing Yu, Shunde Su, Yongming Lin, Hailan Fan, Jun Su, and Chengzhen Wu. 2023. "Impacts of Different Reforestation Methods on Fungal Community and Nutrient Content in an Ex-Tea Plantation" Forests 14, no. 2: 432. https://doi.org/10.3390/f14020432
APA StyleLiang, A., Wen, X., Yu, W., Su, S., Lin, Y., Fan, H., Su, J., & Wu, C. (2023). Impacts of Different Reforestation Methods on Fungal Community and Nutrient Content in an Ex-Tea Plantation. Forests, 14(2), 432. https://doi.org/10.3390/f14020432





